Regeneration dynamics in the laurel forest: changes in species richness and composition
iForest - Biogeosciences and Forestry, Volume 11, Issue 2, Pages 308-314 (2018)
doi: https://doi.org/10.3832/ifor2580-011
Published: Apr 13, 2018 - Copyright © 2018 SISEF
Research Articles
Collection/Special Issue: COST Action FP1202
Strengthening conservation: a key issue for adaptation of marginal/peripheral populations of forest trees to climate change in Europe (MaP-FGR)
Guest Editors: Fulvio Ducci, Kevin Donnelly
Abstract
The recovery and survival of the Macaronesian laurel forest depends on its regeneration strategies. After years of long-term monitoring, both sexual and asexual regeneration appear to be equally important. However, the mechanisms for each are just beginning to be understood. In order to contribute to the understanding of the laurel forest sexual regeneration, we analyzed the species composition of the seedling bank every two weeks over three years in the laurel forest of Anaga (Tenerife, Canary Islands). We compared the species compositions of the seedling bank with the canopy, and analyzed changes in their diversity over this period in different forest stands. We found that species diversity (evenness) is different among plots regardless of the stand. In some cases, plot diversity remained constant over time, while others showed some variations, which were little related to climatic conditions (temperature and precipitation). We also found no relationship between the seedling bank and canopy composition, with shade-intolerant species being more abundant in the former. Although climatic conditions remained constant during the period and other environmental conditions did not vary either, some changes were found in the seedling bank species composition. These were related to the increased degree of conservation of the laurel forest of Anaga (by closing unpaved roads, limiting access, and the abandonment of agriculture) that had negatively affected the density of shade-intolerant species. We suggest that such conservation measures should be maintained and extended to other areas where agriculture has been recently abandoned to allow the potential establishment of laurel forest and late successional species.
Keywords
Conservation, Evenness, Regeneration, Seedling Bank, Species Composition
Introduction
The structural and species composition of forests depend considerably on the reproductive abilities of the dominant tree species that make up its canopy. Indeed, seed dispersal and seedling establishment are important constraints and may represent a major bottleneck in plant population dynamics ([20], [30]). Thus, processes occurring at the seedling level, the most vulnerable stage in the life of a plant species, could have a major impact on the natural regeneration of forests ([25], [29]).
Understanding natural regeneration is of paramount importance to examine the build-up of future forest structure and composition ([11], [38]). The processes involved in tree sexual regeneration can be influenced by many factors, such as variations in seed dispersal intervals, seed quality, wind direction and speed, slope gradients and aspects and soil moisture availability ([41]). In addition, intra- and inter-species competition for water, nutrients, light, and space are also important factors in the regeneration and growth of species ([35]). However, regeneration dynamics are a heterogenic aspect of forests, and environmental conditions significantly affect the survival rate within the seedling bank as well as species composition under similar canopy composition ([16]). Furthermore, natural regeneration can also be affected by human disturbances (deforestation, soil degradation, grazing and fire) and changing species composition of the forest stands ([33], [9]).
The Macaronesian laurel forest is a unique relic ecosystem hosting the highest levels of biodiversity within Europe. It is currently distributed in the archipelagos of Azores, Madeira and the Canary Islands. In the Canary Islands, the laurel forest covers an area of about 10.000 ha, representing only 12% of its original extension before humans arrived on these islands ([13]). Since the arrival of Europeans (15th century), the laurel forest on Tenerife (Canary Islands) has been extensively exploited ([28]) and currently, only 10% of the forest remains in unexploited areas. These areas are well preserved and only small human disturbances have occurred in recent years, as protection of this laurel forest was initiated forty years ago. No data are available about the forest age, but we assume that this evergreen forest is at least a few generations old. This assumption is supported by the lack of reports on government exploitation in this area in the last century ([5]). In addition, aerial pictures taken through time show a similar forest (in terms of extension and structure) without noticeable disturbances. In the research literature, the laurel forest composition has been well described but poorly analyzed in terms of its dynamic processes, a very common situation for montane cloud forests ([17], [22]).
This paper focuses on the sexual regeneration dynamics of the laurel forest in Tenerife, Canary Islands. In order to contribute to the understanding of the laurel forest’s dynamics and diversity, we analysed three forest stands over three years in which seedling bank dynamics were monitored every two weeks. We wanted to test the following hypotheses: (i) laurel forest sexual regeneration is consistent with the canopy composition, thus we are able to explain forest diversity through sexual regeneration; and (ii) species composition and dynamics in the seedling bank remain constant throughout the year.
This paper provides useful information for the development of restoration and afforestation plans in the laurel forest of the Canary Islands and to offer guidance for forest management in order to restore natural forests.
Material and methods
Study site
The study was undertaken in the Anaga Rural Park, which is located in the north-eastern corner of the island of Tenerife, Canary Islands (28° 19′ N, 16° 34′ W - Fig. 1). The park includes a 7-8 million-year-old basaltic massif ([2]) that covers a total of 14.224 ha, spans an altitudinal range from sea level to 1024 m a.s.l (Cruz de Taborno) and represents 7% of Tenerife’s total surface area. The park is characterized by a complex topography dominated by ravines, valleys and cliffs and a central ridge running from northeast to northwest, which results in well-defined northern and southern slopes ([26]). The soils have been classified in the order Entisol, suborder Orthens ([15]). The climate is influenced by the trade winds, which produce a “sea of clouds” on the windward slopes and wetter conditions on the northern than on the southern slopes. The annual precipitation of the park reaches 900 mm, but can be twice this amount if fog drip is taken into account ([24]). The mean annual temperature is close to 15 °C with minimal annual and daily fluctuations and no frost events.
Fig. 1 - Location of Anaga Rural Park in Tenerife and laurel forest stands of El Moquinal and Barranco de los Núñez (windward) and Monte de Aguirre (leeward).
The Park encompasses the best preserved laurel forest remnants and the most biodiverse area of the island. In the Anaga laurel forest, there is a total of 19 tree species ([31]), with Erica arborea, E. platycodon ssp. platycodon, Ilex canariensis, Laurus novocanariensis, Morella faya, Prunus lusitanica ssp. hixa, and Viburnum rigidum being the dominant species. The forest understory is composed of seedlings, saplings and suckers of tree species, and ferns (such as Diplazium caudatum, Dryopteris spp., Woodwardia radicans), with endemic shrubs and forbs (Canarina canariensis, Sideritis spp., Pericallis spp., Geranium reuteri, Isoplexis canariensis) being present in the most open areas ([18]). Nomenclature of plant species follows Acebes et al. ([1]).
Sampling design
In the year 2000, we selected three study sites, representing well-preserved laurel forest stands: “El Moquinal” (plots A, B, C) and “Barranco de los Núñez” (plots D, E, F) on the windward slopes (685-820 m a.s.l.), and “Monte de Aguirre” (plots G, H, J) on the leeward slopes (630-920 m a.s.l.) of the Anaga Rural Park (Fig. 1). At each station, three randomly located permanent plots (10 × 10 m) were established, marked with iron bars at the corners, and these points were geo-referenced with fixed UTM coordinates to record data with a high degree of precision and minimal amount of sampling error. In summary, there were three leeward plots and six windward ones (Tab. 1).
Tab. 1 - General characteristics of the studied plots in the laurel forest of Anaga (Tenerife). Plots A-C were located at the “El Moquinal” sampling site; D-F at “Barranco de los Núñez” and G-I at “Monte de Aguirre”.
| Plot | Altitude (m a.s.l.) |
Slope (°) |
Aspect | Tree density (n ha-1) |
Basal Area (m2 ha-1) | Canopy cover (%) | Light incidence (lux) | Regeneration richness | Canopy richness |
|---|---|---|---|---|---|---|---|---|---|
| A | 685 | 18 | NW (310°) | 1500 | 34.52 | 96 | 1.5 | 8 | 7 |
| B | 790 | 12 | W (260°) | 2900 | 55.89 | 99 | 1.2 | 5 | 4 |
| C | 780 | 20 | NW (45°) | 2600 | 41.95 | 98 | 2.3 | 4 | 5 |
| D | 815 | 27 | N (350°) | 3600 | 58.92 | 98 | 3.0 | 7 | 7 |
| E | 820 | 25 | SW (220°) | 2900 | 36.74 | 98 | 2.8 | 6 | 4 |
| F | 810 | 18 | W (240°) | 1300 | 50.96 | 98 | 3.5 | 7 | 5 |
| G | 630 | 25 | S (190°) | 3000 | 32.98 | 96 | 4.5 | 7 | 4 |
| H | 900 | 45 | S (180°) | 4900 | 48.32 | 96 | 12.0 | 4 | 6 |
| J | 820 | 30 | S (180°) | 1500 | 32.02 | 96 | 3.8 | 9 | 3 |
Starting in winter 2000, within each plot emerging seedlings were permanently tagged by tying a plastic label to the stem and mapped (x, y coordinates within each plot). The seedling bank was monitored every two weeks and seedling establishment and death date were recorded. At the plot level, we also recorded the following parameters: altitude (m a.s.l.), slope (degrees), DBH (diameter at breast height in cm for individuals taller than 1.30 m with a DBH of at least 1 cm), tree density (individuals m-2), seedling density (seedling m-2), basal area (m2 ha-1), canopy height (m) and canopy cover (%, using a spherical densitometer); the two last parameters were measured at each plot corner and the plot centre. Additionally, we measured the percentage of light above the vegetation that reaches the ground surface. Percentage of light penetration was measured using a point quantum sensor, taking nine measurements per plot (one measure each five meters) to cope with spatial variation in light, and two measurements outside the canopy. Sampling was carried out from December 2000 to December 2003. There is a maximum variation of 4 sampled periods at the beginning and at the end of the survey, but all the plots include between 50 and 54 sampling periods.
The nearest meteorological station with complete data series is located around 3-5 km from the three studied sites (Anaga-Taganana meteorological station), but at a lower elevation (300 m a.s.l.). We provide the mean, maximum and minimum temperatures for each month from 1999 to 2004, and the monthly precipitation for the same period, in order to relate possible changes in the seedling bank with changes in weather conditions in the previous years.
Statistical analyses
Environmental characteristics and stand structure of the groups of plots were analysed by the analysis of variance (ANOVA) following the same procedure. Normality of the data was checked with the Shapiro-Wilk test and the homoscedasticity of the data with an F test (p<0.05). The post-hoc Tukey’s test was used to test for significant differences between group means for the different variables. We looked for differences between these groups in elevation, slope, canopy cover, tree density, tree basal area and light penetration.
To measure the distribution of abundance among species in the seedling community, a basic feature of biological plant communities, we calculated the evenness index of Smith & Wilson ([34]) for each sampled period of each plot and represented it graphically in order to determine temporal changes over the study period. For these calculations, we used the seedling density of the species and each different sampling period and location.
Ordination techniques were applied to explain the community variation ([21]), and to evaluate trends over time and space ([19], [37]). We used Detrended Correspondence Analysis (DCA - [23]) using the CANOCO software ([37]) to examine how the seedling bank species composition changed over time in each plot. We included in the analyses all the sampled periods, between 49 and 53 sampled periods per plot (indicated as “Plot_” followed by the letter that identifies the plot). We also included the species composition of the canopy (indicated as “Can_” followed by the plot’s letter). In order to standardize all the information, we based the analyses on the percentage of the species density. All the regeneration periods analysed per plot were represented by the centroid of all the periods sampled.
Basic statistical methods followed Zar ([42]) and were implemented using the SPSS® statistical package ([36]).
Results
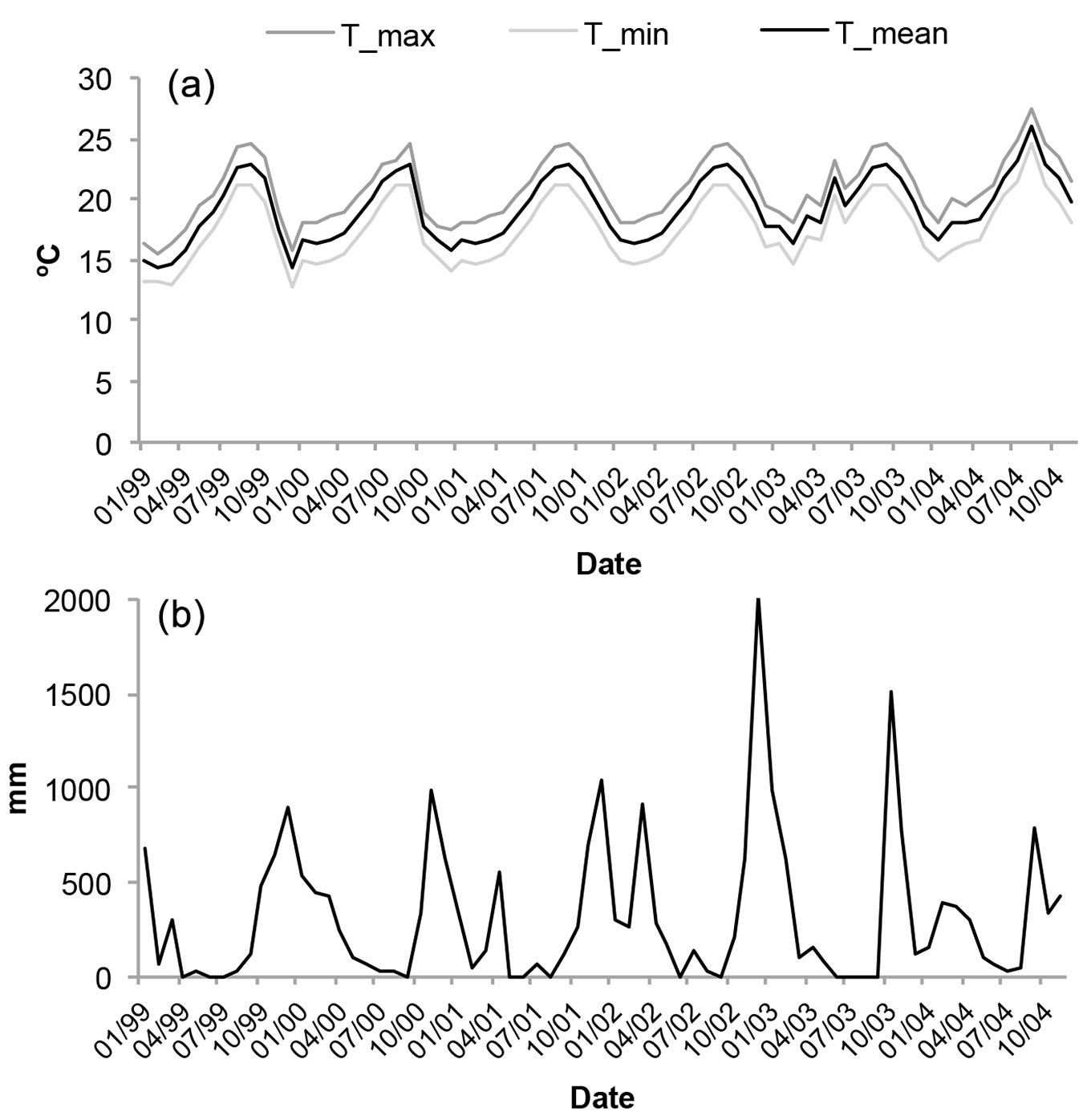
During the sampled period, we did not register any relevant variability for temperatures and precipitation. Only during the summer of 2004 did the average temperature increase by 2 °C, while it remained practically the same in the previous 5 years (Fig. 2a). With respect to precipitation, there is one rainy period during the year, October-November, although winter periods also have some important precipitation (Fig. 2b). November 2002 was particularly intense in rains, as was November 2003, while the rest of the years the precipitation remained regular (900-1000 mm).
Fig. 2 - (a) Mean temperature, mean minimum temperature and mean maximum temperature per month (based on the daily information over the period January 1999 to December 2004). (b) Total monthly precipitation in Anaga Rural Park from January 1999 to December 2004.
Environmental variables (Tab. 1) did not reveal significant differences and neither did other stand structure variables analysed (density, basal area and species richness - p>0.05). Altitude ranged between 685 and 900 m a.s.l., well within the laurel forest elevational belt. Tree density was variable, between 1300 to 4900 ind. ha-1 (26% variation), while basal area showed a variation among plots of almost 55% (32.02 to 58.92 m2 ha-1), pointing to a certain difference in the maturity of the plots. A total of 15 tree species were found in the study, three of them (Ocotea foetens, Rhamnus glandulosa and Visnea mocanera) being absent from the plot canopies and three more (Erica arborea, E. platycodon ssp. platycodon and Morella faya) absent from the seedling banks.
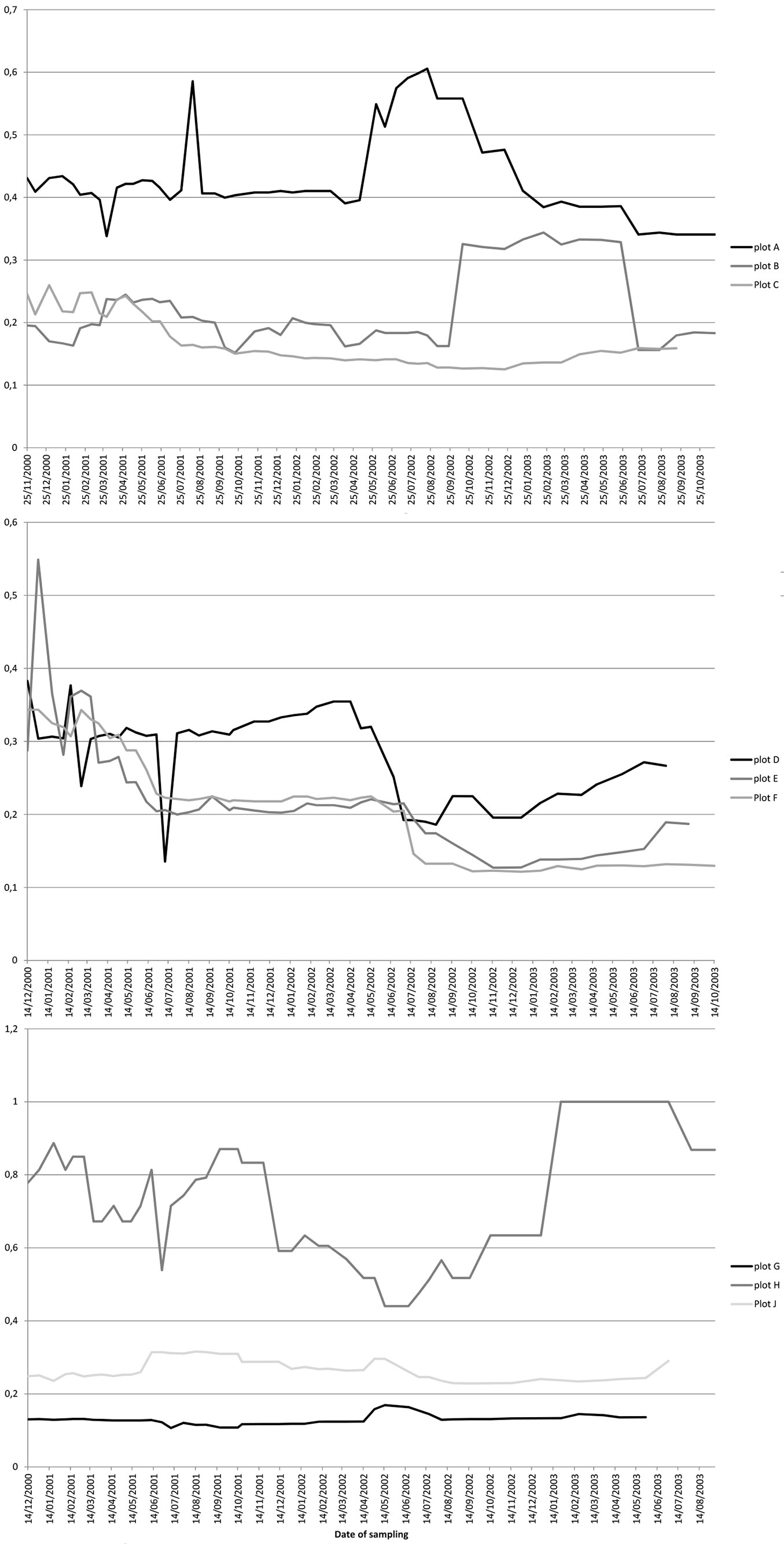
Results of the evenness analysis for the 9 plots (Fig. 3) showed the highest values for plot H, with more than 50% of variability over the three years, and reaching in some cases values of 1 (for a period at the end of 2002), while the other plots (G and J) of the same area (“Monte de Aguirre”) remained constant throughout the three years with low values, between 0.15 and 0.3. In the group of plots A, B and C (“El Moquinal”), plot A had values over 0.4 increasing for a period to 0.6, while plots B and C remained, in general, constant between 0.1 and 0.35. Finally, plots D, E, F (“Barranco de los Núñez”) had a more constant variation, starting at values of 0.5 but decreasing over the three years to 0.1 in the case of plot F and to 0.25 in plot D.
Fig. 3 - Smith and Wilson’s evenness index for the 9 studied plots in the Anaga laurel forest (Tenerife). Each line represents the variation of the index value at each sampling plot from November 2000 to January 2003. Upper panel: “El Moquinal” sampling site (plots A, B, C); middle panel: “Barranco de los Núñez” sampling site (plots D, E, F); lower panel: “Monte de Aguirre” sampling site (plots G, H, J).
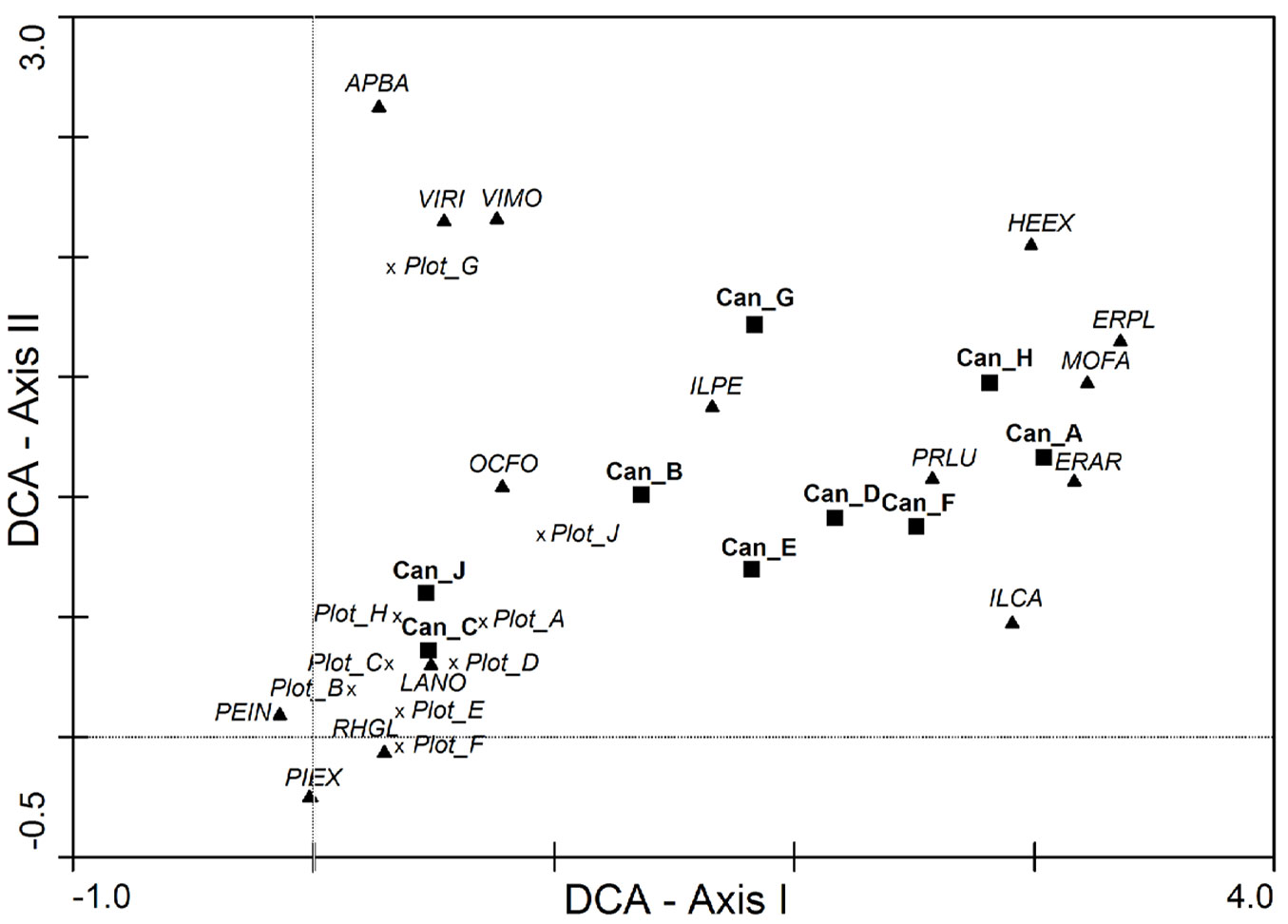
The ordination analysis (Fig. 4) revealed that only plots C and J had some consistency between the seedling bank and the canopy composition: these plots were located near the centroids of the regeneration plots (Plot_A, Plot_B, Plot_C, Plot_D, Plot_E, Plot_F, Plot_H). The rest of the canopy plots (Can_A, Can_D, Can_F, Can_G, Can_H) had pioneer species such as Erica arborea, E. platycodon ssp. platycodon, and Morella faya, but also some mid to late successional species such as Ilex canariensis, I. perado ssp. platyphylla, Prunus lusitanica ssp. hixa and Heberdenia excelsa, as the representative species of the canopy composition. However, the seedling bank composition was characterized by Laurus novocanariensis, Picconia excelsa, Persea indica, Ocotea foetens, and Rhamnus glandulosa, mesic species of well-preserved laurel forest stands ([4]). Some canopy plots were located at a more intermediate position (Can_B and Can_E). There was a gradient of species composition along Axis I from seedling plots to canopy plots. In the case of Axis II, seedling bank of Plot G was discriminated due to the occurrence of Apollonias barbujana, Viburnum rigidum and Visnea mocanera. This plot was characterized by a high tree density and a lower elevation with respect to the others (Tab. 1).
Fig. 4 - Detrended Correspondence Analysis (DCA) of the regeneration (seedling bank) plots and canopy (tree) plots with the species composition based in percentage abundance. Regeneration plot coordinates indicate the centroid of all the sampled periods with a cross (Plot_A, Plot_B, Plot_C, …, Plot_J), canopy plots are represented with a black square (Can_A, Can_B, Can_C, …, Can_J), and the species coordinates is represented with a triangle with the names indicated by the two first letters of their genus followed by the first two letters of the species: Apollonias barbujana (APBA), Erica arborea (ERAR), Erica platycodon ssp. platycodon (ERPL), Heberdenia excelsa (HEEX), Ilex canariensis (ILCA), Ilex perado ssp, platyphylla (ILPE), Laurus novocanariensis (LANO), Morella faya (MOFA), Ocotea foetens (OCFO), Persea indica (PEIN), Picconia excelsa (PIEX), Prunus lusitanica ssp. hixa (PRLU), Rhamnus glandulosa (RHGL), Viburnum rigidum (VIRI) and Visnea mocanera (VIMO). Eigenvalue for axis I: 0.639, eigenvalue for axis II: 0.532, cumulative percentage of variance of species data for axes I and II: 41.6 %.
Discussion
During three years of sexual regeneration monitoring in the laurel forest of Anaga, we found 15 tree species, of which 11 appeared as canopy trees (all except Heberdenia excelsa, Ocotea foetens, Rhamnus glandulosa and Visnea mocanera), while 12 appeared in the seedling banks (all except Erica arborea, E. platycodon ssp. platycodon and Morella faya). The first group of species absent in the plot canopies are non-pioneer species, some of them are considered as very specific to certain environments, such as V. mocanera or H. excelsa for the xeric laurel forest ([4]), while O. foetens prefers the most humid parts of the forest ([12]). Their absence from the canopy within the studied area (900 m2 in total) might be explained by their specificity to certain environments and does not imply they are not present in nearby areas. This means that tree species richness would be better captured by larger sampling areas within the laurel forest, for instance 50 × 50 m plots ([6], [10]). Regarding the species absence from the seedling banks, they correspond to the three pioneer species occurring in the laurel forest ([17]). This is a common situation found in previous studies in this forest, in which human activities (wood extraction, agriculture, charcoal production, etc.) have promoted the occurrence of pioneer species in the forest. However, seeds from these pioneer species only germinate under light conditions, so they cannot be found under a closed canopy ([17]). Therefore, the adult trees of these species are just remnants of the early successional stages of the forest. These trees may persist within the forest for decades; in particular, M. faya is able to regenerate vegetatively by producing basal sprouts, and the trees are usually among the biggest and oldest in the laurel forest ([14]). After several decades of strict conservation of the Anaga Rural Park, the canopy composition cannot be explained based on the sexual regeneration. Pioneer species are now more abundant in other communities like the fayal-brezal (Morella-Erica woody heath - [6]), and are clearly present in the laurel forest seed bank but are unable to germinate ([5]).
In this study, environmental conditions did not significantly differ among the plots within the same stand. However, aspect is an important determinant of species composition ([10], [27]) which is supposed to control species richness or basal area and density. Nonetheless, we did not find any significant difference among stands in terms of species richness, basal area and density. According to these results, local environmental differences seem to be more relevant than those of the stand or even the aspect of the plot. The evenness index revealed differences among groups of plots, confirming that microenvironmental conditions are an important issue in determining species germination ([17]). It is worth noting that while some plots remain constant in their seedling species composition over the period regardless of the weather conditions, some others showed more variability, although this was hardly related with climate (Fig. 2). In this case, we assumed that the environmental conditions were equally suitable for all the species (being all typical of the laurel forest) and equally affect the seedling bank community.
The distribution of seedlings in the forest is dependent on the distribution of parent trees producing seeds, the seed rain, the presence of dispersers and predators and the availability of adequate sites for germination ([32]). Based on the lack of relationships between the seedling bank composition and the tree composition in some plots, the role of dispersers becomes essential to understand these results. In previous studies, the importance of dispersers, mainly birds, in the spreading of fruits has been shown ([40], [3], [8]). However, the environmental pressure in this ecosystem is not so strong as to create a completely dependent relationship of fruit dispersers. Moreover, the fruit production does not differ between species ([8]). Some of the plots (such as plot J and C) are more similar in their canopy species composition and their seedlings than other plots, and even more similar to the seedling bank of the other plots than their own ones (as in the case of plot J). This lack of relationship between species composition of canopy and seedling bank has been previously analysed by models, which predicted a change in the laurel forest canopy in Anaga, with an increase of later successional species (e.g., Lauraceae) and a reduction of pioneer ones (such as Erica arborea, E. platycodon ssp. platycodon and Morella faya). Given the low climate variability and low disturbance regime, shade intolerant species are expected to be displaced to marginal areas in the forest or even to areas affected by anthropogenic disturbance, as long as natural disturbances do not foster the establishment of these pioneer species within the forest ([5], [7], [9]).
Although sexual regeneration of shade-intolerant species has not been recorded in the period analysed, there is evidence that they are present in the seed bank over the whole area of the laurel forest in Anaga ([9]). Similar situations have been reported by other studies concerning seed bank analysis ([39]). Furthermore, some of the early successional species can reproduce mainly by asexual regeneration, thus ensuring their presence in the stand for many generations; therefore, although changes in species composition are expected, these are assumed to be long term. Finally, to fully understand species composition in the laurel forest at the present time, the human intervention has to be considered, as the arrival of Europeans in the Canary Islands five centuries ago had an important impact on the extension of this forest ([28]).
In summary, the enhanced conservation measures applied to the laurel forest of Anaga (by closing unpaved roads, limiting access, and the abandonment of agriculture) had negatively affected the density of shade-intolerant species. We recommend such conservation measures to be maintained and extended to other areas where agriculture has been recently abandoned, in order to allow the establishment of potential laurel forest and late successional species, thus providing better opportunities for the recovery of this plant community.
Acknowledgments
We thank the Rolle-Stiftung and Hotel Océano for their economic support. We are also grateful to the staff of Anaga Rural Park for granting permission to conduct this study and their support for the project. Climatic data were provided by the Agencia Estatal de Meteorología (AEMET). We are also grateful to all the field-work assistants: Cristina Blandino, Alistair Dominguez, Gustavo Morales and Priscila Rodríguez. We also appreciate the comments and corrections on the English provided by Clive Tyrell, professional technical editor.
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Lea De Nascimento
Silvia Fernández-Lugo
Javier Méndez
Guacimara González-Delgado
Eduardo Balguerías
José María Fernández-Palacios
Departamento de Botánica, Ecología y Fisiología Vegetal, Universidad de La Laguna, La Laguna, 38206 Santa Cruz de Tenerife (Spain)
Instituto de Botânica, Governo do Estado São Paulo, Av. Miguel Stéfano 3687, São Paulo (Brazil)
Corresponding author
Paper Info
Citation
Arévalo JR, De Nascimento L, Fernández-Lugo S, Méndez J, González-Delgado G, Balguerías E, Gomes Pereira Cabral E, Fernández-Palacios JM (2018). Regeneration dynamics in the laurel forest: changes in species richness and composition. iForest 11: 308-314. - doi: 10.3832/ifor2580-011
Academic Editor
Fulvio Ducci
Paper history
Received: Aug 03, 2017
Accepted: Jan 12, 2018
First online: Apr 13, 2018
Publication Date: Apr 30, 2018
Publication Time: 3.03 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2018
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 49483
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 41430
Abstract Page Views: 2850
PDF Downloads: 4084
Citation/Reference Downloads: 19
XML Downloads: 1100
Web Metrics
Days since publication: 2877
Overall contacts: 49483
Avg. contacts per week: 120.40
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2018): 2
Average cites per year: 0.25
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Optimum light transmittance for seed germination and early seedling recruitment of Pinus koraiensis: implications for natural regeneration
vol. 8, pp. 853-859 (online: 22 May 2015)
Review Papers
Methods of soil seed bank estimation: a literature review proposing further work in Africa
vol. 15, pp. 121-127 (online: 26 March 2022)
Research Articles
Methods for predicting Sitka spruce natural regeneration presence and density in the UK
vol. 12, pp. 279-288 (online: 23 May 2019)
Research Articles
Investigating the effect of selective logging on tree biodiversity and structure of the tropical forests of Papua New Guinea
vol. 9, pp. 475-482 (online: 25 January 2016)
Review Papers
The forest biodiversity artery: towards forest management for saproxylic conservation
vol. 9, pp. 205-216 (online: 26 October 2015)
Research Articles
Biodiversity conservation and wood production in a Natura 2000 Mediterranean forest. A trade-off evaluation focused on the occurrence of microhabitats
vol. 12, pp. 76-84 (online: 24 January 2019)
Review Papers
Soil seed banks of pioneer tree species in European temperate forests: a review
vol. 11, pp. 48-57 (online: 25 January 2018)
Research Articles
Tropical seedling performance under drought: a functional trait approach for species selection in restoration
vol. 19, pp. 9-17 (online: 10 January 2026)
Research Articles
Bird response to forest structure and composition and implications for sustainable mountain forest management
vol. 19, pp. 18-27 (online: 11 January 2026)
Research Articles
Endangered and endemic species increase forest conservation values of species diversity based on the Shannon-Wiener index
vol. 9, pp. 469-474 (online: 02 January 2016)
iForest Database Search
Google Scholar Search
Citing Articles
Search By Author
- JR Arévalo
- L De Nascimento
- S Fernández-Lugo
- J Méndez
- G González-Delgado
- E Balguerías
- E Gomes Pereira Cabral
- JM Fernández-Palacios
Search By Keywords
PubMed Search
Search By Author
- JR Arévalo
- L De Nascimento
- S Fernández-Lugo
- J Méndez
- G González-Delgado
- E Balguerías
- E Gomes Pereira Cabral
- JM Fernández-Palacios
Search By Keyword