Emerging pests and diseases threaten Eucalyptus camaldulensis plantations in Sardinia, Italy
iForest - Biogeosciences and Forestry, Volume 9, Issue 6, Pages 883-891 (2016)
doi: https://doi.org/10.3832/ifor1805-009
Published: Jun 29, 2016 - Copyright © 2016 SISEF
Research Articles
Abstract
The rapid growth and environmental adaptability of Eucalyptus species has favored their global cultivation for pulpwood production. On the island of Sardinia, Italy, eucalypt plantations were established in the 20th century primarily in areas reclaimed from marshland, but the trees are now grown all over the island as ornamentals or windbreaks, and for timber, pulp and honey production. In recent years, an unusual decline and mortality of unknown etiology has been observed in Eucalyptus camaldulensis (river red gum) plantations throughout the island. Given the ecological and economic importance of eucalypt ecosystems in Sardinia, a survey was carried out in 2013 to determine which insect pests and fungal pathogens are directly involved in these phenomena. Field surveys throughout the island revealed severe infestations with the red gum lerp psyllid (Glycaspis brimblecombei) at all 12 surveyed sites, with the greatest numbers of pre-imaginal stages and adults occurring between May and July. The adult population reached its peak in July, followed 2 months later by the peak population of its specific parasitoid, Psyllaephagus bliteus. Symptoms of leaf chlorosis, crown thinning, shoot and branch dieback, sunken cankers, epicormic shoots and exudations of kino gum were also observed at the 12 field sites. Symptomatic woody samples yielded fungal isolates representing three distinct families: Botryosphaeriaceae, Diaporthaceae and Valsaceae. Morphological and DNA sequence data revealed seven distinct fungal species, namely Diaporthe foeniculina, Neofusicoccum australe, N. luteum, N. mediterraneum, N. parvum, N. vitifusiforme and Valsa fabianae. Two putative new species of Cytospora were also identified. Neofusicoccum australe was the only species recovered from all 12 sites, with isolation frequencies of 51-95%. Pathogenicity trials revealed that all Neofusicoccum species except N. vitifusiforme are directly involved in the etiology of the observed decline in the E. camaldulensis population on Sardinia.
Keywords
Timber Industries, Exotic Species, Biosecurity, Invasive Pathogens and Insects
Introduction
Eucalyptus L’Hér. is a large genus in the family Myrtaceae which includes evergreen trees and shrubs, mostly native to Australia ([14], [46]). The rapid growth of Eucalyptus species and their adaptability to different environmental conditions has favored their widespread cultivation, particularly in the tropics and in the southern hemisphere, providing one of the most important global sources of structural timber, pulp and fuel-wood ([29]).
In Italy, eucalypt plantations cover more than 72.000 ha. The most common species are Eucalyptus camaldulensis Dehnh., E. globulus Labill. and E. occidentalis Endl. The massive introduction of these exotic species began in the first half of the 20th century to protect areas affected by soil erosion and to produce pulpwood for the paper industry ([10], [47]). Eucalypt plantations on the island of Sardinia were estimated to cover 8000-8700 hectares ([47]), but more recent investigations as part of a Sardinian regional program ([15]) provided a more accurate quantification of the area covered by plantations of at least 1 ha, revealing a total of 22.754 ha, 90% of which are less than 400 m above sea level. These were established initially in areas reclaimed from marshland but have subsequently spread all over the island. The trees are cultivated as windbreaks and ornamentals, for the production of pulpwood, biomass and construction timber, and more recently for the production of honey.
Over the last few decades, several insect pests and fungal pathogens have threatened Eucalyptus species in both native and introduced ranges ([52], [23], [75], [11]). In Australia, where forests and woodlands are dominated by eucalypts, several research teams have investigated the prevalent insect pests, whereas studies of exotic plantations have usually focused on the insects of greatest economic importance. The massive introduction of eucalypts into new environments has created problems with exotic pests, including indigenous insects that have adapted to feed on these new hosts. Recent invasions of exotic species have been reported, including sap-sucking insects such as Glycaspis brimblecombei Moore, Blastopsylla occidentalis Taylor and Thaumastocoris peregrinus Carpintero & Dellapé ([12], [74], [67]). Other investigations have considered the gall wasps Ophelimus maskelli Ashmead and Leptocybe invasa Fisher & La Salle, as well as beetles such as Phoracantha semipunctata Fabricius, P. recurva Newman and Gonipterus scutellatus Gyllenhal ([54], [28]).
Glycaspis brimblecombei, the red gum lerp psyllid, is considered the most serious pest of eucalypts in the Mediterranean area ([8], [56], [9], [66]) and E. camaldulensis Dehnh. is the most susceptible to infestation ([13]). Glycaspis brimblecombei was first detected in Italy in 2010 ([36]) and quickly spread through the central-southern areas including Sicily and Sardinia. Eggs are laid on the leaf surface, and the developing nymphs form conical white covering structures (lerps) from lipids, proteins and carbohydrates, and live under the protective structure where they feed by penetrating the vascular tissues and withdrawing sap ([61]). Adults and nymphs also produce large amounts of honeydew, promoting the growth of sooty mold. The resulting damage includes leaf discoloration and, in the case of heavy infestations, severe leaf drop. Psyllaephagus bliteus Riek (Hymenoptera Encyrtidae) is a specific natural enemy of G. brimblecombei and develops solely on this host ([53], [26]). It is present in Italy and has been used for classical biological control strategies ([19]).
One of the most serious diseases affecting eucalypt trees worldwide is Mycosphaerella leaf disease (MLD), which represents a substantial economic threat to commercial plantations ([25], [75], [11]). The predominant symptoms of MLD are leaf spots that often enlarge and coalesce to form blotches on the leaf surface, causing leaf blight, premature defoliation and shoot dieback, and stunting ([43]).
Other severe diseases that pose a threat to eucalypt plantations worldwide include eucalypt rust caused by Puccinia psidii G. Winter, Ceratocystis wilt caused by Ceratocystis spp., certain canker-causing agents such as Chrysoporthe cubensis (Bruner) Hodges (= Cryphonectria cubensis), and species of Botryosphaeriaceae Theiss. & P. Syd. ([22], [32], [58], [63]). The wide distribution and virulence of the latter cause the greatest negative impact on eucalypt trees both in native and introduced ranges ([62], [16], [49]). Several studies have revealed the involvement of some species belonging to the genera Botryosphaeria, Lasiodiplodia and Neofusicoccum in the decline and mortality of Eucalyptus spp. In particular, Botryosphaeria dothidea (Moug.: Fr.) Ces. & De Not. and Neofusicoccum ribis (Slippers, Crous & M.J. Wingf.) Crous, Slippers & A.J.L. Phillips have been commonly associated with different disease symptoms affecting Eucalyptus spp. in temperate climates ([72], [51], [64]), while Lasiodiplodia theobromae (Pat.) Griffon & Maubl. was the species most frequently reported in tropical areas ([60], [57], [48]). However, recent studies based on DNA sequence data have revealed that several strains previously identified as B. dothidea and N. ribis are in fact strains of N. parvum ([62]). Furthermore, several cryptic species have been identified within the L. theobromae species complex and four species are currently known to infect eucalypt trees, namely L. crassispora T.I. Burgess & Barber, L. pseudotheobromae A.J.L. Phillips, A. Alves & Crous, L. rubropurpurea Burgess, Barber & Pegg and L. theobromae ([18], [3], [63]).
Despite the occurrence of pests and pathogens in other countries, neither insect pests nor serious fungal diseases were observed on eucalyptus plantations in Sardinia until 2010. Since then, beekeepers have reported a remarkable reduction in eucalyptus honey yields, which have been attributed to inflorescence damage caused by insects. Subsequent field surveys have demonstrated the pullulation of psyllid infestations and the widespread decline and mortality of young and mature E. camaldulensis trees, with unknown etiology.
Given the economic importance of eucalyptus plantations for the regional economy and the potential losses caused by these emerging pests and diseases, we set out to record the symptoms and study the etiology of the decline events in Sardinian eucalypt plantations, to determine the virulence of the major fungal pathogens, and to investigate the population dynamics of the prevalent insect pest G. brimblecombei and the role of its specific parasitoid P. bliteus.
Materials and methods
Field surveys and sampling procedure
Field surveys were carried out in spring, summer and autumn 2013, at 12 E. camaldulensis plantation areas throughout Sardinia (Tab. 1). Stem and branch cankers were the most frequent symptoms, so the agents responsible at each site and season were determined by selecting five declining trees and collecting samples on the stem or branches from the margin of three active cankers per tree, making a total of 510 cankers.
Tab. 1 - Characteristics of the Eucalyptus camaldulensis plantation survey sites.
| Site | Locality | Latitude (N) | Longitude (E) | Elevation (m a.s.l.) |
Type of plantation |
|---|---|---|---|---|---|
| 1 | Ottana | 40° 13′ 56.82″ | 9° 01′ 54.02″ | 185 | Windbreak |
| 2 | Arborea | 39° 48′ 06.43″ | 8° 37′ 47.20″ | 7 | Plantation |
| 3 | Serramanna | 39° 24′ 48.38″ | 8° 51′ 46.14″ | 30 | Windbreak |
| 4 | Uta | 39° 15′ 24.84″ | 8° 55′ 50.54″ | 6 | Plantation |
| 5 | Siliqua | 39° 16′ 16.32″ | 8° 48′ 52.57″ | 66 | Plantation |
| 6 | San Vito | 39° 20′ 03.84″ | 9° 31′ 51.35″ | 13 | Windbreak |
| 7 | Arbatax | 39° 55′ 42.07″ | 9° 42′ 30.31″ | 13 | Ornamental |
| 8 | Siniscola | 40° 35′ 27.70″ | 9° 42′ 34.52″ | 39 | Plantation |
| 9 | Olbia | 40° 52′ 14.61″ | 9° 30′ 55.81″ | 15 | Windbreak |
| 10 | Ozieri | 40° 36′ 48.18″ | 8° 55′ 49.95″ | 390 | Windbreak |
| 11 | S. Maria La Palma | 40° 39′ 09.62″ | 8° 17′ 25.73″ | 30 | Windbreak |
| 12 | Alghero | 40° 35′ 37.91″ | 8° 17′ 25.12″ | 7 | Ornamental |
The adult G. brimblecombei population was sampled by placing four yellow sticky traps (20 × 20 cm) in each study area from March to December. They were changed every 2 weeks and the number of adults was counted. To monitor P. bliteus parasitism, 16 leaves per station were randomly collected every 2 weeks. The number of unparasitized juvenile stages (neanids and nymphs) and mummies (parasitized nymphs) of G. brimblecombei were counted on the upper and lower side. The percentage of parasitism at each station was calculated as the ratio between the number of mummies and the total number of G. brimblecombei individuals (not taking into account the newly parasitized nymphs without symptoms, thus representing a conservative estimate). The susceptibility of second-instar G. brimblecombei psyllids to parasitism by P. bliteus is minimal due to the size of the lerps ([26]).
Isolation and identification of canker-associated fungi
Eucalyptus samples were taken to the laboratory and the outer bark surface tissue was cut away with a scalpel. Longitudinal and transversal cuts from symptomatic branch samples revealed internal symptoms. Fungi were isolated from chips of inner bark and xylem tissue (~5 mm2) removed from the margin of necrotic lesions with a sterile scalpel. All chips were cultured on potato dextrose agar (PDA, Oxoid Ltd., Basingstoke, UK) in Petri dishes. After incubation at 25 °C for 5-7 days in the dark, fungal colonies were sub-cultured onto half-strength PDA supplemented with autoclaved holm oak twigs and incubated at room temperature with natural daylight until the differentiation of reproductive structures was observed. The contents of conidiomata were dissected, mounted in 100% lactic acid and observed at x400 magnification. Fungal isolates were assigned to groups according to morphological features and representative isolates from each group were used for DNA analysis and pathogenicity tests.
DNA extraction, PCR amplification and sequencing
Genomic DNA was extracted from 5-day-old cultures grown on PDA at 25 °C using Instagene Matrix® (BioRad Laboratories, Hercules, California, USA). The entire internal transcribed spacer (ITS) region of the ribosomal DNA, including the 5.8S rRNA gene, was amplified and sequenced using primers ITS1 and ITS4 ([73]), whereas part of the EF-1α gene encoding translational elongation factor 1 alpha was amplified and sequenced with primers EF446f and EF1035r ([33]). PCR amplification was carried out as described by Linaldeddu et al. ([39]) and the products were purified using the EUROGOLD® gel extraction kit according to the manufacturer’s instructions (EuroClone S.p.A., Pero, Italy). Both strands were sequenced by the BMR Genomics DNA sequencing service (⇒ http://www.bmr-genomics.it). The nucleotide sequences were edited using FinchTV v. 1.4.0 (Geospiza, Inc., Seattle, Washington, USA; ⇒ http://www.geospiza.com/finchtv) and compared with sequences deposited in GenBank using the BLAST algorithm (⇒ http://blast.ncbi.nlm.nih.gov).
Pathogenicity tests
To verify the pathogenicity of each species investigated in this study, a field inoculation trial was conducted in May 2014 on asymptomatic E. camaldulensis trees located in a plantation in the south of Sardinia (Serramanna: 39° 41′ 96″ N, 8° 92′ 71″ E). During the experimental period, the daily mean air temperature was 14.6-32.3 °C and the total rainfall was 0.4 mm.
Six plants (15-20 cm trunk diameter) were inoculated at breast height with a representative isolate of each fungal species, and six uninfected plants were used as controls. The inoculated region of the trunk was surface-disinfected with 70% ethanol and a piece of outer and inner bark was removed with a flamed cork borer and replaced with an agar-mycelium plug taken from the margin of an actively growing colony on PDA. The original bark plug was placed on top of the agar disk and the inoculation site was covered with cotton wool soaked in sterile water and wrapped in a piece of aluminum foil secured with masking tape. Controls were inoculated with a sterile PDA plug applied as described above. After 1 month, the outer bark was carefully removed with a chisel and the necrotic lesion surrounding each inoculation site was measured by placing transparent paper over the top of the lesion and drawing around the perimeter. The image of each lesion was scanned and the area was determined using the Assess v. 2.0 software ([35]).
The re-isolation of inoculated species was attempted by transferring onto PDA 10 pieces of inner bark and wood taken from around the margin of each lesion. Cultures were grown in daylight and room temperature until the development of fungal colonies was observed.
Statistical analysis
Pathogenicity assay data were checked for normality, then subjected to analysis of variance (ANOVA). Significant differences among mean values were determined using Fisher’s least significant differences multiple range test (α = 0.05) after one-way ANOVA using the software package XLSTAT® (Addinsoft, Paris, France).
The abundance of G. brimblecombei and the prevalence of P. bliteus parasitism were analyzed using a two-factor design (station and month) general linear model of ANOVA, and means were separated by Fisher’s least significant differences test.
Results
Field surveys
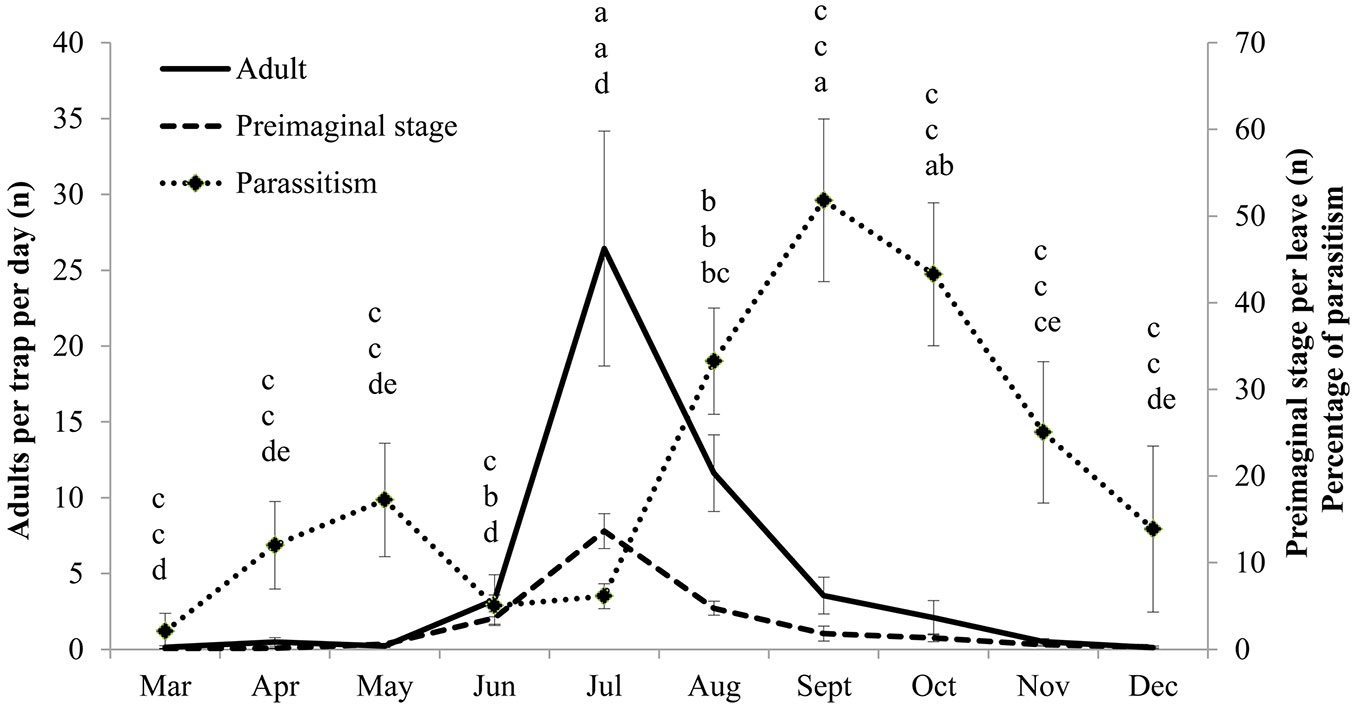
Glycaspis brimblecombei and its parasitoid P. bliteus were monitored at 12 field sites to characterize the population dynamics in each area. Glycaspis brimblecombei infestations were abundant at all the field sites on both declining and asymptomatic trees (Fig. 1). No significant differences were observed among the sites in terms of adult abundance (F=1.60, df=11, P=0.1097), pre-imaginal stage abundance (F=0.84, df=11, P=0.5989) or the level of parasitism (F=1.37, df=11, P=0.1999), thus mean values representing the data collected at all sites are presented in Fig. 2. There was a significant increase in the number of pre-imaginal (F=27.29, df=9, P<0001) and adult G. brimblecombei (F=10.29, df=9, P<0001) between May and July, followed by a decrease in the subsequent months. The sticky traps revealed a peak in the number of adults in July with a mean value of 26 specimens per trap per day. There was a strong correlation between the number of adult females caught in the sticky traps and the immature stages on the foliage samples (r > 0.90). The incidence of parasitism showed a bimodal trend, with the September value (52%) significantly higher than all other months except October (F=6.97, df=9, P<0.0001). Parasitism reached a peak 2 months after the peak population of pre-imaginal stage and adult G. brimblecombei.
Fig. 1 - Life stages of Glycaspis brimblecombei. (a-d): Eggs, neanid, nymph and adult stage; (e): construction of a lerp on a Eucalyptus camaldulensis leaf; (f): adult of the parasitoid Psyllaephagus bliteus; (g) leaves of Eucalyptus camaldulensis heavily infested with Glycaspis brimblecombei.
Fig. 2 - Numbers of adult and pre-imaginal stages of Glycaspis brimblecombei and level of parasitism by Psyllaephagus bliteus. Each point represents the mean of 12 stations (±SE). Different letters among the months indicate significant differences for adult (comparing top letters), preimaginal stages (comparing middle letters) and parasitism level (comparing bottom letters) using general linear model ANOVA (P<0.01) followed by Fisher’s least significant differences test (α = 0.05).
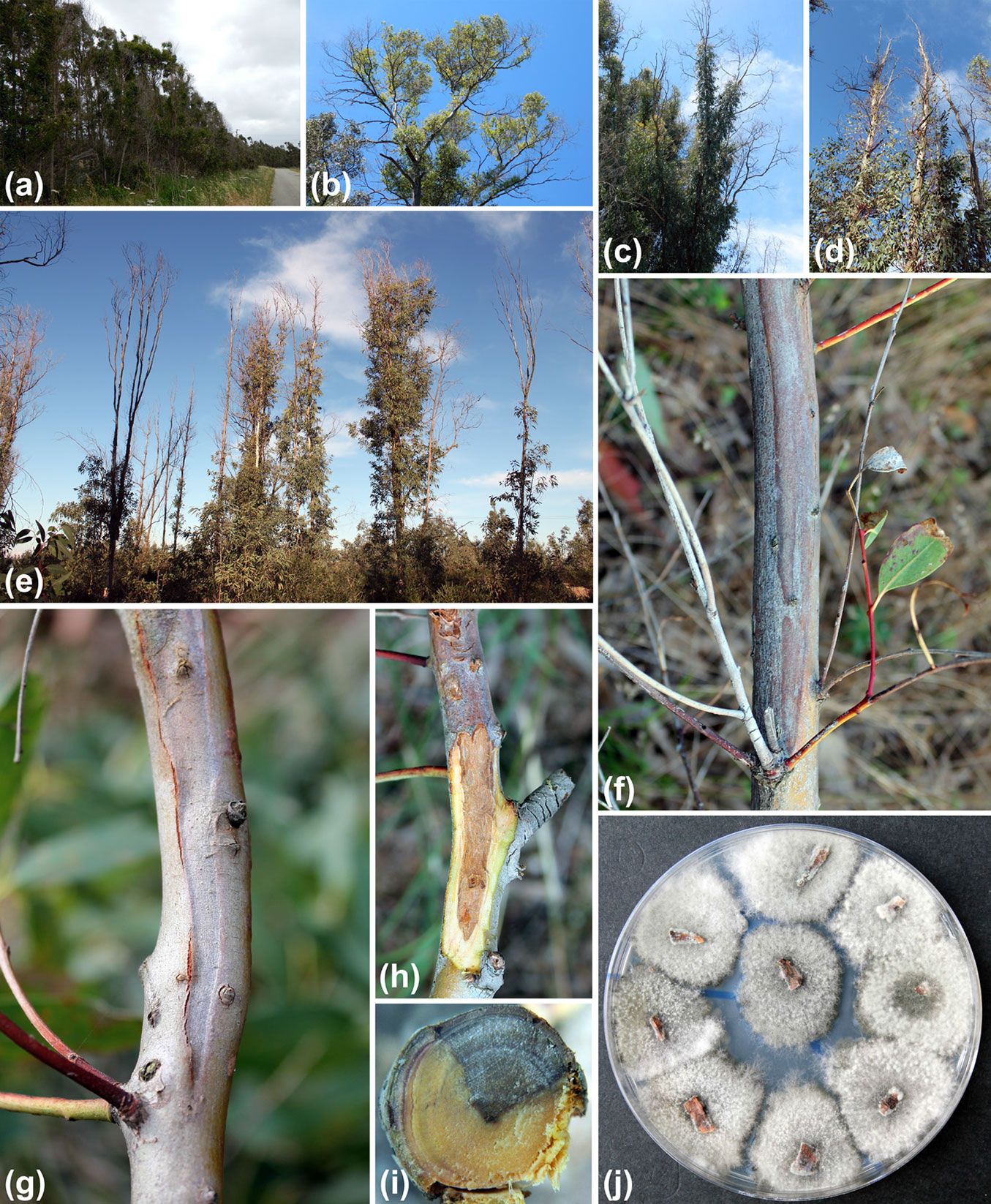
Eucalyptus camaldulensis trees affected by decline and mortality were abundant at all the field sites, suggesting the phenomenon we observed was epidemic. Both young and old trees exhibited various canopy symptoms, including the progressive dieback of twigs and branches associated with the abnormal growth of epicormic shoots (Fig. 3). All declining trees showed extensive sunken cankers on the branches and trunk, often with copious exudations of kino gum. After removing the outer and inner bark from cankers, dark brown necrotic lesions were visible on the xylem tissue. When branch and trunk cankers were cross-sectioned, internal symptoms included characteristic wedge-shaped necrotic lesions which often interested a large part of the section (Fig. 3). Cankers and bark necrosis on branches and trunks spread from the top downwards. In correspondence of necrotic tissues a number of dark pycnidia erupting from the bark were usually visible.
Fig. 3 - Major disease symptoms observed in Eucalyptus camaldulensis trees. (a-e): Crown thinning, dieback of twigs and branches associated with an abnormal growth of epicormic shoots along stems and branches; (f, g): sunken bark cankers; (h): wood necrosis and discoloration visible after bark removal; (i): cross section of a branch showing a characteristic wedge-shaped necrotic sector in the wood; (j): colonies of Neofusicoccum australe growing on PDA following isolation from bark and wood chips.
Isolation and identification of canker-associated fungi
The symptomatic woody samples yielded 489 fungal isolates representing the families Botryosphaeriaceae, Diaporthaceae Höhn. ex Wehm. and Valsaceae Tul. & C. Tul. Botryosphaeriaceous fungi were the dominant component, accounting for 391 of the isolates. On the basis of morphological features and DNA sequence data (ITS and EF-1α), five distinct species were identified, namely Neofusicoccum parvum (6 isolates), N. vitifusiforme (12 isolates), N. luteum (14 isolates), N. mediterraneum (20 isolates) and N. australe (339 isolates). For each species BLAST searches against GenBank showed 99-100% identity to reference sequences of representative strains including those of ex-type cultures. New sequences were deposited in GenBank [accession numbers: KP142959 - KP142961 (ITS), KP142978 (EF-1α) for N. parvum; KP142962 - KP142966 (ITS), KP142979 (EF-1α) for N. vitifusiforme; KP142949 - KP14 2953 (ITS), KP142976 (EF-1α) for N. luteum; KP142954-KP142958 (ITS), KP142977 (EF-1α) for N. mediterraneum; KP142944 - KP1429 48 (ITS), KP142975 (EF-1α) for N. australe].
Neofusicoccum australe was the only species recovered from all survey sites in all sampling seasons, 339 of the 510 cankers processed yielded colonies of this pathogen. The isolation frequency (number of cankers with positive isolations of each fungus divided by the total number of cankers examined x 100) of N. australe ranged from 51% to 95% (Tab. 2). The other four Neofusicoccum species were isolated with a lower frequency and were often found at only a few sites (Tab. 2). The isolation frequency of N. australe declined between May and October, whereas N. mediterraneum showed an opposite trend. The other species were predominantly isolated in the spring (Tab. 2).
Tab. 2 - Isolation frequencies (%) of fungal species at each survey site and season. (ns): no sample taken.
| Fungal species |
Sampling period |
Site | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| Cytospora sp.1 | Spring | - | 6.7 | - | 26.7 | - | 13.3 | - | - | - | - | - | - |
| Summer | - | - | - | - | - | - | - | - | - | - | - | ns | |
| Autumn | - | - | - | - | - | - | - | - | - | - | - | ns | |
| Cytospora sp.2 | Spring | - | - | - | - | 13.3 | - | 20 | - | - | - | - | - |
| Summer | - | - | - | - | - | - | - | - | - | - | - | ns | |
| Autumn | - | - | - | - | - | - | - | - | - | - | - | ns | |
| Diaporthe foeniculina | Spring | - | - | - | - | - | - | - | - | - | - | - | 20 |
| Summer | - | - | - | - | - | - | - | - | - | - | - | ns | |
| Autumn | - | - | - | - | - | - | - | - | - | - | - | ns | |
| Neofusicoccum australe | Spring | 46.7 | 93.3 | 80 | 46.7 | 66.7 | 46.7 | 53.3 | 100 | 80 | 80 | 100 | 80 |
| Summer | 66.7 | 40 | 40 | 86.7 | 60 | 73.3 | 53.3 | 93.3 | 80 | 80 | 93.3 | ns | |
| Autumn | 53.3 | 26.7 | 66.7 | 46.7 | 80 | 46.7 | 46.7 | 93.3 | 26.7 | 40 | 93.3 | ns | |
| Neofusicoccum luteum | Spring | - | - | - | - | - | - | - | - | - | - | - | - |
| Summer | - | 26.7 | - | - | - | - | - | - | 20 | - | - | ns | |
| Autumn | - | 6.7 | - | 6.7 | - | 6.7 | - | 6.7 | 6.7 | 6.7 | 6.7 | ns | |
| Neofusicoccum mediterraneum | Spring | - | - | - | - | 6.7 | - | - | - | - | 20 | - | - |
| Summer | 20 | - | - | - | - | 7 | - | - | - | 20 | - | ns | |
| Autumn | - | - | - | 40 | - | 20 | - | - | - | - | - | ns | |
| Neofusicoccum parvum | Spring | - | - | - | - | - | - | - | - | - | - | - | - |
| Summer | 6.77 | - | - | - | 26.7 | - | - | - | - | - | - | ns | |
| Autumn | - | - | - | - | 6.7 | - | - | - | - | - | - | ns | |
| Neofusicoccum vitifusiforme | Spring | 40 | - | - | 26.7 | - | - | - | - | - | - | - | - |
| Summer | - | - | - | 13.3 | - | - | - | - | - | - | - | ns | |
| Autumn | - | - | - | - | - | - | - | - | - | - | - | ns | |
| Valsa fabianae | Spring | - | - | - | - | - | 13.3 | - | - | - | - | - | - |
| Summer | - | 46.7 | 60 | - | - | 20 | 46.7 | 6.7 | - | - | 6.7 | ns | |
| Autumn | 46.7 | 66.7 | 33.3 | 6.7 | 13.3 | 26.7 | 53.3 | - | 66.7 | 53.3 | - | ns | |
The remaining 98 isolates were found to represent four distinct species: Diaporthefoeniculina (Sacc.) D. Udayanga & L.A. Castlebury (three isolates, GenBank KP1429 70), two unidentified Cytospora species (seven isolates of species 1, GenBank KP14 2967-KP142968; five isolates of species 2, GenBank KP142969) and Valsa fabianae G.C. Adams, M.J. Wingf. & Jol. Roux (83 isolates, GenBank KP142971 - KP142974). Valsa fabianae was the second most frequently isolated fungus after N. australe. It was isolated from 11 sites and the isolation frequency increased from the spring to the autumn sampling. The other three species were isolated at a relatively low frequency from one or a few surveyed sites and only during the spring (Tab. 2).
Pathogenicity tests
The pathogenicity tests highlighted significant differences in aggressiveness among the nine species obtained from branch cankers (Tab. 3). All the Neofusicoccum species except N. vitifusiforme proved to be aggressive pathogens on E. camaldulensis. At the end of the experimental period, all trees inoculated with N. australe, N. luteum, N. mediterraneum or N. parvum displayed dark brown bark lesions that spread up and down the trunk from the inoculation site and also penetrated a few millimeters into the wood. The average lesion size differed significantly according to the species, e.g., the lesions caused by N. mediterraneum (mean = 25.25 cm2) were significantly larger than those caused by N. luteum (mean = 18.61 cm2), N. parvum (mean = 11.08 cm2) and N. australe (mean = 8.9 cm2). All the other fungal pathogens caused small necrotic lesions confined to the inoculation point with no statistically significant difference from the control inoculations. All the fungal species were successfully re-isolated from symptomatic wood and inner bark tissues from all inoculated trees (Tab. 3).
Tab. 3 - Mean lesion area ± standard error (SE) caused by fungal species on Eucalyptus camaldulensis trees. (1): Values within column followed by the same letter do not significantly differ according to Fisher’s least significant differences multiple range test (P < 0.05). (2): Number of samples from which the fungus was re-isolated out of 10 samples per tree examined.
| Species | Isolate | Mean lesion area (cm2) ± SE1 |
Reisolation2 |
|---|---|---|---|
| Cytospora sp. 1 | CAD049 | 3.23 ± 0.22 d | 60 |
| Cytospora sp. 2 | CAD050 | 1.95 ± 0.17 d | 60 |
| Diaporthe foeniculina | CAD051 | 1.55 ± 0.09 d | 60 |
| Neofusicoccum australe | CAD028 | 8.90 ± 1.50 c | 60 |
| Neofusicoccum luteum | CAD029 | 18.61 ± 1.67 b | 60 |
| Neofusicoccum mediterraneum | CAD026 | 25.25 ± 1.98 a | 60 |
| Neofusicoccum parvum | CAD025 | 11.08 ± 3.32 c | 60 |
| Neofusicoccum vitifusiforme | CAD027 | 2.14 ± 0.12 d | 60 |
| Valsa fabianae | CAD052 | 1.38 ± 0.09 d | 60 |
| Control | - | 1.53 ± 0.04 d | - |
Discussion
This study describes the first survey of pests and diseases threatening E. camaldulensis plantations in Sardinia. Our results have characterized the population dynamics of the principal insect pest, G. brimblecombei, and have clarified the symptomatology and etiology of the severe decline affecting this species. In particular, our data revealed that G. brimblecombei infestations have assumed epidemic proportions at all 12 survey sites and that this pest currently represents the main entomological threat to E. camaldulensis plantations in Sardinia. Its natural enemy P. bliteus was also found at each site, but was not sufficient to limit the population increase of G. brimblecombei which started in May.
Morphological analysis and DNA sequencing revealed nine different fungal species in V-shaped cankers, including D. foeniculina, N. australe, N. luteum, N. mediterraneum, N. parvum, N. vitifusiforme, V. fabianae and two putative new species of the genus Cytospora.
Neofusicoccum species accounted for more than 80% of the isolates, making this by far the most common genus associated with branch cankers in declining Sardinian E. camaldulensis trees. Almost all the Neofusicoccum species we identified have previously been detected on eucalyptus trees ([30], [6], [16], [24]) with the exception of N. luteum, which is presented here as a eucalypt pathogen for the first time.
Neofusicoccum australe was the most frequently isolated fungal species, and our field survey and pathogenicity test data clearly revealed that it is the major cause of stem and branch canker in Sardinian E. camaldulensis plantations. The high frequency of N. australe agrees with earlier studies conducted in native eucalypt forests, where the dominance of this pathogen prompted the hypothesis that it is native to the southern hemisphere ([16], [17]). In Italy, N. australe was previously reported in association with olive (Olea europaea L.) drupe rot, declining grapevine (Vitis vinifera L.) plants and declining juniper trees ([37], [5], [41]).
The other four Neofusicoccum species were isolated at a lower frequency and from only a few sites. Neofusicoccum luteum was previously isolated from eucalypts in Australia by Smith & Stanosz ([65]). However, the definitive identification of Botryosphaeriaceae species was challenging in the past and more than one species has erroneously been described as N. luteum over the years. When the ITS sequence of isolate 96-130 (= ATCC56125) studied by Smith & Stanosz ([65]) was used as a BLAST query, we found 100% identity with sequences from the ex-type culture of N. australe (AY339262), suggesting that the isolate is N. australe rather than N. luteum. Although the importance of N. luteum as a eucalypt pathogen is unclear, it infects a wide range of fruit and forest trees worldwide ([45], [2], [7]) and has recently been isolated from declining tree heath (Erica arborea L.) shrubs in their natural range in Sardinia ([42]).
Neofusicoccum mediterraneum was originally isolated from eucalypts in Greece ([24]) but was later identified as an aggressive pathogen of olive drupes in southern Italy, grapevine and English walnut (Juglans regia L.) in California ([37], [68], [69]). To our knowledge, this is the first report of this pathogen in Sardinia. In the pathogenicity tests, N. mediterraneum was the most aggressive species, but because it was only found at a few of the survey sites its impact is marginal compared to that of N. australe.
Neofusicoccum parvum is emerging as a common and cosmopolitan species on diverse host plants, and is now recognized as an aggressive pathogen of grapevine ([70]), mango (Mangifera indica L. - [44]) and walnut ([20]). Neofusicoccum parvum is known to infect several eucalypt species including E. globulus, E. grandis W. Hill ex Maiden and E. saligna Sm. ([30]), E. pellita F. Muell ([6]) and E. urophylla S.T. Blake ([49]). In particular, Gezahgne et al. ([30]) found that N. parvum is the major cause of botryosphaeria canker in Ethiopian eucalypt plantations. In our investigation, N. parvum was isolated only sporadically from our field sites suggesting it has only a marginal role in the etiology of the decline of Sardinian E. camaldulensis plantations. However, it is interesting to note that only the trees artificially inoculated with N. parvum showed copious exudations of kino gum at the end of the experimental period.
Neofusicoccum vitifusiforme was originally thought to be restricted to grapevine ([71]), but it was later isolated from olive ([37]), peach (Prunus persica [L.] Batsch), Chinese plum (Prunus salicina Lindl. - [27]) and the northern highbush blueberry (Vaccinium corymbosum L.) ([34]). Furthermore, both Lazzizera et al. ([37]) and Phillips et al. ([55]) showed that N. vitifusiforme is phylogenetically indistinguishable from Dichomera eucalypti (G. Winter) B. Sutton, and have suggested that D. eucalypti is the synanamorph of N. vitifusiforme. Eucalyptus spp. can therefore be regarded as additional hosts for N. vitifusiforme. Although the plurivorous nature of N. vitifusiforme has now been established, there are still conflicting reports regarding its pathogenicity. In particular, it has been reported as an aggressive pathogen of grapevine and olive drupes in Italy, apple (Malus domestica Borkh.) in South Africa and blueberry in China ([37], [34], [21], [50]), as a weak pathogen of grapevine in South Africa and E. globulus in Australia ([71], [16]) and as nonpathogenic on grapevine and pear trees (Pyrus spp.) in South Africa by Cloete et al. ([21]). In our study, only four of the nine isolated species were pathogenic on E. camaldulensis as judged by the formation of necrotic lesions that were significantly larger than controls. The pathogenicity tests therefore suggest that N. vitifusiforme, together with D. foeniculina, V. fabianae and the two unidentified Cytospora species, could represent a component of the endophytic eucalypt mycoflora.
The genus Diaporthe (teleomorph of Phomopsis) includes a great number of endophytic, saprotrophic and phytopathogenic fungi from a wide range of woody and herbaceous hosts ([31]). In this study, D. foeniculina was isolated with a relatively low frequency at a single survey site and only during the spring. This represents the first report of the species on E. camaldulensis.
Eucalyptus spp. are known to host a large number of species of Cytospora and their Valsa teleomorphs, some of which are thought to cause stem cankers. These genera have a long and confusing taxonomic history because species identification was initially based solely on morphological features. The phylogenetic analysis of different genomic regions has prompted the taxonomic reassessment of many species within these genera ([1]). We isolated two species of Cytospora (here reported as Cytospora species 1 and 2) with low frequencies at a small number of sites, and solely in the spring samples. We have not named these species because further investigation is necessary to achieve the unequivocal identification of these taxa. Valsa fabianae (anamorph Cytosporaeuca lypticola van der Westh.) was recently isolated from cankered and dead branches of several Eucalyptus species in Australia, Uganda and South Africa ([1]). More recently, this species was isolated from symptomatic and asymptomatic leaves of E. globulus trees affected by MLD in Spain ([59]). In our study, V. fabianae was isolated from 11 sites and the isolation frequency increased from the spring to the autumn.
Conclusions
Our results have shown that N. australe is the main pathogen associated with cankers and branch dieback in Sardinian E. camaldulensis plantations. The high frequency of trees at different life stages with cankers on their stems and branches, as well as fresh exudates of kino gum, suggests that the disease is still in its epidemic phase. Abiotic factors that may facilitate the outbreak of N. australe infections remain unknown, as well as potential synergistic interactions with other Neofusicoccum species and plant stress caused by G. brimblecombei. Our data, together with the results of previous studies on the etiology of decline affecting holm oak (Quercus ilex L.), narrow-leaved ash (Fraxinus angustifolia Vahl) and Phoenician juniper (Juniperus phoenicea L. - [38], [40], [4]), emphasize how fungal species in the Botryosphaeriaceae family represent a growing threat to forest ecosystems in Sardinia.
Acknowledgements
This study was financially supported by the “Regione Autonoma Sardegna, Tavolo Tecnico Difesa Fitosanitaria Piante Forestali, Assessorato Difesa Ambiente - Servizio Tutela del suolo e politiche forestali” in the context of the research project “Programma triennale di controllo biologico della Psilla lerp dell’eucalipto Glycaspis brimblecombei e monitoraggio delle problematiche fitosanitarie dell’eucalipto in Sardegna”, coordinated by Prof. Ignazio Floris, University of Sassari, Italy. Franco Buffa and Antonio Deidda gratefully acknowledge the Sardinian Regional Government for the financial support of their PhD scholarships. Bruno Scanu acknowledges the Sardinian Regional Government for the financial support of the research project “P.O.R. Sardegna F.S.E. Operational Programme of the Autonomous Region of Sardinia, European Social Fund 2007-2013 - Axis IV Human Resources, Objective l.3, Line of Activity l.3.1”. The authors would like to thank Dr. Richard M Twyman for English language editing.
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Online | Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Authors’ Info
Authors’ Affiliation
Franco Buffa
Benedetto Teodoro Linaldeddu
Claudia Pinna
Bruno Scanu
Vitale Deiana
Alberto Satta
Antonio Franceschini
Ignazio Floris
Dipartimento di Agraria, Sezione di Patologia vegetale ed Entomologia, Università degli Studi di Sassari, v.le Italia 39, I-07100 Sassari (Italy)
Corresponding author
Paper Info
Citation
Deidda A, Buffa F, Linaldeddu BT, Pinna C, Scanu B, Deiana V, Satta A, Franceschini A, Floris I (2016). Emerging pests and diseases threaten Eucalyptus camaldulensis plantations in Sardinia, Italy. iForest 9: 883-891. - doi: 10.3832/ifor1805-009
Academic Editor
Alberto Santini
Paper history
Received: Aug 11, 2015
Accepted: Mar 05, 2016
First online: Jun 29, 2016
Publication Date: Dec 14, 2016
Publication Time: 3.87 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2016
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 62439
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 50104
Abstract Page Views: 6045
PDF Downloads: 4763
Citation/Reference Downloads: 71
XML Downloads: 1456
Web Metrics
Days since publication: 3507
Overall contacts: 62439
Avg. contacts per week: 124.63
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2016): 18
Average cites per year: 1.80
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Short Communications
Local spread of an exotic invader: using remote sensing and spatial analysis to document proliferation of the invasive Asian chestnut gall wasp
vol. 5, pp. 255-261 (online: 24 October 2012)
Research Articles
Gnomoniopsis castaneae associated with Dryocosmus kuriphilus galls in chestnut stands in Sardinia (Italy)
vol. 10, pp. 440-445 (online: 24 March 2017)
Research Articles
Natural spread of Verticillium wilt as effective constraint on Ailanthus altissima invasion
vol. 18, pp. 391-398 (online: 22 December 2025)
Research Articles
Essential environmental variables to include in a stratified sampling design for a national-level invasive alien tree survey
vol. 12, pp. 418-426 (online: 01 September 2019)
Research Articles
Assessing escapes from short rotation plantations of the invasive tree species Robinia pseudoacacia L. in Mediterranean ecosystems: a study in central Italy
vol. 9, pp. 822-828 (online: 25 May 2016)
Research Articles
Shear modulus of old timber
vol. 10, pp. 446-450 (online: 24 March 2017)
Research Articles
Seven Ulmus minor clones tolerant to Ophiostoma novo-ulmi registered as forest reproductive material in Spain
vol. 8, pp. 172-180 (online: 13 August 2014)
Research Articles
Arbuscular mycorrhizal colonization in black poplar roots after defoliation by a non-native and a native insect
vol. 9, pp. 868-874 (online: 29 August 2016)
Review Papers
Forests and climate change - lessons from insects
vol. 1, pp. 1-5 (online: 28 February 2008)
Research Articles
Comparison of timber-house technologies and initiatives supporting use of timber in Slovenia and in Sweden - the state of the art
vol. 10, pp. 930-938 (online: 07 December 2017)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword