Can bark stripping cause red heartwood formation in beech stems?
iForest - Biogeosciences and Forestry, Volume 11, Issue 2, Pages 251-258 (2018)
doi: https://doi.org/10.3832/ifor2147-011
Published: Mar 12, 2018 - Copyright © 2018 SISEF
Research Articles
Abstract
Injuries to standing trees caused by logging and the subsequent changes in biochemical composition and anatomy of affected tissues lead to wood quality loss, thus lowering the commercial value of roundwood. In this study, we investigated the influence of various factors that could help mitigate or prevent the spread of infections in the stem caused by injuries. A total of 112 beech logs (tree age: 42-143 years) from ten forest stands at three different sites in central Slovakia were examined, and the extent of discolouration and decay zones in each stem was measured, along with cambial age, stem diameter, injury width, and injury closure period. The results showed that the width of physiologically active wet sapwood and the width of the inactive dehydrated zone in the stem are important factors influencing red heartwood formation. We found no significant differences in the extent of discolouration and decay among different stands and sites. Stem diameter and injury width did significantly affect the penetration of infection through sapwood, and red heartwood formation was significantly affected by cambial age and injury width, while stand age, site slope, beech proportion in the stand and injury closure did not show any significant effect. Binary logistic models were applied to assess the probability of pathogen penetration through sapwood into the stem dehydrated zone as a function of injury width and stem diameter, as well as the probability that this could lead to red heartwood formation based on injury width and cambial age of beech stems.
Keywords
Sapwood Width, Dehydrated Zone Width, Discoloration Depth, Decay Depth, Red Heartwood Formation
Introduction
Mechanical injury to tree stems caused by forest thinning and tree felling often determines the devaluation of beech roundwood. Bark stripping activates a variety of physiological defence reactions, which modify the biochemical characteristics of the affected tissues in the living trees. Discolouration is a visible symptom reflecting the presence of polyphenols in the affected tissue, which are probably due to the interaction between the enzymes of pioneer pathogens and the atmospheric oxygen ([1]). This indicates that exposure to air and the initial colonisation by microorganisms have already taken place. Simultaneously, polyphenols also protect the threatened tissues against further invasion of pathogens and retard the decaying process of wood caused by fungi ([23]).
Sapwood is the outer zone of the stem which has an effective defence system to protect the vital functions of transport and reserves, restrict air embolism during the later stages, and limit the spread of pathogens ([7]). However, aging and maturation in some diffuse-porous species does not cause genetically-controlled metabolic changes leading to the transformation from sapwood to heartwood in the centre of the stem ([32]). The central non-coloured zone of the stem, known as “ripewood” ([22]) or “dehydrated zone” (especially in beech - [34]), is free of tyloses and polyphenolic compounds. These tissues show a reduced moisture content and a limited number of vital parenchyma cells ([15], [22]). Several developmental and environmental factors affect the formation and size of the dehydrated zone ([34], [4], [10], [25]); however, the age of the tree is a dominant factor in this process ([22], [3]).
Injury of a living stem enables pathogens to enter through the injured surface and penetrate deeper into the surrounding xylem tissues. Faced with the risk of drying out and an expanding microbial infection, the sapwood efficiently defends its high moisture content, by forming tyloses in the vessels and triggering primary biochemical reactions. The capacity for defence is predominantly influenced by the physiological state of the tree ([8], [33]). If the pathogens entering through an injury overcome the strongest anatomical and biochemical barriers of the sapwood, a massive longitudinal spread of discolouration and decay can affect the dehydrated zone. The presence of discoloured wood in the middle of a stem, initiated by an injury, reduces the ratio of white coloured wood and thus lowers its commercial value ([2]). Apart from terms such as “discoloured wood” and “decay wood” ([32]), the terms “red heartwood”, “wound heartwood”, “splash heartwood” and “abnormal heartwood” ([27]) are frequently used to describe this phenomenon.
Discolouration and decay in the centre of beech stems (hereafter referred to as red heartwood) are predominantly caused by infection and oxygen penetrating through broken and dead branches ([21], [4], [19], [18], [40], [29], [35], [36]). However, there is a lack of detailed information on whether bark stripping caused by logging can lead to red heartwood formation in beech.
The aim of this work was to evaluate: (i) if there is a link between the internal extent of discolouration and decay in the stem (in radial direction) and the external features on the injured surface; (ii) if there is a link between the extent of discolouration and decay in the stem (in radial direction) and different environmental conditions; (iii) what is the probability of discolouration and decay, caused by air penetration and microbial invasion (in radial direction) spreading through the sapwood into the central part of the stem; and (iv) what is the probability that the microbial infection entering the injury and penetrating into the dehydrated zone through sapwood is capable of causing extensive red heartwood formation.
Material and methods
Site characteristics and sample material
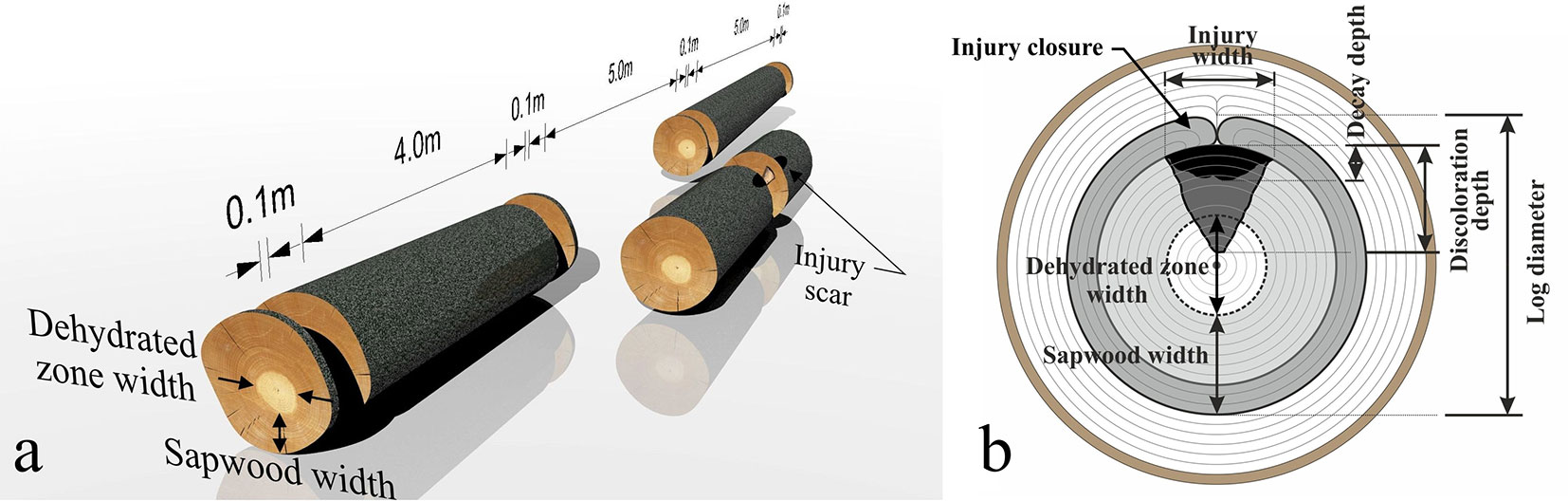
Sample material was obtained from 10 different forest stands at three diffent locations belonging to the Forest Enterprise of the Technical University in Zvolen, Slovakia, situated in the orographic unit of Kremnické vrchy (48° 35′ 32.6″ N, 19° 03′ 45.0″ E - Tab. 1). The occurrence of fungi in the investigated forests was determined according to the phytopathological methodology of permanent monitoring plots (10 × 10 m) in combination with random selection of transects with an area of 100 × 10 m ([13] - Tab. S1 in Supplementary material). Trees were felled continuously during the vegetation and dormant period from February 2012 to March 2013. A total of 248 sample beech trees aged from 42 to 143 years with DBH ranging from 22.7 to 61.6 cm, were obtained. This equates to approximately 25 trees per forest stand. Subsequently, the trees were cut into logs (3-5 m in length - Fig. 1a).
Tab. 1 - Main characteristics of the sampled stands at the three investigated sites. (Aa): Abies alba; (Ag): Alnus glutinosa; (Apl): Acer platanoides; (Aps): Acer pseudoplatanus; (Fe): Fraxinus excelsior; (Cb): Carpinus betulus; (Pa): Picea abies; (Ps): Pinus sylvestris; (Qp): Quercus petraea; (Qp): Quercus cerris; (Um): Ulmus montana; (*): concerning only beech.
| Characteristics | Site | Mláčik | Bukovina | Trnie | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stand | 813 B | 737 | 352 | 806(4) | 744A(1) | 626 B | 203(1) | 427 A | 545 | 339 | |
| Mean age (years)* | 70 | 110 | 115 | 135 | 160 | 110 | 130 | 65 | 90 | 110 | |
| Mean DBH (cm)* | 26 | 42 | 56 | 42 | 42 | 48 | 47 | 25 | 34 | 55 | |
| Mean height (m)* | 23 | 32 | 32 | 29 | 26 | 28 | 30 | 24 | 29 | 29 | |
| Beech proportion (%) | 80 | 51 | 49 | 50 | 9 | 75 | 15 | 85 | 55 | 51 | |
| Other species (%) | Cb-20 | Aa-22, Fe-16, Aps-6, Pa-5 |
Qp-41, Qc-10 |
Cb-50 | Aps-43, Fe-25, Aa-13, Apl-10 |
Cb-15, Qp-5, Qc-3, Ps-2 |
Qp-50, Aa-33, Pa-2 |
Qp-5, Cb -5, Ag-5 |
Qp-25,Pa-15, Aa-5 |
Aps-29,Apl-12, Cb-8 |
|
| Site quality* | medium | good | good | poor | poor | medium | medium | good | good | medium | |
| Altitude (m a.s.l.) | 300-450 | 675-825 | 430-460 | 500-650 | 680-800 | 475-555 | 590-690 | 380-460 | 445-590 | 500-580 | |
| Exposure | SE | SE | E | E | SE | NE | SE | NE | E | E | |
| Slope (%) | 40 | 35 | 15 | 45 | 30 | 15 | 25 | 30 | 60 | 40 | |
| Thinning date | 07-2012 | 03-2013 | 01-2013 | 02-2012 | 04-2012 | 07-2012 | 09-2012 | 02-2012 | 05-2012 | 09-2012 | |
| Number of fell trees | 23 | 28 | 25 | 23 | 27 | 22 | 26 | 24 | 26 | 24 | |
| Number of injuriies | 16 | 8 | 7 | 9 | 11 | 10 | 12 | 17 | 11 | 11 | |
Fig. 1 - (a) Scheme of stem cutting. (b) Graphical representation of the independent and dependent variables measured in this study (b).
Measurement of sapwood and dehydrated zone width at the time of felling
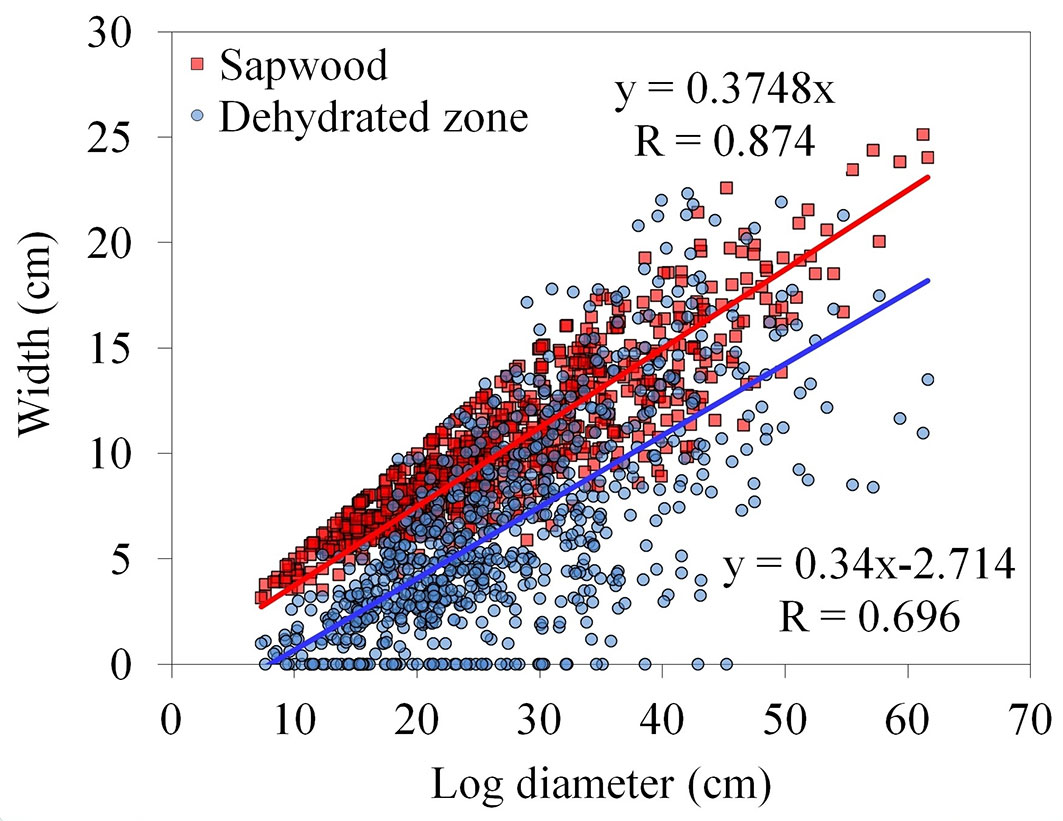
From each log, a 10 cm thick cross-section was cut (1063 cross sections in total - Fig. 1a). Within 30 minutes, a central dehydrated zone appeared on the freshly felled cross-sections, whilst the wetter outer zone was considered to be sapwood. Both zones, together with the stem diameter (measured from all the cross-sections along the tree height), were measured at the time of felling. Linear regression relationships between sapwood width and stem diameter, as well as between dehydrated zone width and stem diameter, were then determined (Fig. 2). Following these steps, the logs were used for estimating sapwood and dehydrated zone width at the time of injury closure.
Fig. 2 - Relationship between stem diameter and sapwood width (red points) or dehydrated zone width (blue points).
Measurement of injury-related variables
A total of 112 logs with visible wound scars were selected and sectioned through the middle of the injury (Fig. 1a). All injuries were caused by thinning operations, and were located randomly along the entire length of the tree stems. The length of injuries ranged from 0.12 to 0.86 m. The centripetal discolouration depth and the decay depth on the log cross sections were determined as dependent variables, while the cambial age, stem diameter (both obtained at the time of injury closure), injury width and injury closure period were determined as independent variables (Fig. 1b). Cambial age and injury closure period were counted, whereas the remaining injury characteristics were measured. Cambial age denotes the total number of annual growth rings up to injury closure, whereas the injury closure period denotes the number of annual growth rings, starting with the origin of the injury and ending by closure of injury by lateral calluses.
Estimation of sapwood and dehydrated zone width at the time of injury closure
Stem diameters at the time of injury closure were measured on the faces of the cross-sectioned discs that contained discoloured and decayed zones only. Then, the probable sapwood width and dehydrated zone width at the time of injury closure were estimated (Fig. 1b), according to a linear regression relationship (Fig. 2).
Estimation of sapwood penetration
A discrete variable was designed to indicate if discolouration or decay have exceeded the sapwood boundary in each stem. The ratio r1 between the discolouration depth and the sapwood width, and the ratio r2 between the decay depth and the sapwood width, were calculated according to the following formulae (eqn. 1):
where DiD is the discolouration depth (cm), DeD is the decay depth (cm), SW is the sapwood width (cm). If ri >1, the sapwood boundary has been exceeded and the value “yes” was assigned to the discrete variable. If ri <1, the sapwood boundary was not overcome and the discrete variable was given the value “not”.
Estimation of red heartwood occurrence and size
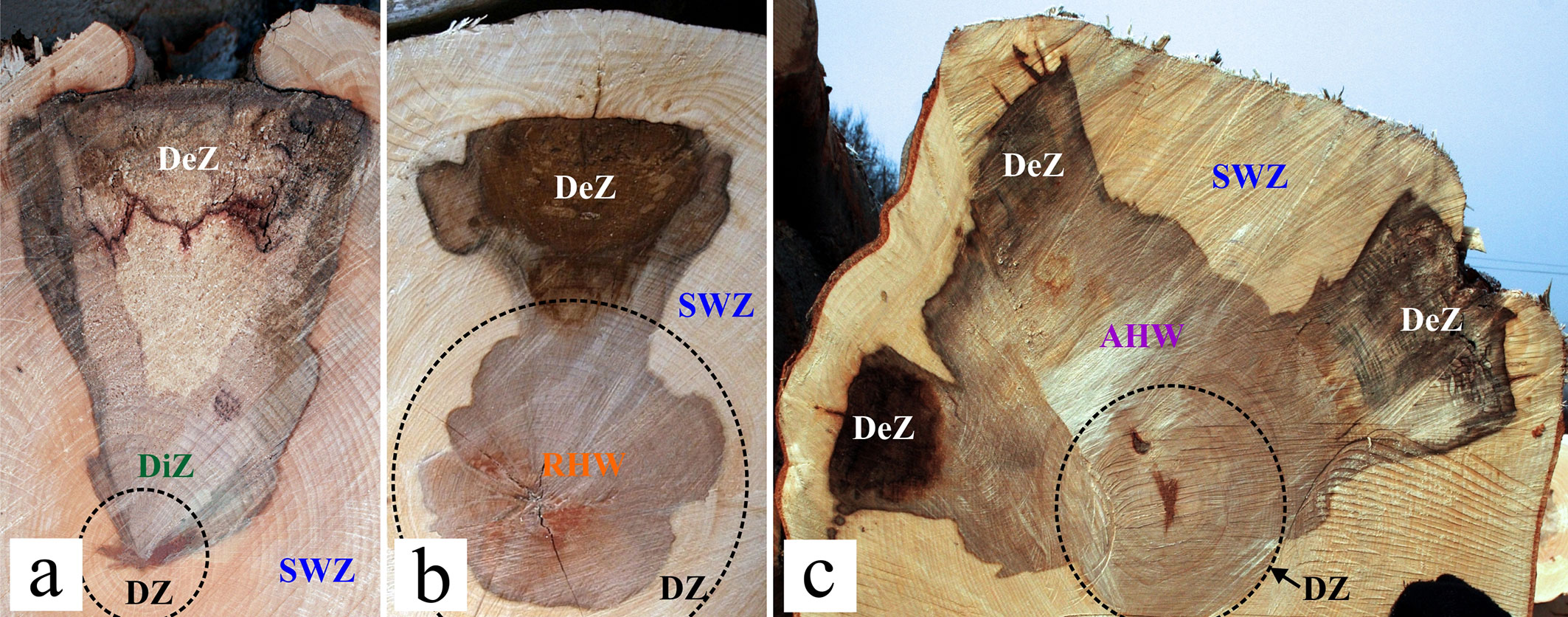
The presence of red heartwood was assessed visually. Injuries with only wedge-shaped discoloured zones and unstained central dehydrated zones (Fig. 3a) were classified as injuries that did not cause the formation of red heartwood (discrete value “not”). On the other hand, injuries with wedge-shaped discoloured zones, centripetally expanding in width, and associated with central round discoloured zones (red heartwood - Fig. 3b) were classified as injuries that had a causal relationship with the formation of red heartwood (discrete value “yes”). Abnormally large, irregular-shaped heartwood exceeding the dehydrated zone was also included in the category of red heartwood (Fig. 3c).
Fig. 3 - Classification of injuries: (a) Injuries not causing red heartwood formation; (b) Injuries causing red heartwood formation; (c) Injuries causing abnormal heartwood formation. (SWZ): sapwood zone (a wet marginal zone visible on freshly-felled cross sections of the tree trunks); (DZ): dehydrated zone (a paler and drier central part of the stem visible on freshly-felled cross sections of older tree trunks); (DiZ): discoloured zone (a wedge-shaped and pale brown-coloured zone in sapwood, sometimes exceeding dehydrated zone boundary); (DeZ): decayed zone (a wedge-shaped, dark brown or white-coloured zone localized in marginal parts of discoloured zones); (RHW): red heartwood (smaller central round-shaped and reddish brown-coloured zone formed in dehydrated zone); (AHW): abnormal heartwood (a large irregular-shaped and brown-coloured zone exceeding of dehydrated zone boundary and deeply extending into the sapwood zone).
The dimension of red heartwood was assessed by measuring its width in two perpendicular directions. The size of red heartwood was then calculated as the average value from the two measurements.
Statistical analysis
Descriptive statistics were calculated for each measured variable. Multiple linear regression analysis was applied using the following model (eqn. 2):
where yi represents the dependent variables (y1: discolouration depth; y2: decay depth), b0 is the intercept, b1-b4 are the regression coefficients, and x1-x4 are the predictors (independent variables, x1: cambial age, in years; x2: stem diameter, in cm; x3: injury width, in cm; x4: injury closure period, in years).
The correlation coefficients of the dependent variables with the predictor variables were calculated, and the significance of correlation and regression coefficients was tested for all regressions. Multiple analysis of variance (ANOVA) and post-hoc Duncan’s test were used to assess possible differences across different forest stands and sites. The software STATISTICA® ver. 10.0 (Stat Soft, Tulsa, OK, USA) was used for all analyses.
The probability of exceeding the sapwood boundary, and the probability of formation of discolouration and decay in the centre of the stem using the investigated factors as predictors were evaluated by binary logistic regression analysis. The binary logistic regression analysis was used to predict the probability of the discrete dependent variable y, which has a non-linear relationship with the linear predictor x. For the final transformation of the dependent variables into a linear one, a logarithm of odds (logit) was calculated. Then, the linear logistic models were described by the equation (eqn. 3):
where pi,j is the dependent variable (p1,j is the probability that discolouration exceeds the sapwood boundary, p2,j is the probability that decay exceeds the sapwood boundary; p3,j is the probability of formation of discolouration and decay in the centre of the stem), b0 is the intercept, b1-b7 are the regression coefficients, and x1-x7 are the independent variables (x1: forest age, in years; x2: beech proportion, in %; x3: slope, in %; x4: cambial age, in years; x5: stem diameter, in cm; x6: injury width, in cm; x7: injury closure period, in years).
By means of reverse transformation, a non-linear relationship between the probability and the vector of independent variables was achieved (eqn. 4):
The open-source data-mining software Tanagra ver. 1.4.5 (⇒ http://eric.univ-lyon2.fr/~ricco/tanagra/) was used to calculate the odds ratio in the logistic anaysis and test for the statistical significance of the regression coefficients ([26]). The global significance of the logistic models was tested by model fit statistics, model chi-squared test (likelihood ratio) and pseudo-R-like tests. Subsequently, the coefficients of the binary logistic regression models were tested by means of the Wald’s chi-squared test, these being defined as the square root of ratios between the coefficients and their decisive errors ([14]).
The logistic analyses were carried out in two steps. First, all the seven predictors were included in the initial analyses. Subsequently, the initial models were adjusted, discarding the non-significant variables previously included. The adjusted models were then used to assess the relationship between transition probabilities and the significant independent variables, according to eqn. 4.
Results
Extent of discolouration and decay
In 48 stems (of the 112 total) only wedge-shaped discoloured zones were present, whilst in the remaining 64 stems, decayed zones were also present (Tab. 2). The average depth of the decayed zones was lower than the average depth of the discoloured zones in all investigated samples; they reached approximately half the depth of the discoloured zones. Also between these variables, a relatively strong linear correlation relationship was found (y = 0.58·x; r = 0.76 - Fig. S1 in Supplementary material). Moreover, stems containing decay zones showed significantly higher average values of injury width and injury closure period. On the contrary, there was no significant difference as far as cambial age and stem diameter were concerned (Tab. 2).
Tab. 2 - Descriptive statistics (mean ± standard deviation) of all variables categorised according to the pathological status of injuries.
| Variable | Discoloration zone only |
Discoloration and decay zone |
Prob. (t-test) |
Total cases |
|---|---|---|---|---|
| n | 48 | 64 | - | 112 |
| Cambial age | 53.9 ± 19.5 | 59.8 ± 20.9 | 0.632 | 50.3 ± 19.3 |
| Stem diameter | 26.8 ± 12.6 | 27.8 ± 9.8 | 0.064 | 23.2 ± 10.0 |
| Injury width | 4.4 ± 2.6 | 6.6 ± 3.1 | <0.001 | 5.6 ± 3.1 |
| Injury closure period | 5.6 ± 2.6 | 7.9 ± 3.8 | <0.001 | 6.9 ± 3.5 |
| Discoloration depth | 2.3 ± 2.4 | 6.7 ± 5.7 | <0.001 | 4.8 ± 5.1 |
| Decay depth | - | 3.7 ± 3.6 | - | 2.4 ± 3.3 |
| Sapwood width | 8.8 ± 4.3 | 8.6 ± 3.3 | 0.775 | 8.7 ± 3.7 |
| Dehydrated zone width | 5.3 ± 3.9 | 5.1 ± 3.0 | 0.753 | 5.2 ± 3.4 |
Despite all combinations of the investigated variables were considered, significant effects were observed only for linear regression models with a maximal of two independent variables (b1 and b2 - Tab. 3). The high values of the correlation coefficients confirmed the relatively strong impact of injury width and injury closure period on the extent of discolouration and decay in radial direction. On the other hand, cambial age and stem diameter had a weak, or no impact. Although their individual effect was weak, some multiple regression models including both variables as predictors showed a relatively strong significance (Tab. 3).
Tab. 3 - Significant linear regression and results of the correlation analysis between the investigated variables and the extent of discoloration and decay. (ns): non significant; (*): p<0.05; (**): p<0.01; (***): p<0.001.
| Dependent variable (y) | Independent variables |
Intercept | Regression coefficients |
Correlation coefficient (r) |
||
|---|---|---|---|---|---|---|
| (x1) | (x2) | b 0 | b 1 | b 2 | ||
| Discoloration depth (n=112) | Cambial age | - | ns | 0.11*** | - | 0.401*** |
| Stem diameter | - | ns | 0.15** | - | 0.289** | |
| Injury width | - | -1.92** | 1.19*** | - | 0.728*** | |
| Injury closure period | - | -1.27** | 0.88*** | - | 0.610*** | |
| Cambial age | Injury width | -3.36*** | 0.04* | 1.10*** | 0.741*** | |
| Cambial age | Injury closure period | -4.17*** | 0.07*** | 0.78*** | 0.663*** | |
| Stem diameter | Injury closure period | -3.09** | 0.09* | 0.83*** | 0.636*** | |
| Decay depth (n=64) | Cambial age | - | ns | 0.07** | - | 0.395** |
| Injury width | - | ns | 0.74*** | - | 0.642*** | |
| Injury closure period | - | ns | 0.51*** | - | 0.536*** | |
| Cambial age | Injury closure period | ns | 0.05* | 0.44*** | 0.596*** | |
The extent of discolouration and decay increased with the length of injury closure period. Discolouration depth accelerated with age, whereas decay depth at first also accelerated with age, but only up to 15 years, and afterwards decreased (Tab. S2 in Supplementary material).
Extent of discolouration and decay across different stands and sites
The extent of discolouration and decay differed only slightly among localities. Higher average values were found only in Bukovina. However, no significant difference in the extent of discolouration nor decay was observed in stems from different sites (Tab. 4). On the other hand, other values that could affect the extent of discolouration and decay were significantly different among the investigated sites.
Tab. 4 - Mean ± standard deviation of the variables considered in the three study sites and significance of their differences after ANOVA.
| Variable | Mláčik | Bukovina | Trnie | Prob. |
|---|---|---|---|---|
| n | 51 | 22 | 39 | - |
| Cambial age | 59.3 ± 21.9 | 67.3 ± 20.7 | 48.9 ± 14.8 | 0.0017 |
| Stem diameter | 27.6 ± 11.5 | 32.9 ± 12.7 | 24.0 ± 7.8 | 0.0087 |
| Injury width | 5.7 ± 2.7 | 7.2 ± 4.4 | 4.8 ± 2.4 | 0.0142 |
| Injury closure period | 6.7 ± 3.6 | 8.5 ± 4.0 | 6.2 ± 2.9 | 0.0471 |
| Discolouration depth | 4.2 ± 4.5 | 6.9 ± 7.4 | 4.4 ± 4.0 | 0.0950 |
| Decay depth | 2.0 ± 3.0 | 2.7 ± 4.3 | 2.0 ± 3.1 | 0.6494 |
Further analyses revealed a higher extent of discolouration and decay in stands with high average age, low proportion of beech versus other species, and low slope (stands 744 A1 and 203 (1) - Tab. 1, Tab. 5). High values of the extent of discolouration and decay were also found in other older stands, like 339 and 806 (4). Furthermore, injuries were more frequent in stems with higher cambial age in the stands 744 A1 and 203 (1). The average width of injuries was also higher, whilst the injury closure period in all investigated forests varied by only four years (Tab. 5).
Tab. 5 - Descriptive statistics for the studied variables in different forest sites and stands.
| Variable | Mláčik | Bukovina | Trnie | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 813 B | 737 | 352 | 806 (4) | 744 A1 | 626 B | 203 (1) | 427 A | 545 | 339 | |
| n | 16 | 8 | 7 | 9 | 11 | 10 | 12 | 17 | 11 | 11 |
| Cambial age | 39.1±12.5 | 58.0±17.7 | 66.0±16.8 | 62.3±14.2 | 82.9±17.2 | 59.4±18.3 | 73.8±21.0 | 42.3±11.7 | 46.5±13.4 | 61.6±13.3 |
| Stem diameter | 19.7±6.9 | 25.0±6.9 | 33.2±7.3 | 28.3±9.9 | 37.1±14.8 | 30.8±13.2 | 34.7±12.5 | 22.6±7.3 | 22.0±6.7 | 28.0±8.8 |
| Injury width | 4.5±2.0 | 5.2±2.2 | 3.9±1.4 | 7.1±3.4 | 7.6±2.6 | 5.8±4.4 | 8.3±4.2 | 4.5±1.9 | 4.9±3.1 | 5.0±2.4 |
| Injury closure period | 5.8±2.7 | 8.1±5.4 | 5.1±1.8 | 7.8±4.3 | 7.4±3.2 | 7.2±3.5 | 9.6±4.3 | 6.1±2.3 | 6.6±4.1 | 6.1±2.4 |
| Discoloration depth | 2.3±2.1 | 4.2±3.5 | 3.5±5.1 | 5.3±4.9 | 6.7±6.1 | 3.7±4.6 | 9.6±8.3 | 3.5±2.9 | 3.8±3.1 | 6.2±5.6 |
| Decay depth | 1.0±1.9 | 1.7±2.6 | 0.9±1.5 | 2.3±2.2 | 4.2±4.6 | 0.6±1.2 | 4.5±5.1 | 1.0±1.9 | 2.1±2.8 | 3.3±4.5 |
Probability of exceeding sapwood width
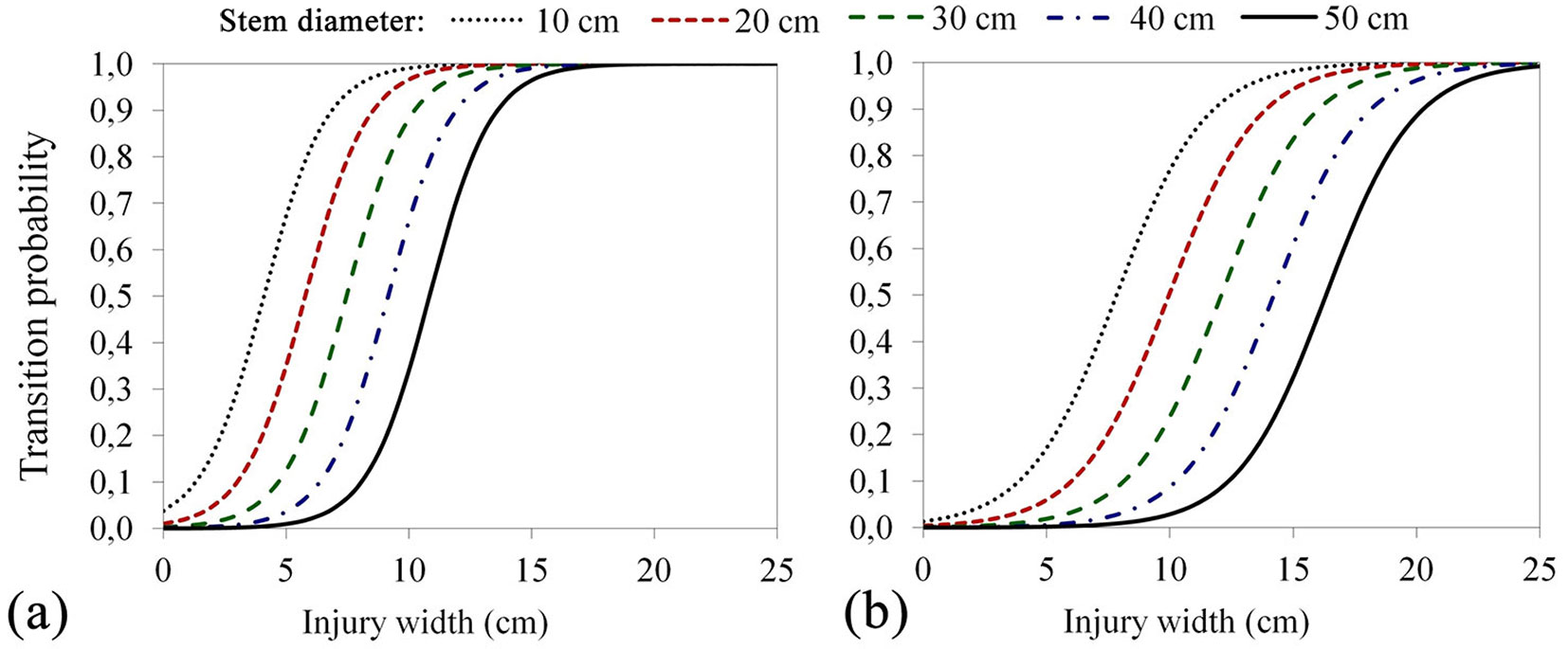
The analysis of probabilities confirmed that only some of the investigated factors had a significant effect on the exceeding of discolouration and decay through the sapwood in the dehydrated zone. Globally-significant logistic models showed significant coefficients only for two predictors, namely stem diameter and injury width, whereas other independent factors, such as forest age, beech proportion, slope, cambial age, and injury closure period, did not show any significant effect (Tab. S3 and Tab. S4 in Supplementary material). Therefore, the final equation describing the transition probability of discolouration (p1) was defined as follows (eqn. 5):
while the final equation describing the transition probability of decay (p2) was as follows (eqn. 6):
where x1 is the stem diameter and x2 is the unjury width in both the above equations.
By increasing the stem diameter, the average value of the odds to exceed the sapwood decreases by only 1.14 times (1:0.88) in the case of discolouration, and 1.12 times (1:0.89) in the case of decay. On the other hand, the increase in width of the injury raises the average value of the odds by 2.21 times (2.21:1) in the case of discolouration, but only 1.74 times (1.74:1) in the case of decay (Tab. S3, Tab. S4 in Supplementary material). The variation of transition probabilities as a function of stem diameter injury width is displayed in Fig. 4.
Fig. 4 - Transition probabilities of exceeding the sapwood boundary for (a) discoloration and (b) decay in beech stems as a function of injury width (x-axis) at different stem diameters (different coloured lines).
Probability of formation and extent of red heartwood
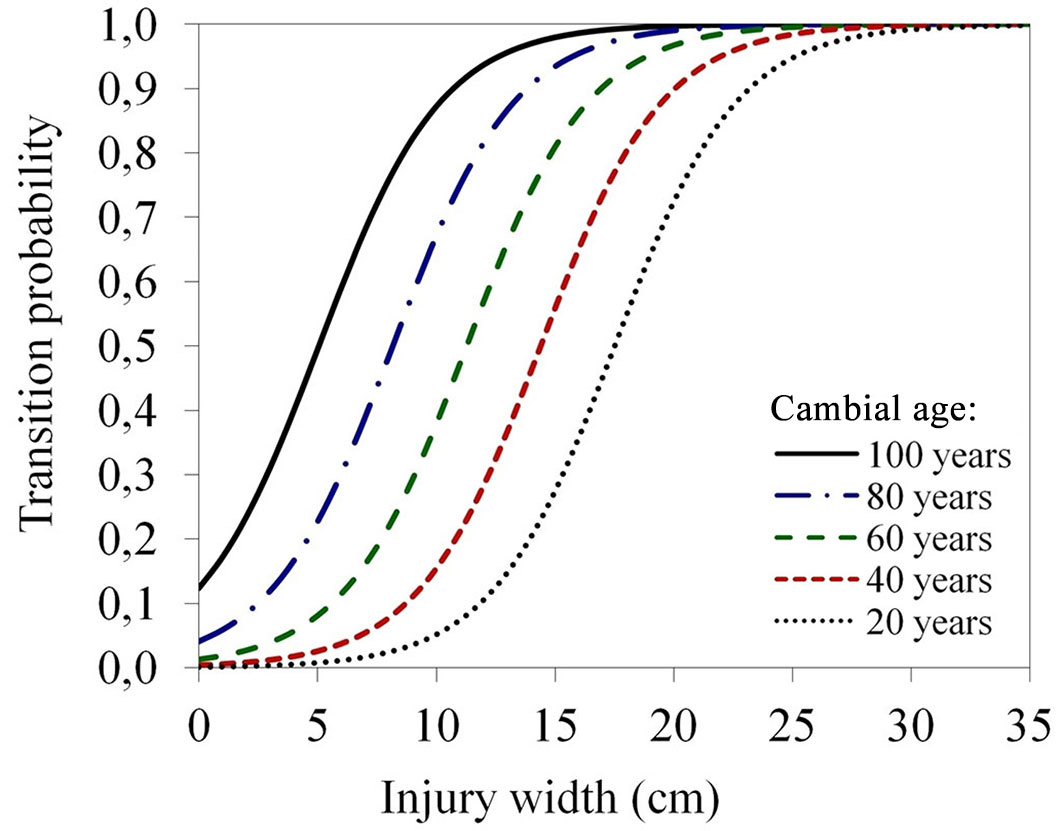
Globally-significant logistic models of red heartwood formation confirmed the significance of two coefficients only, namely cambial age and injury width, while other investigated factors had no significant effect on red heartwood formation. The final equation describing the transition probability of red heartwood formation (p3) was defined as follows (eqn. 7):
where x1 is the cambial age of the stem and x2 is the unjury width.
With increased cambial age, the average odds of red heartwood formation increased by 1.06 times only (1.06:1); however, the increase in width of the injury raised the average odds by 1.47 times (1.47:1 - Tab. S5 in Supplementary material). The transition probability of red wood formation as a function of cambial age and injury width is displayed in Fig. 5.
Fig. 5 - Transition probability of red heartwood formation in beech stem as a function of injury width (x-axis) at different cambial ages (different coloured lines).
The results confirmed the assumption that a relatively strong correlation (r = 0.635, p = 0.002) exists between the size of the dehydrated zone and red heartwood. In most cases, the average value of red heartwood width was less than that of the estimated dehydrated zone. Only in three out of 20 cases the width of red heartwood was higher (Fig. S2 in Supplementary material). Also, cambial age had a relatively strong effect on red heartwood width (r = 0.645, p = 0.003 - Fig. S3 in Supplementary material). Therefore, the higher the cambial age of the stem, the greater the red heartwood width; however, injury width had a very weak effect (r = 0.163, p = 0.026 - Fig. S4).
Discussion
All investigated injuries contained at least a minimal extent of discolouration, contrasting to the findings of Schumann & Dimitri ([31]), Hecht et al. ([12]) and Schulz ([30]), who reported that only 64%, 70% and 82% of 3-year-old open wounds contained discolouration in beech, respectively. Injuries with a lower average width and a shorter time period of injury closure did not contain decayed zones (Tab. 2). Decayed zones were present only in larger injuries with a longer time period of injury closure. According to Shortle et al. ([33]), decay in living trees occurs only in wood that has previously been discoloured. Also, it is generally known that larger and older wounds are more likely to be colonised by fungi ([39], [37]). In our case, 57% of injuries contained decay zones; however, Schumann & Dimitri ([31]) found only 25% of wounds due to logging which were infected by fungi. The extent of decay in radial direction was present in 49% of our cases, where they expanded by only 1 cm over three years. This means that beech is more resistant to wound-invading microorganisms, as compared with other species ([5]). However, discolouration at later stages of wood decay poorly inhibits the spread of decay. On the other hand, the reaction zone represents a strong and persistent static barrier against decay lesions ([24]). Therefore, the lower depth of decay compared to discolouration is probably due to a delay in the spread of wood decay (Fig. S1 in Supplementary material).
The injury width has a dominant impact on the extent of discolouration and decay of beech stems (Tab. 3), similarly to birch ([36]), whereas the impact is weak in ash ([38]). The injury closure period is also positively and linearly correlated with the extent of both discolouration and decay in beech (Tab. 3). The prolonged invasion of pathogens via open injuries enables the infection to penetrate deeper into the wooden tissues of the stem, whereas a weaker compartmentalisation allows an increase in the spreading rate of the discolouration and decay. Contrary to Vasaitis et al. ([36]), we found no significant difference in stem discolouration between open and occluded injuries in beech. Also, there was no significant correlation, as found in birch ([36]) or in ash ([38]). in this study, cambial age and stem diameter had the weakest correlation (Tab. 3), similar to the findings of Vasiliauskas & Stenlid ([38]) in ash.
Our results indicated that the extent of discolouration and decay depth in injuries was similar across different localities and stands. Small differences in some forest stands were influenced by a different cambial age, trunk diameter, injury width and injury closure period. Such differences could also be affected by the physiological state of individual trees ([8], [33]), the season of injury ([6], [7]), climatic conditions in the year of injury, different micro-organisms, pathogens, or saprophytes etc. Indeed, we observed a higher extent of discolouration and decay only in stands 744 A1 and 203 (1) from Bukovina (Tab. 4 and Tab. 5).
Biochemical processes and the moisture content of intact sapwood in beech represent a relatively strong defence barrier against the invasion of pathogens. On the other hand, the centripetal decreasing humidity and the sap flow density in beech stems ([11]) may facilitate the penetration of air and pathogens into the inner parts. Overall, the difference in the extent of discolouration and decay at different heights along the stem was only 0.1-0.4 m in depth (max 10 cm) over a three year-exposure ([6]), whereas it does not exceed 1.5-2 m during longer-term exposure ([8], [9]).
We found that not all injuries that exceeded the sapwood caused the formation of red heartwood. In the 32% of discoloured cases and 9% of decayed cases that exceed the sapwood, less than half of them (19%) led to red heartwood formation. In terms of typology, according to Sachsse ([27]), only 2.7% of red heartwood cases are categorised as abnormal heartwood (Fig. 3c). Further, all the monitored factors had no significant effect on the probability of exceeding the sapwood (Tab. S3 and Tab. S4 in Supplementary material) and forming red heartwood (Tab. S5).
Injured stems having a large diameter showed a lower probability that infection will exceed the sapwood, as pathogens have to travel a longer distance. Conversely, wider injuries have odds that are significantly higher (Fig. 4a and Fig. 4b). Cambial age and injury width also have a significant impact on the probability of red heartwood formation (Fig. 5, Tab. S5 in Supplementary material). Previous studies reported that tree age and diameter at breat height have a dominant effect on the frequency and size of red heartwood ([16], [17], [18], [40]). Krempl & Mark ([20]) also assumed that large wounds could be the initiation point of red heartwood formation. Sachsse & Simonsen ([28]) stated that the transformation from “wound heartwood” (discolouration of sapwood) to red heartwood may gradually occur in trees older than 75-90 years. Age-related alteration of vital parenchyma rays and moisture content in the dehydrated zone lead to an increased susceptibility to red heartwood formation in older trees. This is also supported by our findings that cambial age and dehydrated zone width are correlated with red heartwood width (Fig. S2 and Fig. S3 in Supplementary material), though the effect of injury width on red heartwood width was weak (Fig. S4). According to Fürst et al. ([10]), further studies should focus on the starting age/diameter of “dry heartwood” formation (analogous to dehydrated zone, or ripewood) in European beech to elucidate the above relationship. Indeed, the “dry heartwood” has the potential for later red heartwood formation, and thus can be used as an indicator for the risk of later wood quality loss.
Conclusions
We conclude that only large stem injuries caused by bark stripping during thinning operations have the potential to initiate red heartwood formation in European beech. In particular, the width of sapwood and the dehydrated zone may affect such process. In this study, no significant difference in discoloration, decay and red heartwood formation were found across different sites and beech stands.
Although thinning can promote the growth of standing trees, intense interventions may cause frequent and large injuries on the stems, thus increasing the risk of infection that could cause an intensive red heartwood formation in aged beech trees. Therefore, the adoption of appropriate thinning strategies could reduce massive stem injuries and the probability of red heartwood formation in older target trees.
Acknowledgements
We acknowledge the support from the Scientific Grant Agency of the Ministry of Education SR and the Slovak Academy of Sciences Grant No. 1/0822/17. This work was also supported by funding from the Slovak Research and Development Agency under the contracts no. APVV-16-0177 and no. APVV-0744-12.
References
CrossRef | Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Olga Mišíková
Department of Wood Science, Technical University in Zvolen, T.G.Masaryka 24, 960 53 Zvolen (Slovakia)
Department of Integrated Forest Protection and Landscape, Technical University in Zvolen, T.G.Masaryka 24, 960 53 Zvolen (Slovakia)
Institute of Foreign Languages, Technical University in Zvolen, T.G.Masaryka 24, 960 53 Zvolen (Slovakia)
Corresponding author
Paper Info
Citation
Račko V, Mišíková O, Hlaváč P, Deáková V (2018). Can bark stripping cause red heartwood formation in beech stems?. iForest 11: 251-258. - doi: 10.3832/ifor2147-011
Academic Editor
Giacomo Goli
Paper history
Received: Jun 21, 2016
Accepted: Dec 27, 2017
First online: Mar 12, 2018
Publication Date: Apr 30, 2018
Publication Time: 2.50 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2018
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 48162
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 41101
Abstract Page Views: 2673
PDF Downloads: 3193
Citation/Reference Downloads: 21
XML Downloads: 1174
Web Metrics
Days since publication: 2882
Overall contacts: 48162
Avg. contacts per week: 116.98
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2018): 2
Average cites per year: 0.25
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Earlywood vessel features in Quercus faginea: relationship between ring width and wood density at two sites in Portugal
vol. 8, pp. 866-873 (online: 27 April 2015)
Short Communications
Effect of intensive planting density on tree growth, wood density and fiber properties of maple (Acer velutinum Boiss.)
vol. 9, pp. 325-329 (online: 22 October 2015)
Research Articles
Analysis and evaluation of the impact of stand age on the occurrence and metamorphosis of red heartwood
vol. 10, pp. 605-610 (online: 15 May 2017)
Research Articles
Mechanical and physical properties of Cunninghamia lanceolata wood decayed by brown rot
vol. 12, pp. 317-322 (online: 06 June 2019)
Review Papers
Moisture in modified wood and its relevance for fungal decay
vol. 11, pp. 418-422 (online: 05 June 2018)
Research Articles
Effect of forwarder multipassing on forest soil parameters changes
vol. 15, pp. 476-483 (online: 24 November 2022)
Research Articles
Bioactivity of ethanol extracts from Eucalyptus bosistoana F. Muell. heartwood
vol. 12, pp. 467-473 (online: 14 October 2019)
Research Articles
Density, extractives and decay resistance variabilities within branch wood from four agroforestry hardwood species
vol. 14, pp. 212-220 (online: 02 May 2021)
Research Articles
The natural recovery of disturbed soil, plant cover and trees after clear-cutting in the boreal forests, Russia
vol. 13, pp. 531-540 (online: 18 November 2020)
Research Articles
Physical and mechanical characteristics of poor-quality wood after heat treatment
vol. 8, pp. 884-891 (online: 22 May 2015)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword