Nearest neighbour relationships in Pinus yunnanensis var. tenuifolia forests along the Nanpan River, China
iForest - Biogeosciences and Forestry, Volume 10, Issue 4, Pages 746-753 (2017)
doi: https://doi.org/10.3832/ifor2405-010
Published: Aug 01, 2017 - Copyright © 2017 SISEF
Research Articles
Abstract
Forest stand structural diversity can be examined at different scales. Small-scale structural changes are the basis of forest structural diversity and habitat heterogeneity, and play a key role in biodiversity conservation. Most research on forest structure has focused mainly at stand level and above, with little attention paid to fine-scale structure and correlations among different forest stand attributes. We set up four permanent plots within a secondary forest community of Pinus yunnanensis var. tenuifolia mixed forests along the Nanpan River in southern China. We analyzed their nearest-neighbor relationships using a bivariate distribution of stand spatial structural parameters (SSSP) with the aim of understanding the processes that drive structural diversity in the development of a secondary forest community. Our results revealed that communities with different disturbance histories and species compositions differed in the level of species mixture. Large, small, and medium-sized trees were well mixed within the community, both conspecific and heterospecificindividual with varying densities. All plots exhibited a uniform size differentiation pattern. Trees with different dominance levels or mixture levels were randomly distributed within the plots, and only few of these displayed clumped or regular distribution. Pearson’s correlation analysis revealed that distribution patterns may be related to species composition and diameter differentiation, though their relationship was very weak. The results of this study are relevant to optimize forest management activities in the studied stands, and promote tree growth, regeneration and habitat diversity at the fine scale.
Keywords
Bivariate Distribution, Nearest Neighbour Trees, Pinus yunnanensis, Secondary Forest, Structure Diversity
Introduction
Stand structure is a basic forest attribute resulting from forest dynamics and biophysical processes ([39], [34]), and is thought to be a driving factor of tree growth. Natural forests usually have complex physical structures, species diversity, and integrated biochemical cycles, which provide a number of benefits, including increased productivity, resistance to invasive alien species, and ecosystem resilience ([21], [31]). Therefore, a complex stand structure is often viewed as a reference state of the forest and a template for forest management ([4], [38]). Recently, much attention has been paid to the value of forest ecosystems and the diversified benefits and multiple functions provided by the forest stands. More structured forests can increase their biodiversity and strengthen forest ecosystem services ([28]). However, although classic forest management models aimed at improving the forest structural diversity are based on principles drawn from the knowledge of natural ecosystem process ([40]), the resulting management principles are not yet able to meet the forest management needs. Therefore, a deeper understanding of forest structural diversity and the differences between forest types is required ([31]).
Forest structural diversity exists at a variety of spatial scales, which can be roughly divided into four levels (in descending order): delta, gamma, beta, and alpha. The delta and gamma levels include landscape-scale and large forest areas ([33], [22]), comprise all forest community types, and are considered in studies on habitat fragmentation ([19]). The beta level, or medium scale, enables the comparison between forest stands. The alpha level is the smallest scale, and depends on stand-level characteristics. Patch dynamics generally deal with small or very fine scales ([11], [12], [5]). These classifications are directly related to the appearance of the forest community. Many studies have been carried out at landscape and especially stand levels ([4], [34], [5], [22]). This may be because stand-level data are relatively easy to acquire and interpret, particularly due to the availability of tools and techniques designed for forest management at this scale.
However, as forest management technologies and the knowledge of forest ecosystem diversity improve, a better understanding of forest structural diversity at smaller scales is increasingly needed, with particular regards of the relationships taking place among nearest neighbour trees and their spatial distribution ([33]). This information is important for three major reasons: (i) it allows a better understanding of the processes underlying the forest community succession, including the competition between trees of different sizes ([8], [36]); (ii) it reveals the cycle of aging and death in dominant tree groups and regeneration and growth from the understory ([29]); and (iii) it is related with the chemical and physical intraspecific and interspecific interactions ([6], [26]). Forest management can exploit these mechanisms to determine the ratio of tree species, control distances between individuals, and plan harvesting and regeneration. It may also help clarify the relationships between forest stand structure and the type and quantity of other organisms living in the forest, particularly those vulnerable to changes in habitat ([43], [17]). To our knowledge, understanding of forest structural diversity at different scales is still incomplete. In particular, little attention has been paid to the spatial relationships of nearest neighbour trees, which could be revealed through an analysis of natural forests of different types and ages.
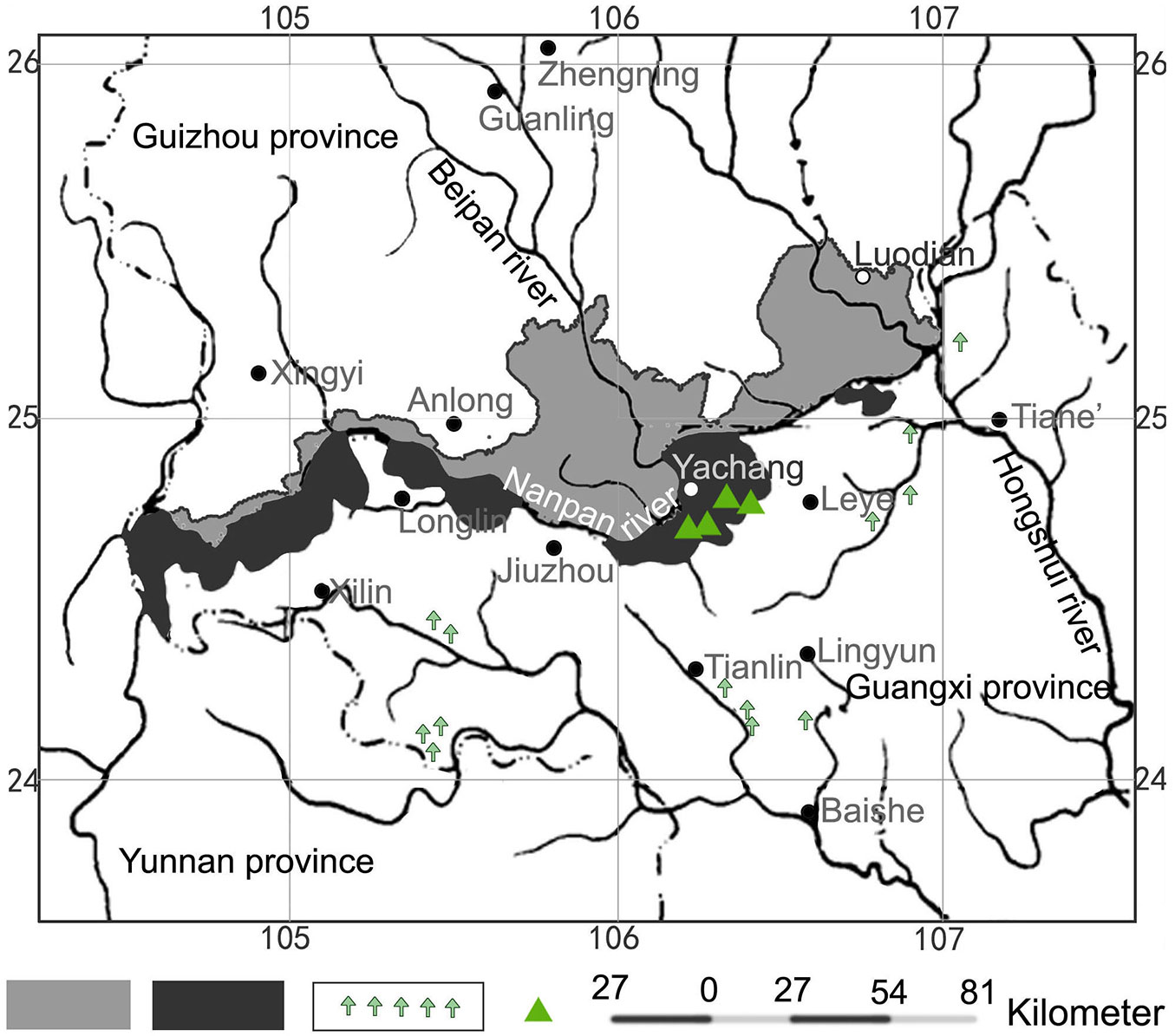
Pinus yunnanensis var. tenuifolia is a geographical variety of Pinus yunnanensis (Franch.) that has spread down the Nanpan River valley. Historically, its range was the long and narrow area formed by the riverbanks of the Nanpan and Beipan Rivers, as well as the Hongshui River ([23]). P. yunnanensis var. tenuifolia is a dominant species influencing the habitats and species composition of the plant community ([44]). Its leaves are thin, tender, and glossy, an adaptation to the dry, hot local climate of these river valleys. Most of the primary P. yunnanensis var. tenuifolia forests disappeared after the excessive cutting carried out between 1950 and 2000. The high quality of the wood materials produced by this species induced the Chinese government to protect these forests. Nonetheless, after the completion of the Longtan Dam in the lower reaches of the Hongshui River in 2005, flooding destroyed a large proportion of the P. yunnanensis var. tenuifolia forests. Subsequently, the population redistribution occurred after the flooding resulted in the deforestation of mountain areas for farming and grazing livestock. Only sparse secondary forests of P. yunnanensis with different age structures and disturbance histories have remained in Guangxi province (Fig. 1). Since 2006, the remaining forest resources were protected with the establishment of the Yachang Orchid National Nature Reserve. At present, however, we still know very little of the present situation and characteristics of such P. yunnanensis forests. Previous studies were conducted in very limited areas with the aim of examining their geographical distribution before cutting ([9]), the community composition ([44]), and soil fertility characteristics ([46]), as well as to inventory the understory and tree harvest ([2]).
Fig. 1 - Distribution of P. yunnanensis var. tenuifolia in Guizhou (gray) and Guangxi provinces (black). Green arrows indicate sporadic distribution of the species within the Guangxi province. Green triangles indicate our study sites.
As current P. yunnanensis var. tenuifolia forests show different tree species compositions and experienced different disturbance type and severity, we hypothesized that their nearest neighbour relationships differed from each other. The aims of this study were to: (1) explore the variation of nearest neighbour relationships between trees along the secondary succession of these forests; (2) analyze the degree of correlation between various stand spatial properties; (3) determine the structural diversity of P. yunnanensis var. tenuifolia secondary forests and clarify its dynamics.
Materials and methods
Study site
Our study area was located in the Yachang National Orchid Reserve, Leye, Baise, Guangxi Zhuang Autonomous Region (24° 44′ 16″ - 24° 53′ 58″ N; 106° 11′ 31″ - 106° 27′ 04″ E), belonging to the border area of the Yunnan, Guizhou, and Guangxi provinces located in southwest China. It is also the transition zone from the Yunnan-Guizhou plateau area to the hilly mountains of Guangxi. This reserve is 26.2 km in length (east-west) and 18 km in width (north-south), with a total area of 22.062 ha. Its terrain is mainly medium- to low-elevation mountains that trend roughly from northwest to southeast, with many valleys and overlapping peaks. Panguwang Mountain is 1971 m in elevation, and the lowest point in the valleys is approximately 400 m a.s.l. The climate is subtropical monsoon, and is strongly influenced by monsoon circulation and foehn effects. A humid (marine) climate prevails in summer, whereas a cold (mainland) climate is typical in winter. The average annual temperature is 16.3 °C, with a maximum temperature of 41.1 °C and a minimum temperature of -3 °C, with occasional frost and snow in winter. Rain falls mainly in summer; the average annual rainfall is approximately 1051.7 mm. Significant drought occurs in spring and autumn. In valleys lower than 500 m a.s.l., cinnamon soil predominates, and at higher altitudes (500-1000 m) hilly red soils are common. Mountain yellow brown soil appears only above 1000 m in altitude, and develops into meadow soil at the tops of some mountains. This area is known for a great number of wild orchids, including Tiankeng groups, as well as some unique and rare Chinese plants such as the Handeliodendron bodinieri (Levl.) Rehd. and the tree fern Alsophila spinulosa (Wall. ex Hook.) R. M. Tryon.
Our study sites were located at the Huaping, Langquan, and Yigou conservation stations. These are situated in secondary forests in areas where primary P. yunnanensis var. tenuifolia forests had been severely damaged. They had experienced different types of disturbance and recovery, resulting in two main types of species composition (Fig. 1). Without human disturbance, the pine-oak mixed forests at Langquan preservation station naturally developed on the burn blank where virgin forest had been completely destroyed at 1987. P. yunnanensis var. tenuifolia and other several oak species dominate the plant community of 18 tree species. Many standing dead trees and fallen logs remain in different stages of decay, and the ground is covered by a thick litter layer. The canopy cover is full and tree density is high, with little vertical structure. The slope of the forest floor is steep, and tree species change notably with increases in altitude, shifting toward oak seedlings. At the Huaping conservation station, pine-Keteleeria mixed forest regenerated following intense thinning in 1970. P. yunnanensis var. tenuifolia and Keteleeria davidiana (Bertr.) Beissn. are the dominant species. Plant species richness is very high; in addition to the 40 species of trees and shrubs, there are also six types of climbing plants. K. davidiana and Q. variabilis seedlings are abundant. The pine-oak mixed forests at the Yigou conservation station are dominated by both P. yunnanensis var. tenuifolia and Q. variabilis, which regenerated following clear-cuts in 1960 on lands once covered by primary forest. During its recovery period, approximately half of the P. yunnanensis var. tenuifolia population on medium slopes were cut for sap in 1990, and some oaks were harvested for fuel; only a few trees were cut on the steeper slopes at higher altitudes. A large number of dead Lyonia ovalifolia var. elliptica, L. ovalifolia var. lanceolata, and P. yunnanensis var. tenuifolia remain on the forest floor. The forest features a total of 27 species, 4 varieties of orchids, and a thin litter layer. Although plant species compositions differ between these forests, they share barren, dry, weakly acidic soils with high gravel content. There are few traces of cutting and regeneration in the P. yunnanensis var. tenuifolia population (Tab. 1, Tab. 2).
Tab. 1 - The main characteristics of P. yunnanensis var. tenuifolia communities.
| Parameter | Plot a | Plot b | Plot c | Plot d |
|---|---|---|---|---|
| Site | Daping | Yigou | Hua ping | Yigou |
| Location | 106°19′ 4.2″ N, 24°51′ 15.9″ E |
106°14′ 14.6″ N, 24°47′ 25.4″ E |
106°23′ 10.6″ N, 24°49′ 54.2″ E |
106°14′ 9.5″ N, 24°27′ 28.9″ E |
| Mean altitude (m) | 1068.5 | 770.3 | 1253.9 | 749.9 |
| Mean slope (°) | 26 | 22 | 10 | 25 |
| Slope position | up | up | medium | medium |
| Aspect | Southeast | Southwest | Northwest | Southwest |
| Soil type | rendzina | mountain yellow soil | rendzina | mountain yellow soil |
| Gravel content | 40.6% | 35.4% | 53.9% | 38.1% |
| Community type | Pine-oak mixed forest | Pine-oak mixed forest | Pine-other conifer mixed forest | Pine-oak mixed forest |
| Canopy cover | 0.90 | 0.80 | 0.85 | 0.80 |
| density (trees ha-1) |
2931 | 1592 | 1340 | 1340 |
| Plot area (m2) | 10000 | 6400 | 8000 | 5600 |
| Basal area (m2 ha-1) | 33.39 | 22.55 | 32.88 | 26.26 |
| Litter thickness (cm) | 5.0-7.0 | 1.0-3.0 | 3.0-5.0 | 3.0-5.0 |
| Disturbance type and return time | No human interference | Little pine resin harvest (25 years ago) |
Intensive selection harvest (35 years ago) |
Some pine resin and oak selection harvest (25 years ago) |
| Regeneration type | Natural, following fire | Natural, following clear-cut | Natural, following harvest | Natural, following clear-cut |
Tab. 2 - The main floristic composition of the four studied plots (a-d).
| Trees | Shrubs | Herbs | Vines |
|---|---|---|---|
| Albizia kalkora (Roxb.) Prain Betula alnoides Buch-Ham. Cyclobalanopsis glauca (Thunb.) Oerst. Cyclobalanopsis glaucoides Schottky Diospyros kaki Thunb. var. silvestris Makino Kalopanax septemlobus (Thunb.) Koidz. Keteleeria davidiana (Bertr.) Beissn. Liquidambar formosana Hance Meliosma veitchiorum Hemsl. Phyllanthus emblica L. Pinus yunnanensis var. tenuifolia Quercus variabilis Bl. Rhus chinensis Mill. Schima wallichii (DC.) Choisy Toona ciliata M. Roem. Vernicia fordii (Hemsl.) Airy-Shaw Wendlandia uvariifolia Hance |
llex crenata Thunb. Buddleja officinalis Maxim. Cerasus yedoensis (Matsum.) Yu et Li Coriaria nepalensis Wall. Eurya japonica Thunb Eurya distichophylla Hemsl. Glochidion eriocarpum Champ. ex Benth. Glochidion puberum (L.) Hutch. Lespedeza Formosa (Vog.) Koehne Lyonia ovalifolia (Wall.) Drude var. elliptica Lyonia ovalifolia (Wall.) Drude var. lanceolata (Wall.) Hand.-Mazz. Mahonia fortunei (Lindl.) Fedde Myrica rubra (Lour.) S. et Zucc. Pyrus calleryanar Decne. Rhus sylvestris Sieb. & Zucc Sapium discolor (Champ. ex Benth.) Muell.-Arg. Trachycarpus fortunei (Hook.) H. Wendl. |
Asplenium trichomanes L. Sp. Cleisostoma fuerstenbergianum Kraenzl. Cyclosorus acuminatus (Houtt.) Nakai Cymbidium ensifolium (L.) Sw. Dendrobium officinale Kimura & Migo Dicranopteris linearis (Burm.f.) Underw. Miscanthus floridulu (Labnll.) Warb Monotropa uniflora Linn. Pteridium aquilinum var. latiusculum (Desv.) Underw. ex Heller Vanda concolor Bl. Woodwardia japonica (L. f.) Sm. |
Ficus tikoua Bur. Gynostemma pentaphyllum (Thunb.) Makino Lonicea confusa (Sweet) DC. Parthenocissus tricuspidata (Siebold & Zucc.) Planch. Rubus feddei H. Lév. & Vaniot |
Plot establishment
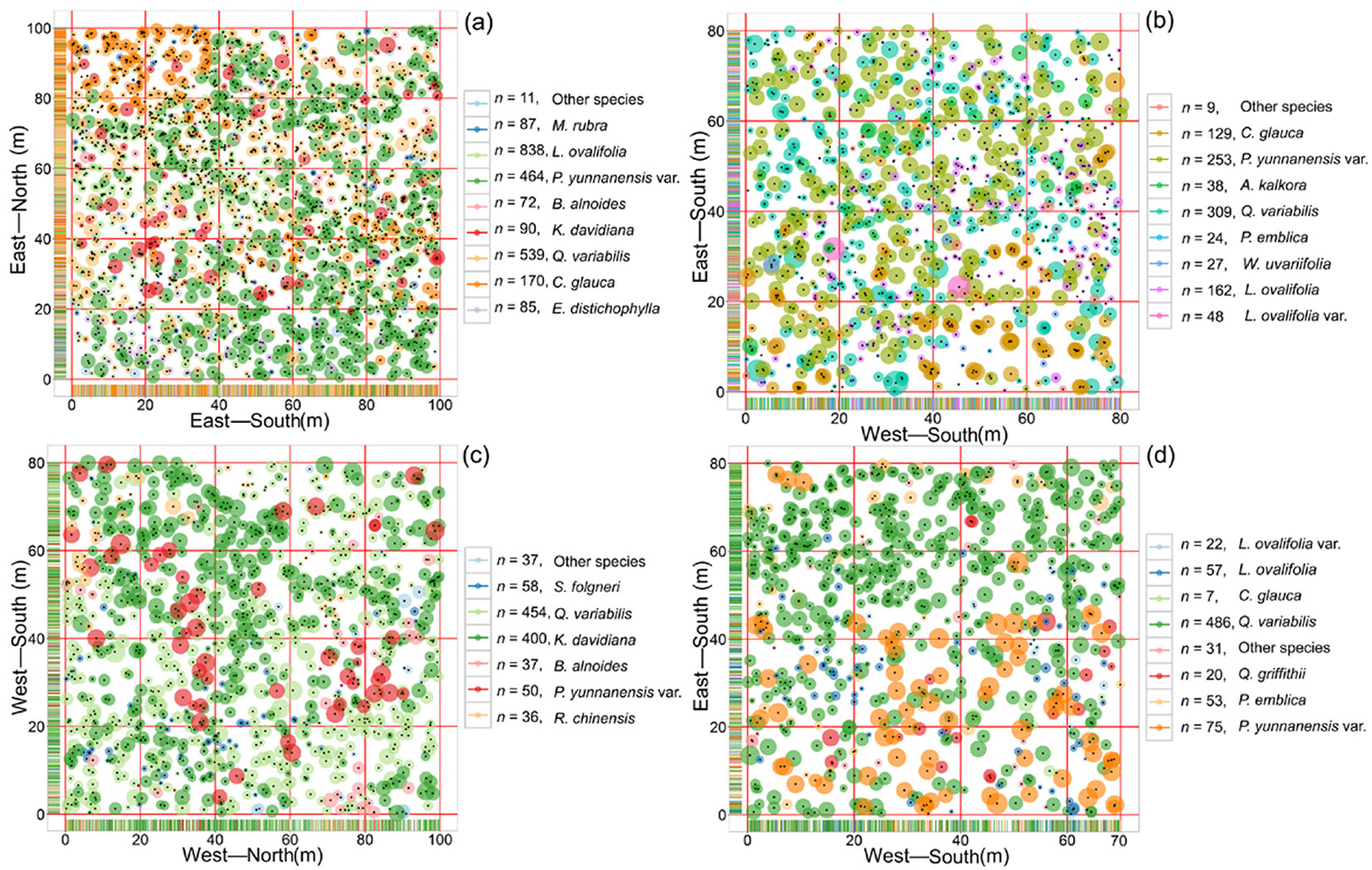
During 2015 to 2016, we selected two different types of P. yunnanensis var. tenuifolia communities from the conservation stations described, using a typical sampling method (Fig. 2). We used GPS to locate areas large enough for sampling, then used a total station (model NTS-372R10, South Surveying and Mapping Instrument Co., Guangzhou, China) to divide each area into several subplots (20 × 20 m each) to reduce measurement errors and ensure that closure error for the area was limited to 1/400. We located the precise position in three dimensions of any tree with a diameter at breast height > 5 cm, and marked the tree with a unique identifier. We also recorded tree characteristics such as tree species, height, crown shape, canopy area, and health status. A total of four plots measuring 100 × 100 m, 100 × 80 m, 80 × 80 m, and 80 × 70 m were established (Fig. 2).
Fig. 2 - Distribution patterns in the four studied plots. Colors of circles indicate tree species and their sizes indicate the diameter. (n): number of tree for each species.
Data analysis
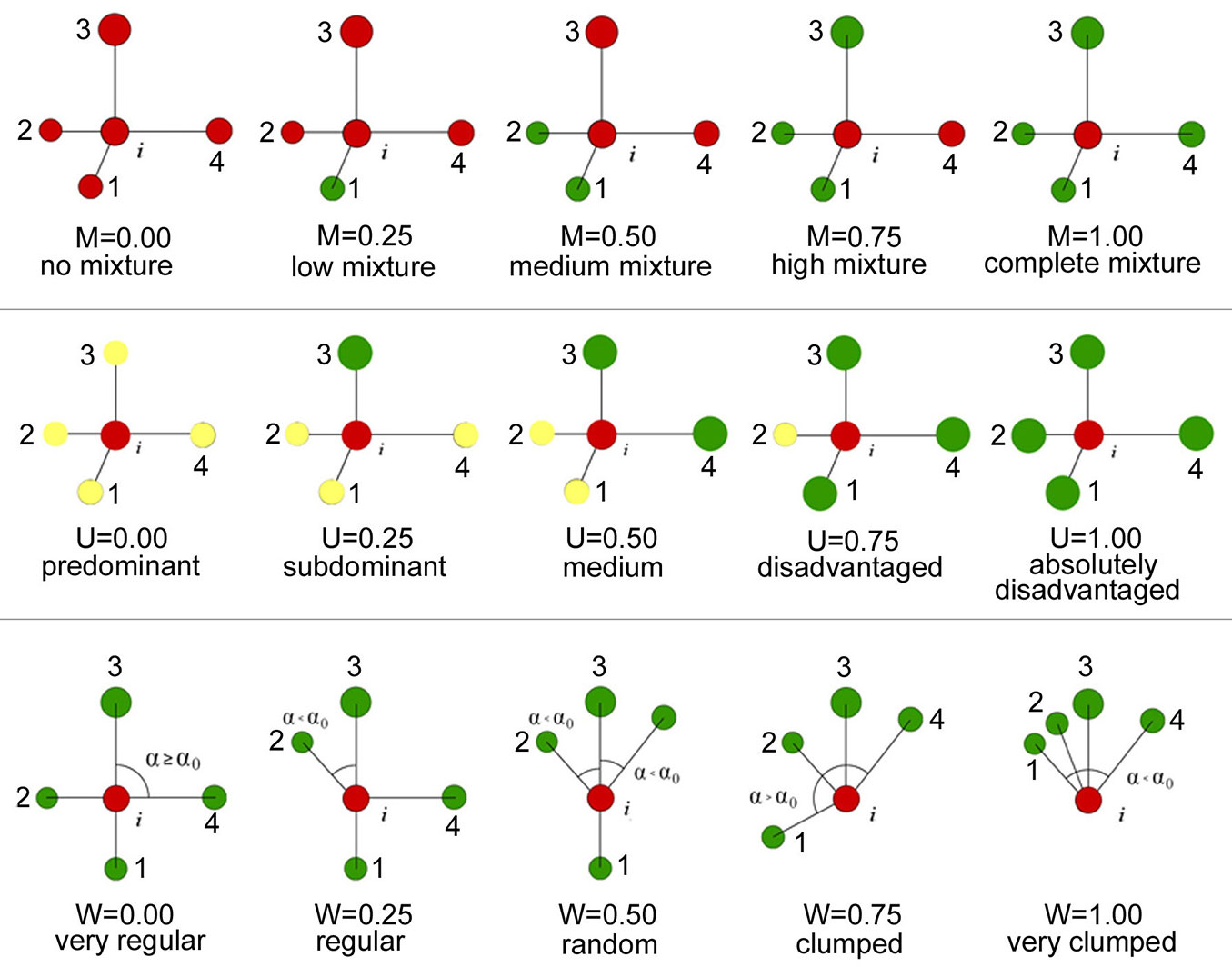
Fig. 3 - Fig. 3 - Indices of stand structure (M, U and W) using nearest-neighbors spatial relationships based on a 5-tree group. Each tree i has a unique Mi, Ui, Wi that takes one of the following values: 0.00, 0.25, 0.50, 0.75, 1.00.
In this paper, we define fine-scale forest structure as the level at which the nearest neighbors can affect each other. We focus on three aspects: tree species composition, diameter differentiation, and distribution pattern; and adopted a group of stand spatial parameters to analyze them: mingling (M), dominance (U), and uniform angle index (W). The parameters M (eqn. 1) and U (eqn. 2) may be regarded as modified versions of Gadow’s mixture and the T index ([14]), respectively. They accurately describe tree species segregation and size differentiation ([25], [30]). Mingling (M) describes the probability of a reference tree i and its four nearest neighbors belonging to the same species; its mean value ranges from 0 to 1, with higher values indicating higher levels of species mixture (Fig. 3). Dominance (U) describes the comparative sizes of a reference tree i and its four nearest neighbors. Its value ranges from 0 to 1, with higher values indicating taller trees in a given unit (Fig. 3). The uniform angle index W (eqn. 3) describes the degree of scatter in the four nearest neighbors to a reference tree i. Its distribution pattern can be evaluated by comparing the angle formed between any two neighbor trees and the reference tree with a standard angle (a0 = 72°). Its value ranges from 0 to 1, with greater values indicating more clustering ([45], [15] - Fig. 3). Mean W values in the interval 0.475-0.517 imply a random distribution pattern. Otherwise, the distribution pattern is clumped (mean W > 0.517) or regular (mean W < 0.475, [15] - eqn, 1, eqn. 2, eqn. 3):
When calculating the Mi, Ui and Wi for each tree, we used the nearest neighbor edge-correction conception (NNS1) to eliminate edge effects ([35]). Each tree has a unique Mi, Ui and Wi that takes values of 0.00, 0.25, 0.50, 0.75 and 1.00 (Fig. 3). Therefore, for the whole plot, M, U and W meet the requirement of two discrete variable joint in pairs ([37]). Three permutations can be obtained by joining the three parameters each other, that is, the M-U, M-W and U-W bivariate distributions. Each distribution contains 5 × 25 combinations. They have much greater power to detect the spatial characteristics of adjacent trees, compared with univariate analysis. Their ability to provide effective information is far superior to most traditional indices ([25], [27]). We adopted this modified method to analyze the nearest neighbor relationships in our four plots. Moreover, we applied the Bonferroni’s correction ([45]) when analyzing the Pearson’s correlation of different levels of the same factor, e.g., in Fig. 4a the correlation between none mixture (M = 0.00) and low mixture axes (M = 0.25). The modified critical P value (α = 0.005) was calculated by dividing the critical P value (here the significance level = 0.05) by the number of comparison [n (n-1) / 2 = 5]. All the calculations were performed in R 3.22 (R code provided by Dr. Zhang Gongqiao and colleagues - see Box 1 in Supplementary material), and figures were prepared using the “corrplot” package. Parts of the results of the correlation analysis are reported in the Supplementary material (Fig. S1 - Fig. S6).
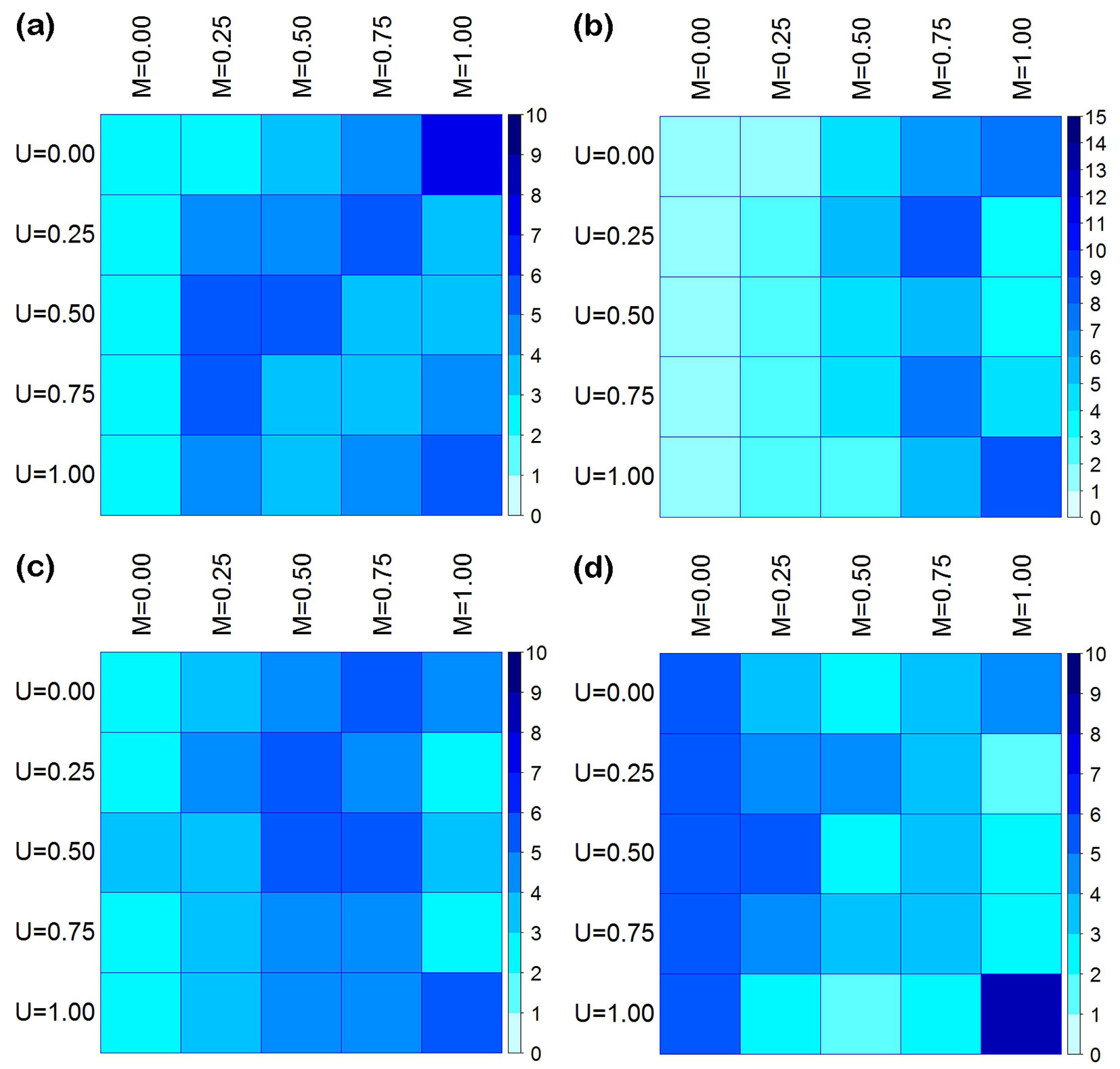
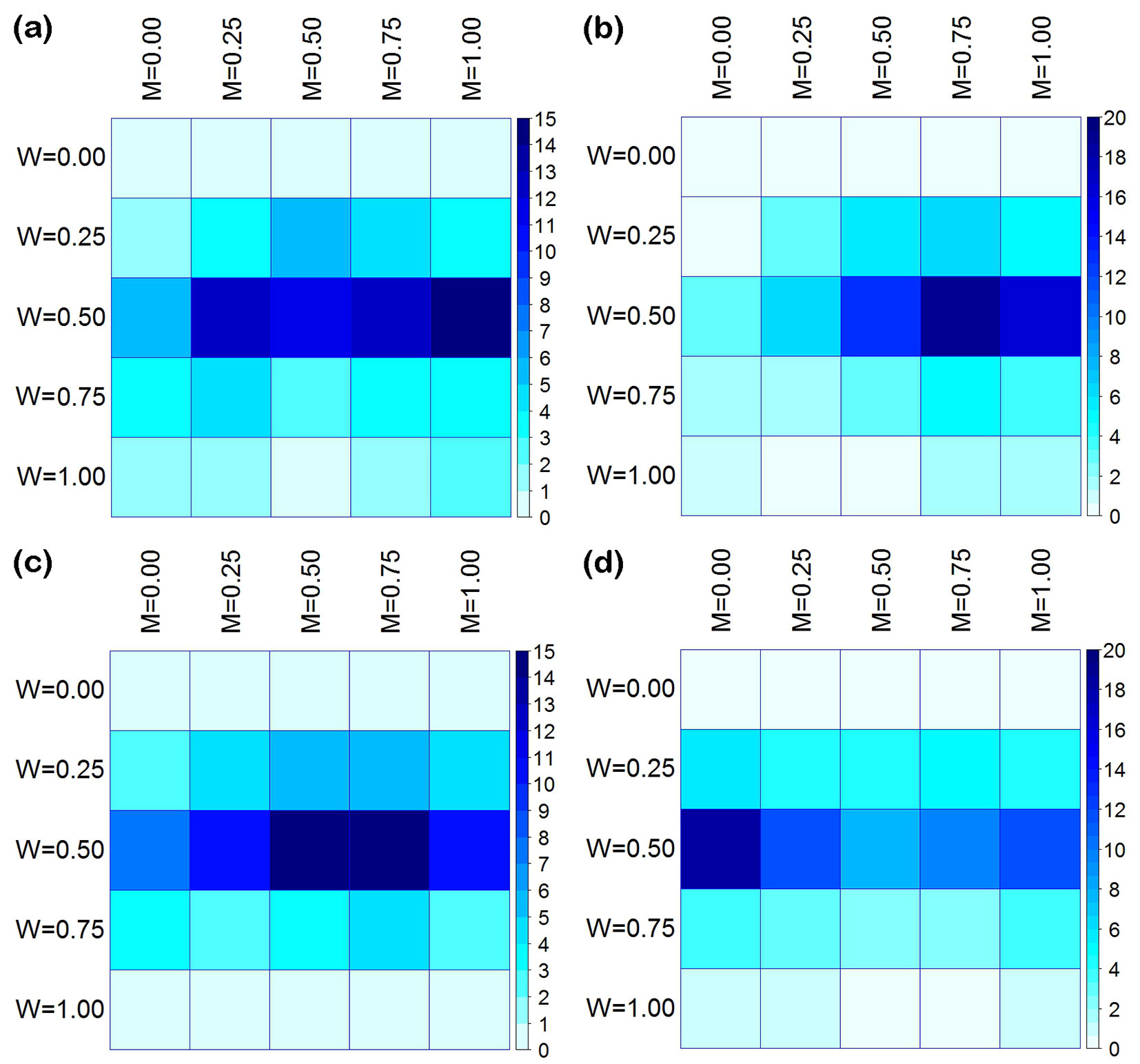
Fig. 4 - The joint distribution (%) of mingling (M) and dominance (U) in the four measured plots (a, b, c, d). The intensity of color is proportional to the number of trees falling into each combination (the darker the color, the higher the number).
Results
M-U bivariate distribution
The individual trees sampled in the four studied plots were spread over all combinations of the M-U bivariate distribution, but with different overall patterns (Fig. 4). In plot d, most trees were concentrated on the low-mixture axis (M = 0.00) and few trees on the medium-mixture axis (M = 0.50). In contrast, in plots a, b, and c, very few trees were located on the low-mixture axis while most appeared on the medium-mixture axis. For the same degree of mixture, the number of trees with different U values was different. The correlation coefficients for M were low in magnitude and there was no significant correlation (p > 0.005 - Fig. S1 in Supplementary material). The number of trees on each U axis (U = 0.00-1.00) was similar and was about 1/5 of the total number of trees in the plot. On the same U axis, however, the number of trees with different M values differed considerably and had only a weak and not significant correlation (p > 0.005 - Fig. S2 in Supplementary material).
M-W bivariate distribution
The M-W bivariate distribution of each plot displayed similar overall characteristics (Fig. 5). Trees populated each M axis (M = 0.00-1.00), and the number of tree on each M axis first increased, then decreased as W increased. Within the same plot, however, the ranges of change were different, some showed a close correlation (p < 0.005 - Fig. S3 in Supplementary material), while others differed from each other (p > 0.005, Fig. S3). Among all W axes (W = 0.00-1.00), most of the individuals in each plot were located on the random distribution axis (W = 0.50). The number of trees falling into the axes W = 0.25 and W = 0.75 were similar and then decreased. The uniform distribution axis (W = 0.00) and clump distribution axis (W = 1.00) had the lowest numbers, approaching zero. Their average values were Wa = 0.530, Wb = 0.506, Wc = 0.489, and Wd = 0.491, respectively. The correlations for the four plots were very weak (p > 0.005 - Fig. S4 in Supplementary material).
Fig. 5 - The joint distribution (%) of mingling (M) and uniform angle index (W) in the four measured plots (a, b, c, d).
U-W bivariate distribution
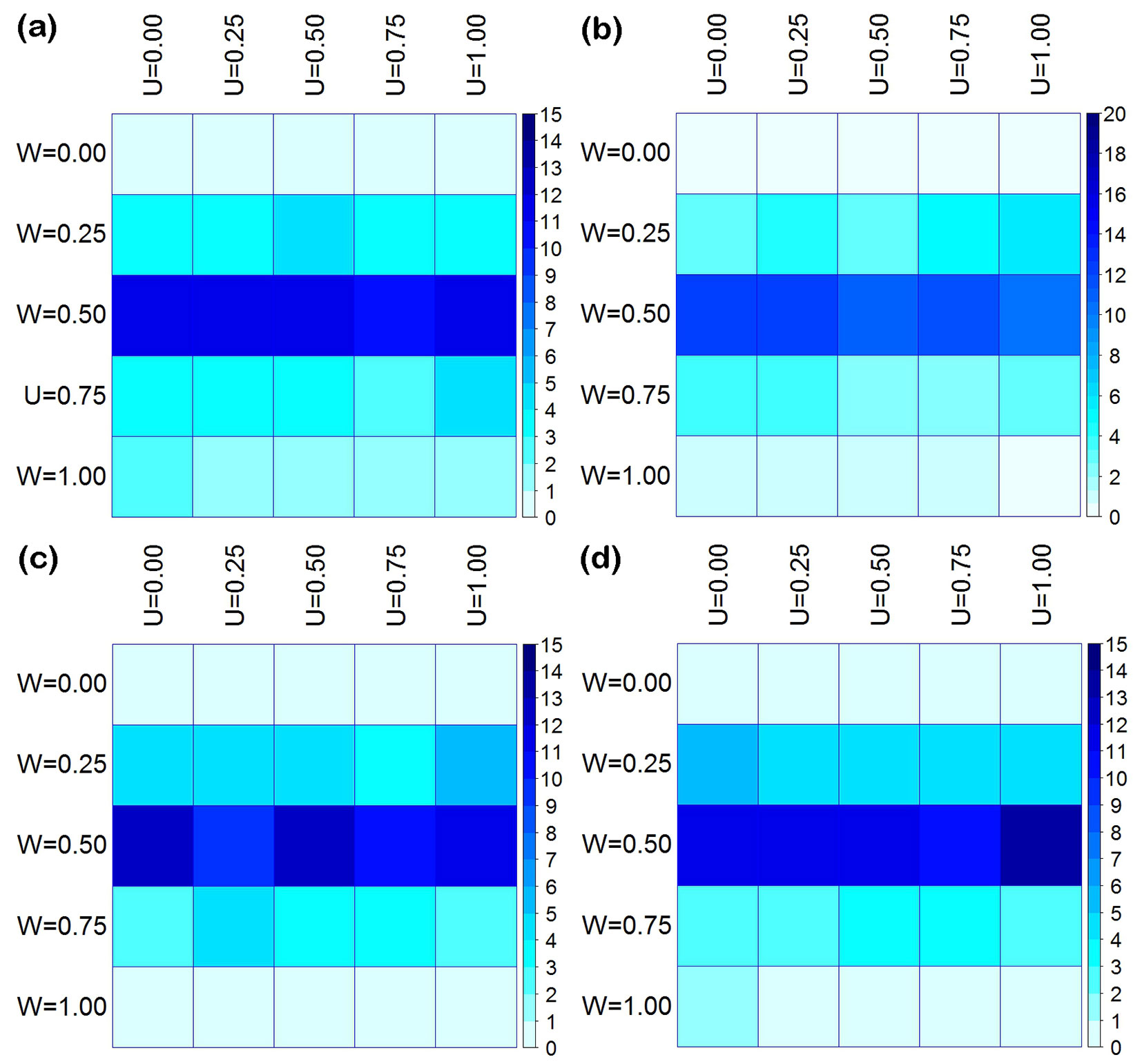
The U-W bivariate distribution for the four measured plots also exhibited a high degree of similarity and symmetry (Fig. 6), as did the M-W bivariate distribution. The number of individuals on each U axis (U = 0.00-1.00) was similar to a normal distribution, i.e., the value increased and then decreased with increasing W values. Within the same plot, each U axis contained similar numbers of individuals. Their correlation coefficients were high and the correlation was significant (p < 0.005 - Fig. S5 in Supplementary material) except some of them in plot b. There was a significant difference in the numbers of trees between different W axes (W = 0.00-1.00). Among these, the random distribution axis (W = 0.50) contained most trees, followed by the regular distribution axis (W = 0.25) and the partial clump distribution axis (W = 0.75). The regular distribution axis (W = 0.00) and the clump distribution axis (W = 1.00) contained the fewest trees. The correlation coefficient among different W axes was weak and not significant (p > 0.005 - Fig. S6 in Supplementary material).
Fig. 6 - The joint distribution (%) of dominance (U) and uniform angle index (W) in the four measured plots (a, b, c, d).
Discussion
With the acceleration of deforestation, habitat fragmentation and biodiversity loss have become topics of frequent discussion worldwide ([1]). Generally, forest tree diversity is investigated only in terms of species abundance and percent composition, and less attention is paid to the physical spatial structure of the forest community. In fact, different individual trees in three-dimensional space constitute the frame that supports ecosystem diversity and can change within time and space. Changes in spatial structure at fine scales determine the complexity of the forest stand and affect key processes related to tree canopies and the diversity of habitat space and species ([11], [5]). Fine-scale heterogeneity is particularly important when forest ecosystems face anthropogenic disturbance, and may help improve the ability of ecosystems to resist disasters and to recover after a severe disturbance ([10], [7]). It is impossible to maintain environmental and ecological conditions promoting biodiversity if we neglect the detailed spatial characteristics of the forest ([3]). Although some researchers hold different concept on the scale of small-scale forest structure ([5] vs. [42]), studies are increasingly focusing on space-time dynamics at this level ([16], [22]).
In this study, plots a and c were stands of moderately mixed composition, plot b had greater diversity, and plot d had a relative low mingling value (Fig. 4, Fig. 5). Regardless of the size of the trees, these plots always maintained similar mingling values. The reference trees had both conspecific and heterospecific neighbors, but the ratios of these differed between plots (Fig. 4). Tree size differentiation was very uniform. Trees of different sizes or neighbors were randomly distributed over all; some exhibited clumped or regular distribution (Fig. 5, Fig. 6). A significant correlation was detected among more than trees with different types of neighbors (Fig. S3 in Supplementary material) and different dominance levels (Fig. S5), suggesting that the distribution pattern associated with species mingling and size differentiation. However, the distribution pattern was rarely influenced by neighboring species or size differentiation (Fig. S4, Fig. S6 in Supplementary material). Additionally, the relationship between size differentiation and neighboring species was very weak (Fig. S1, Fig. S2 in Supplementary material).
The nearest neighbor relationships of the P. yunnanensis var. tenuifolia may be related to species composition, the number or proportion of each species or their distribution pattern within the plot. Each community was rich in tree species, but they differed from each other in species composition. A small number of tree species accounted for a large part of the total number of trees (Tab. 2). For example, L. ovalifolia, Q. variabilis, and P. yunnanensis var.tenuifolia accounted for 78.14% of the total number of trees in plot a; K. davidiana and Q. variabilis accounted for 79.66% of the trees in plot c; Q. variabilis accounted for 63.86% of the trees in plot d; and P. yunnanensis var. tenuifolia and Q. variabilis accounted for 55.15% of the trees in plot b. Abundant species such as L. ovalifolia resulted in crowded plots (Fig. 2), which greatly reduced the degree of segregation. These characteristics are consistent with the modeling results obtained by Graz ([13]) and the phenomena detected in natural Scots pine (Pinus sylvestris L.) forests ([18]), i.e., a single tree species with a large number of individuals will reduce the overall degree of mixture.
The nearest neighbor relationships of P. yunnanensis var. tenuifolia communities may be related to the length of development across species (Tab. 1). With the exception of plot a which showed a partial-regular distribution (Wa > 0.517), the other plots studied (b-d) showed random patterns of horizontal distribution. The transition from clump to random distribution observed in secondary communities matches the expectations based on forest succession laws ([26]). At the same time, we observed three levels of species mingling (low, medium, and high) in our four plots, suggesting that the distribution pattern reaches its climax earlier than stands with higher levels of mingling during the entire succession process. To form a highly mixed state, a forest community should include enough tree species with a scattered distribution ([13]). The transition from a pioneer community (composed of P. yunnanensis var. tenuifolia, Q. variabilis, and L. ovalifolia) to the secondary forest (including several shade-tolerant species like T. ciliata, E. distichophylla, M. rubra, and S. superba) is a long process lasting for decades. Despite the distribution patterns of pioneer and shade-tolerant species are very close to those of primary or long-undisturbed forests, the isolation of tree species in our study is still limited ([16], [32], [40]). Similar results have also been reported for other forests ([41], [24], [47]). Furthermore, Kint ([18]) pointed out that species mixing was constantly changing in aging Scots pine forests in European temperate areas, while species mingling was steadily increasing across the whole community. Based on the above evidence, it appears that the continual increase of species mixing in natural forests is a natural phenomenon; whether it will stop and at what level has not yet been elucidated.
Unlike species mixture, size differentiation can occur in the early stages of succession and is related to the distribution pattern ([36]). Tree distribution pattern deeply affect the spatial resources available for each individual tree, strongly influencing its growth ([20]). Consistently with these perspective, trees diameter differentiation in our four plots showed high consistency, considering their distribution patterns (Fig. 6, Fig. S5 in Supplementary material). Getzin et al. ([12]) further argued that distribution patterns were related to tree size and were simultaneously influenced by competition. The result of competition between nearest neighbors must lead to changes in size differentiation, death, and new distribution patterns. This could explain the diameter differentiation and random distribution patterns observed in the P. yunnanensis var. tenuifolia community. After 40 years since protection of these reforestated areas was established, trees with different dominance levels in Korean pine-broadleaf forests show very similar quantities and distribution patterns ([25]). Individuals of different ages in natural secondary oak forests with large numbers of pioneer species have been shown to have obvious similarity in distribution patterns ([24]). These studies found that diameter differentiation and random distribution patterns are indicative of early successional stages. However, in this study we did not examine the order of uniform size differentiation or the random distribution pattern. More detailed study is required on the different succession stages in this context.
Conclusion
In forest management, the structure and development dynamics of aging forests are usually an important reference for forest restoration and its promotion toward a greater complexity. However, reference primary forests are very rare nowadays; most forests are secondary and established on sites where the native forests suffered from severe disturbances. Therefore, understanding the structural diversity of secondary forests with different characteristics and development stage is of great importance in forest management.
We used the bivariate distribution of SSSP to analyze the nearest-neighbor relationships of P. yunnanensis var. tenuifolia secondary forests along the Nanpan River. Our results revealed similarities and differences in spatial structure and tree species composition among different development stages of these forests. We also examined the spatial correlation of size differentiation, species mixture, and distribution patterns of P. yunnanensis var. tenuifolia secondary forests, aimed to reveal the ecological processes driving the structural development. The results of this study are an important contribution to forest management optimization, providing a reference in terms of species mixture, distribution patterns and size differentiation, to be pursued for further promoting tree growth, regeneration, and habitat diversity at the fine scale in the studied forest communities.
List of abbreviations
SSSP: Stand spatial structural parameters; M: mingling; U: dominance; W: uniform angle index; NNS1: nearest neighbor edge-correction conception.
Acknowledgements
We thank Li Fengting, Lan Huangxu, Cai Xiaoqing, Long Jiafeng, Pan Ting, Lu Zijin, Tang Wenyan, Zhu Deyi from Guangxi University for providing help in data collection. This paper was financially supported by the National Science Foundation of China (grant no. 31400542), Guangxi Natural Science Foundation (grant 2016GXNSFBA380 233) through the project “Structure characteristics of Pinus yunnanensis var. tenuifolia and its influence on litter (2016-2019)”.
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Sufang Yu
Yehong Luo
Xianyu Yao
Shaoming Ye
College of Forestry, Guangxi University, Daxue East Road 100, Xixiangtang District, Nanning, Guangxi Province 530004 (China)
Research Institute of Forestry; Chinese Academy of Forestry, Key Laboratory of Tree Breeding and Cultivation, State Forestry Administration, Box 1958, Beijing 100091 (China)
Corresponding author
Paper Info
Citation
Li Y, Hui G, Yu S, Luo Y, Yao X, Ye S (2017). Nearest neighbour relationships in Pinus yunnanensis var. tenuifolia forests along the Nanpan River, China. iForest 10: 746-753. - doi: 10.3832/ifor2405-010
Academic Editor
Matteo Garbarino
Paper history
Received: Feb 12, 2017
Accepted: Jun 04, 2017
First online: Aug 01, 2017
Publication Date: Aug 31, 2017
Publication Time: 1.93 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2017
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 48665
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 40543
Abstract Page Views: 3272
PDF Downloads: 3707
Citation/Reference Downloads: 15
XML Downloads: 1128
Web Metrics
Days since publication: 3110
Overall contacts: 48665
Avg. contacts per week: 109.54
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2017): 19
Average cites per year: 2.11
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
The patterns of nearest neighbor trees in a temperate forest
vol. 15, pp. 315-321 (online: 23 August 2022)
Research Articles
Distribution factors of the epiphytic lichen Lobaria pulmonaria (L.) Hoffm. at local and regional spatial scales in the Caucasus: combining species distribution modelling and ecological niche theory
vol. 17, pp. 120-131 (online: 30 April 2024)
Research Articles
Modeling extreme values for height distributions in Pinus pinaster, Pinus radiata and Eucalyptus globulus stands in northwestern Spain
vol. 9, pp. 23-29 (online: 25 July 2015)
Research Articles
Local ecological niche modelling to provide suitability maps for 27 forest tree species in edge conditions
vol. 13, pp. 230-237 (online: 19 June 2020)
Research Articles
Chitosan exhibits variable effects on pine (Pinus sylvestris L.) and alder (Alnus glutinosa L.) growth and secondary metabolism
vol. 17, pp. 236-244 (online: 06 August 2024)
Research Articles
Rapid assessment of wind storm-caused forest damage using satellite images and stand-wise forest inventory data
vol. 6, pp. 150-155 (online: 08 April 2013)
Research Articles
Modelling diameter distribution of Tetraclinis articulata in Tunisia using normal and Weibull distributions with parameters depending on stand variables
vol. 9, pp. 702-709 (online: 17 May 2016)
Research Articles
Ensemble modeling of Pinus cembroides Zucc. distribution under future CMIP6 climate scenarios in northern Mexico
vol. 19, pp. 1-8 (online: 10 January 2026)
Short Communications
Biodiversity inventory of trees in a neotropical secondary forest after abandonment of shaded coffee plantation
vol. 10, pp. 303-308 (online: 23 February 2017)
Research Articles
Estimating crown defoliation of Scots pine (Pinus sylvestris L.) trees using small format digital aerial images
vol. 6, pp. 15-22 (online: 14 January 2013)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword