Sweetgum: a new look
iForest - Biogeosciences and Forestry, Volume 8, Issue 6, Pages 719-727 (2015)
doi: https://doi.org/10.3832/ifor1462-008
Published: Jun 23, 2015 - Copyright © 2015 SISEF
Review Papers
Abstract
Sweetgum (Liquidambar styraciflua L.) is the only species of its genus in the Western hemisphere. The species is a relatively early successional species with wide seed dispersal, fast growth and is considered one of the most adaptable tree species in North America, growing across a wide range of soil types, altitudes, and hydrologic conditions. This species has routinely been considered a lesser desired species by many forest managers trying to grow tree plantations or even in natural stands because the species tends to rapidly invade and dominate a site. However, because of sweetgum’s adaptability, ease of propagation and field planting, and fast growth rate, the tending of sweetgum as a potential crop for improved markets has been reinvigorated. Managing sweetgum also opens the possibility of development of new products and markets that supplement the traditional markets and can produce further value-added products. Increasingly, sweetgum is not viewed with as much antipathy amongst foresters and its potential as valuable resources is being rediscovered.
Keywords
Sweetgum, Liquidambar styraciflua L., Fast-growing species, Potential crop, Value-added products
Historical prospective
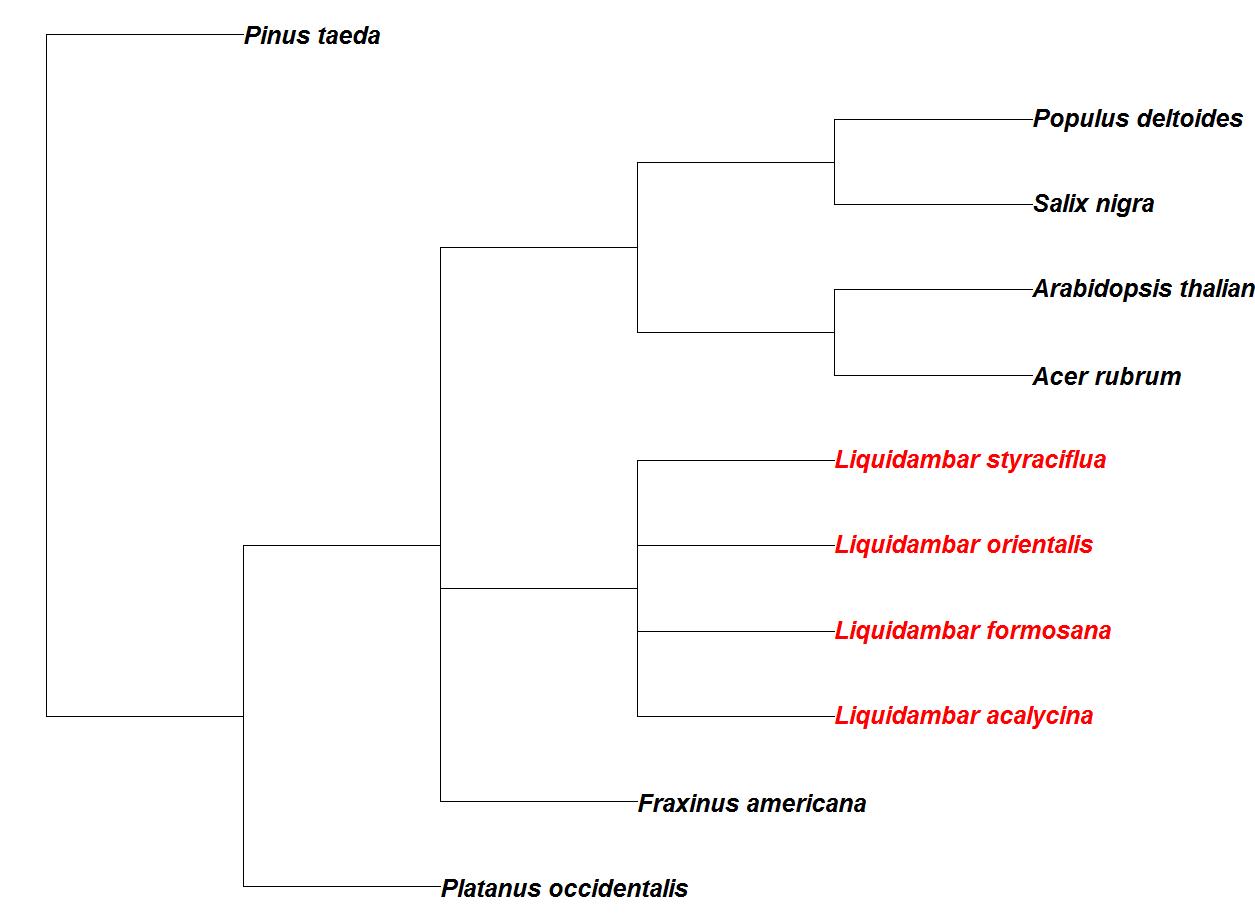
The North American species sweetgum (Liquidambar styraciflua L.) is one of only four species in the Liquidambar genus, also including species formosana, orientalis, and acalysina. The L. formosana, or Formoson gum, inhabits southeastern Asia and is named for Formoson (Taiwan) Island; while the smaller statured species L. acalysina inhabits the mainland of Southeast Asia. Oriental sweetgum, L. orientalis, is native to the eastern Mediterranean region, including Turkey and the island of Rhodes. The genus first appears in the fossil record with many other flowering plants in the Paleocene epoch, ancestors to modern lineages arose in the Oligocene epoch ([37]). The genus reached its widest distribution by the Miocene epoch ([74]) and is highly diverged from other economically important fast-growing species that are currently common in the southeast United States (Fig. 1). There is extremely old evidence of L. styraciflua inhabitation of North America, probably moving into the continent during the existence of an ancient North Atlantic land bridge that predates the more commonly known North Pacific land bridge ([26]). A relic of this ancient migration is also evident in modern genetic divergence data, in which sweetgum is actually a closer relative to the western Asia/Europe species, L. orientalis, than the two east Asian species, L. formosa and L. acalycina. The genetic identity based on a proportion of 22 loci similar between the two species is 0.51. While it potentially maintained contact with the east Asian species via the North Pacific land bridge, the North American species had lower genetic identities of 0.43 and 0.48 with L. formosa and L. acalycina, respectively ([26], [27], [40]). Interestingly, the lowest genetic similarities were between the Europe/west Asia species and the two east Asia species ([26]).
Fig. 1 - A dendrogram created from the NCBI taxonomy browser (⇒ http://www.ncbi.nlm.nih.gov/Taxonomy/taxonomyhome.html/index.cgi) using other tree species that are common to southeast United States’ forests. Species of the Liquidambar genus are in red.
Though the species have been isolated for thousands of years, they are derived from a common ancestor in the Miocene era that favored sites of mesic, warm temperate areas ([26]). The end of the Miocene saw global cooling and the beginning of glaciation in the Pleistocene epoch. These climatic changes limited the genetic exchange among the species progenitors and greatly limited each species geographical range on the landscape ([80]). Though having a long period of separation, the species have similar morphologies thought to be due to evolutionary stasis and have similar ideal growing conditions (i.e., mesic and warm - [26]).
The North American sweetgum has migrated significantly since becoming isolated from the other Liquidambar species but has maintained its preference for the same prehistoric growing conditions. In the last 20 000 years of the late Quaternary period comprising the Holocene epoch, sweetgum has migrated in iterations from north to south with glacial movements ([79]). These glacial periods were at their maximum between 21 000 and 14 000 years ago pushing mesic, warm climate species like sweetgum to the far south including the extreme gulf coast, Florida, and Mesoamerica ([61], [82]). As glaciers began to recede and the climate warmed and became wetter, the suite of present day hardwood species began to move north again. Only in the last 3000 years did sweetgum appear to begin to dominate the southeast forests based on fossilized pollen samples ([79]).
Silvics, silviculture, and management overview
Silvics
Presently, sweetgum grows from central Florida to eastern Texas. Its extreme northern range extends to Connecticut and southern Illinois, south to the Gulf of Mexico coast with scattered locations through central America ([35]). In the past, the central American populations were classified as their own species, Liquidambar macrophylla ([56]); however, through the 20th century there emerged a consensus in literature that the group should be lumped into L. styraciflua. The species is regarded as one of the most adaptable hardwood species in regards to site conditions, growing best on moist alluvial clays and loamy soils associated with river floodplains ([35]). The species is a major component of four forest cover types, a minor component of over 20 others, and is commonly associated with other species such as maples (Acer rubrum and A. negundo), various hickories (Carya glabra, C. laciniosa, C. ovate, and C. tomentosa), and shortleaf and loblolly pine (Pinus echinata and P. taeda, respectively).
Sweetgum enters into these site associations via wind-blown seeds which are particularly adapted to wind dissemination through the presence of small winged structures on the seed. The seeds emerge after fruiting bodies mature by September through November. The seeds are small and prolific in which a kilogram of seed average 180 000 seeds compared to the associated species P. taeda which may only average up to 40 000 seeds kg-1 ([4]). Germination of the seeds can occur quickly as they exhibit only a shallow dormancy ([53]). Indeed with no cold stratification necessary, seeds may begin to germinate after an average of 7-8 days in a germinator and can reach 90% germination after only 11 days ([60]).
The initial growth traits can lead to rapid colonization of a site, and within various forest types, sweetgum is considered a shade intolerant species and grows very rapidly to reach sunlight ([46]). On a site cleared of overstory, sweetgum will tend to dominate the site early and continue to be a major component in the early to mid-successional stages of a forest which can persist in southeastern U.S. forests for 200 years or longer ([25]). Cleared sites are also quickly re-colonized by sweetgum through prolific sprouting that produces vigorous and persistent new trees ([77]). On sites that are primarily regenerated with pine species, sweetgum is able to take the place of pines as the pine cohort begins to senesce as the stand goes through natural successional changes. Thus, sweetgum can become the dominant cover species until they are replaced by more shade-tolerant species ([24]).
Control of unwanted sweetgum
Sweetgum can be considered a weed or nuisance tree in the context of some stand management objectives (Fig. 2). In a study on the Yazoo National Wildlife Refuge, sweetgum was the most common species into the area designated for cherrybark (Quercus pagoda), Nuttall (Q. nuttallii), Shumard (Q. shumardii), water (Q. nigra), and willow oak (Q. phellos) regeneration. This study led to the recommendation of planting additional oak seedlings to ensure these heavy-seeded species were present for wildlife management objectives ([1]). During initiation of southern pine stands that dominate timber production in the southeast U.S., sweetgum is a definite concern. The presence of sweetgum can lead to greater competition for limited resources such as water, reducing survival and growth of the desired pine trees. Sweetgum can reduce soil moisture at depths of 60-90 cm in the soil which can directly adversely affect potential growth of the planted P. taeda stand ([48]). Especially during times of limited water which is common from June to September in much of the pine-sweetgum range, the effect of increased competition by sweetgum can lead to exacerbated water stress and changes in stomatal conductance, the exchange of carbon dioxide molecules through the leaf stomata, which affects photosynthesis ([57]).
Fig. 2 - Graduate student holding a desirable three-year-old loblolly pine that is under intense competition from fast growing sweetgum that naturally seeded into the area after the pine was planted. Sweetgum can be seen in both the foreground and background and is already taller and crowding out the planted loblolly pine.
Removal of sweetgum is often conducted during the initial stages of stand development. This allows desired species to establish and be more competitive with later incursions by sweetgum. Control of juvenile sweetgum can be conducted effectively through use of various herbicides such as glyphosate (e.g., Roundup™) which can be applied via aerial, tractor, ATV, or manual back pack sprayer depending on the site, size of area, and affordability. Indeed, non-specific, broadcast spraying of a site can be very effective in control of sweetgum. D’Anieri et al. ([18]) reported that sweetgum translocated 48% of applied foliar glyphosate to roots in comparison to common associates P. taeda and Acer rubrum L., 3% and 13%, respectively. Efficacy of glyphosate treatments is very good with 1.68 kg m-2 glyphosate application effectively controlling 64-93% of sweetgum after one year ([38], [83]). Bacon & Zedaker ([2]) found that removal of just two-thirds of woody competition (largely sweetgum) and herbaceous control could result in a 53% increase in loblolly pine stem volume growth in the second year. With only total woody control (no herbaceous control), there was still a 10% increase in pine volume production. Several other herbicides are notably effective at controlling sweetgum, including imazapyr, hexazinone, and triclopyr ([16], [52]). As for all herbicide treatments, the costs of the herbicides must then be carried for the length of the stand rotation (e.g., 25-35 years for an average managed loblolly pine stand), thus affecting decisions on sweetgum removal.
Advances in propagation and growth
While sweetgum may be regarded as of marginal value, current and potential product markets may make sweetgum more favorable in the future. Currently it is used as a component in natural forest stands in floodplain areas and as an ornamental in some landscaping plans. To propel sweetgum as a primary candidate for some of these uses, artificial propagation of sweetgum will be necessary to ensure that superior sweetgum trees are effectively and cheaply produced. Hare ([23]) successfully propagated cuttings that were taken from mature sweetgum (species formosa and styraciflua) crowns. In early May, shoots were harvested and planted in a perlite-vermiculite mixture with temperature and water control. After two months, up to 36% of cuttings rooted. Greater survival percentages are achievable by taking a more traditional approach and using cuttings from sprouts. Vegetative shoots from severely pruned L. styraciflua plants resulted in 86% of cuttings rooted and 56% of cuttings surviving and deemed plantable 25 weeks after the cutting was rooted ([59]). Rieckermann et al. ([59]) also found that a weekly nitrogen (N) application of 25-50 mg l-1 was optimal for promoting cutting development. Using severely pruned plants or hedging techniques, sweetgum can be quickly propagated. These mini-cutting techniques involve initial propagation of cuttings to produce mini-stumps that are hedged and produce successive integrations of sprouts (averaging about 2.5 sprouts per 30 days) used as micro-cuttings ([76]). After 10 intervals of harvesting mini-cuttings, only 6% of the mini-stumps had died. This method was able to produce close to 3000 mini-cuttings per square meter per year and is a viable method to quickly expand germplasm for field plantings ([76]).
Sweetgum has also been successfully propagated in vitro for many years. One of the earliest reports of an in vitro method was in 1980 using solid and liquid media ([67]). The researchers based their initial work on established tobacco protocols and found that bud differentiation would occur with cytokinin/auxin ratios of (0.8-1.6 mg l-1 benzyadenine (BA) and 0.1-1.0 mg l-1 naphthaleneacetic acid (NAA), while root formation was conducted on media with a decreased ratio (0.2 mg l-1 BA and 1.0-4.0 mg l-1 NAA). Researchers germinated seedlings, excised the hypocotyl, and put sections of the tissue in the above cultures. Some cultures were transferred to the same mediums without agar (i.e., liquid state) and maintained in a shaker. In both solid and liquid form, successful somatic embryogenesis was achieved.
This early success was followed by mature and juvenile plants successfully propagated using excised shoot tips which were surface-sterilized with sodium hypochlorite ([71]). Both mature and juvenile plants responded best when first placed in a multiplication me- dia composed of Woody Plant Medium (WPM), basal salt supplemented with 0.2 mg l-1 BA and 0.05 mg l-1 NAA. Explants from successful multiplication were then rooted in WPM supplemented with 0.5-1.0 mg l-1 indole-3-butyric acid (IBA). While propagation was successful, large differences in rooting percentages (33-100%) were evident among parent stock ([71]).
A protocol using the same hormones was used by Brand & Lineberger ([5]) on leaf tissue. Woody Plant Medium (WPM) basal salt supplemented with 2.5 mg l-1 BA and 0.1 mg l-1 NAA was used to elicit shoot formation from leaf tissue. Shoot formation was increased by damaging the leaf surface by cutting the lamina with razor. The authors found later in at least one variety, leaves used from intact plants produced over four times the amount of adventitious shoots than plants from in vitro use but was constant as the leaves aged ([6]). In conjunction with development of in vitro techniques for propagation, molecular transformation techniques have been developed which allow for the potential of production of genetically modified sweetgums. These could be engineered with advantageous traits such as superior growth, regulation of seed production, and modified chemical synthesis. Sullivan & Lagrimini ([70]) were able to transform sweetgum plantlets with three different plasmids via Agrobacterium tumefaciens infection. Leaves were excised and transversely cut at the midrib and incubated with the bacteria for three days in BA supplemented 2.5 mg l-1 BA WPM media. Afterwards, the bacterium was killed with cefotaxime and the leaf tissues were transferred to fresh media. Callus tissue initially formed followed by shoots which were excised and transferred to larger vessels. Plasmids have also been successfully transformed into sweetgum cultures via gold particle bombardment. Using established bombardment protocols, Kim et al. ([32]) were able to generate nodule cultures which were receptive to the gene transfer treatment. They found that sweetgum nodules established from seedling hypocotyls should be proliferated using a liquid medium and had desirable traits including rapid growth of colonies, uniform cell size, and ability to produce regenerative tissue.
Sweetgum also appears to be easily transferred from in vitro to greenhouse and subsequent field conditions. In vitro plants were noted to have physiological abnormalities including limited cytoplasm content, large leaf vacuoles, decreased starch content, and increased photosynthetic rates ([39], [78]). Plantlets must be acclimated to field conditions and allowed time for their morphology and cellular physiology to transform into normal field-grown conditions. Transferred to soil and hardening off is easily conducted by maintaining high-humidity by using a plastic bag covering on the plant ([78]). Using such a technique allows for acclimation after about four weeks. Brand & Lineberger ([5]) were also able to easily root and acclimate shoots produced from leaf tissue culture.
Silviculture and management
The next stage of sweetgum propagation would be to plant the newly propagated plants in nursery-like conditions for outplanting to true field conditions. Allowing the propagules to acclimate and develop roots is critical for eventual outplanting. Kormanik ([34]) found that seedlings with more than six lateral roots exceeding 1 mm in diameter outperformed lower grade seedlings. These best seedlings only exhibited dieback in 41% of seedlings and had 79% survival after the first year; whereas, the two lower grades had 67% and 89% dieback and 67% and 51% survival after one year. Larger seedlings, in regards to root collar diameter (RCD), are also good indicators of later growth. Increases of RCD from 4-8 to 12-16 mm led to three-year increase in volume of 90% ([44]).
During the seedling time ex vitro, the roots also have the added benefit of being exposed to beneficial mycorrhizal. These fungal infections resulted in 80x greater above ground biomass after the seedling were grown for one year in nursery conditions ([9]). Indeed, without mycorrhizal presence, seedlings are ill adapted to the competitive field environment and the plants will either die or be severely stunted ([36]). With mycorrhizal, sweetgum performs well with N fertilization (560 kg N ha-1) in nursery conditions. Additionally, a fertilizer, such as ammonium nitrate, is preferable over ammonium sulfate, as it does not acidify the soil as severely ([8]).
Once deployed in plantations, establi-shed sweetgum thrives with fertilization. Through most of sweetgum’s range, N is one of the most limiting factors to growth in forest ecosystems and N additions consistently result in increased stand net primary production and increased fine root production ([12], [54], [28]). In a young 4-year-old study sweetgum plantation, Guo et al. ([20]) applied N and phosphorous (P) treatments and found that a N+P (229.8 kg ha-1 and 137.9 kg ha-1, respectively) combination treatment or N alone treatment at 229.8 kg ha-1 generally performed the best in regards to tree height, diameter and crown length. These effects were very evident after the first year-post treatment (i.e., ages 6 and above) and were maintained through the course of the study when the stand was 15-years-old. In a 9-year-old plantation, sweetgum produced the most aboveground biomass at highest fertilization levels in the study that were equivalent of 400 kg N ha-1 ([51]); however, annual incremental growth is measurable for only one to two years ([49]). Still, the need for fertilization is site specific and each site should be tested for its inherent nutrients. Scott et al. ([64]) found that on a converted agriculture field rich in nutrients, there was no effect on sweetgum growth from the addition of N and P, while trees on a forest cut-over site responded with a 60% increase in biomass.
Deployment of whole plantations of sweetgum can generate rapid biomass production which can be used for many of the outlined products. Volume production ranged from 8.4 m3 ha-1 year-1 to 18.62 m3 ha-1 year-1 with no fertilizer up to 399 kg ha-1 in a Mississippi study after nine years ([50]). In the same study, increases in dimensional attributes after nine years were 9, 14, 28, 39, and 40% for height, DBH, basal area, stem volume, and woody biomass, respectively ([49]). In South Carolina with irrigation and fertilization four-year growth rates of sweetgum ranged from 2.4 to 5.1 Mg ha-1 year-1 ([17]). On a marginal agriculture site in Georgia, sweetgum grown without any amendments produced average of 12.3 Mg ha-1 after six years, while maximal amendments (irrigation+fertilization+pest control) resulted in 62.6 Mg ha-1.
While sweetgum is capable for producing rapid growth suitable for short rotation crops, sweetgum is a valued hardwood lumber species. As such, there is renewed interest in sweetgum as a valuable component to predominantly oak stands by exploiting its natural place in river floodplain ecosystems. In an attempt to mimic natural stand succession, Lockhart et al. ([42]) planted cherrybark oak (Quercus pagoda) and sweetgum seedlings in mixtures. In these mixtures, cherrybark oak seedlings were surrounded by two rows of sweetgum (Fig. 3). Initially the stand was dominated by sweetgum as would be expected based on its early and aggressive growth. After 21 years, the sweetgum began to stagnate and the cherrybark oak ascended into the upper canopy. This planned species succession offers potential benefits of a harvest of sweetgum timber before the cherrybark oaks are harvested which can be 60-80 years in some cases. While cherrybark oaks are competing with sweetgum for light, the sweetgum serves as a “trainer” tree to increase the bole quality of cherrybark oak. This echoes an earlier study by Clatterbuck & Hodges ([14]) which found a lack of different species like sweetgum around the desirable cherrybark oak trees caused the cherrybark oak to have poor form (e.g., many branches, straightness deficiencies) and to be commercially less desirable.
Fig. 3 - Sweetgum were planted and allowed to grow for 28 years around potentially more valuable cherrybark oaks. The crowns of sweetgum (narrow crowns in the center of the picture) are expected to help shear off limbs of the cherrybark oak, improving their quality. The sweetgum also serve as a source of income before the cherrybark oak is finally harvest which may be over 60 years.
Mid-rotation removal of sweetgum is warranted. Johnson & Krinard ([30]) found that in a two mixed sweetgum-red oak stands (red oaks were composed of Q. pagoda, Q. phellos and Q. nigra), approximately 25% of the sweetgum trees died between ages 18 and 23. This was immediately following the maximum observed density (3879 trees per hectare) on the site at which time light became a limiting factor. This increased competition and decline in sweetgum led to the red oak trees growing twice as fast in diameter and 60% faster in height between ages 23 and 29. The growth changes led to the prediction of oaks overtaking the sweetgum in total height by ages 30 to 35. Similar trends were all observed by Clatterbuck et al. ([13]), who found that oaks in their old field stand overtook sweetgum as early as age 15 to 25 years.
A final regeneration harvest necessitates a system that lead to maximal light interception for sweetgum. A clearcut method is optimal for even-age regeneration of a stand. This system will favor in the early years of regeneration shade-intolerant and light-seeded species such as sweetgum and river birch ([46]). A seed-tree method would also have the same effect, while leaving trees on site may be a waste as the species is so light seeded that blown in seed from adjacent areas or regeneration from root sprouts is usually assured ([46]). The shelterwood system as well as the uneven-age system, single-tree selection, are usually not favored for final harvest if the goal is sweetgum regeneration as they favor more shade tolerant species such as elms and maples ([29]). Indeed, even the uneven-age system group selection usually does not produce openings in the canopy large enough to maintain a shade intolerant species such as sweetgum ([15]). Thus final harvest options, with the intent of sweetgum regeneration, are very limited.
Commercial products
Early uses
Sweetgum has chemical properties that are beneficial for a large array of ailments as detailed by Lingbeck et al. ([41]). These benefits have been harnessed from early times with several Native American tribes documented as using sweetgum for medicinal purposes. Hamel & Chiltoskey ([22]) documented several uses among the Cherokee tribe including using rosin and inner bark for diarrhea, flux, and dysentery, salve from leaves used for wounds, sores, and ulcers, and infusions of bark were used as a sedative and given to people with nervousness. The Choctaw and Houma tribes of Louisiana both used a root mixture for dressing cuts and wounds ([10], [69]). These tribes also used the solidified sap as chewing gum. This practice may even continue today and certainly was prevalent during the early 20th century based on personal communications with several people from Louisiana born during the great depression.
Many of these same uses of sweetgum were kept after European colonization. During the civil war, a field guide for medical officers was commissioned for the Confederate States and published after the war. Sweetgum was mentioned many times in this book ([58]). In the guide, the author mentions many of the same uses for patients including boiling equal amounts of red oak and sweetgum into syrup, adding spirits, and digesting the concoction to alleviate diarrhea and dysentery. The manual also recommend mixing the extract with tea or water. Ironically, water extraction is successfully used in current times to extract shikimic acid from sweetgum bark as a precursor to Tamiflu® ([43]). The leaves of the sweetgum were said to contain high tannin levels to provide a powerful astringent. One interesting comment in the guide is a reference to an acid obtained from sweetgum. The author states that he disagrees with the English assentation that benzoic acid is prevalent but rather cynamic acid. This early reference to “cynamic acid” is probably the same cinnamic acid that has since been more recently “rediscovered” and studied across the Liquidambar species for its antioxidant, antipathogenic, and antimicrobial properties ([31], [55], [68], [21]).
Timber
While much emphasis has dealt with the removal of sweetgum from future timber streams, sweetgum can be utilized for pulp, furniture grade lumber, pallets, veneer and panels. The heartwood of the sweetgum trees is reddish brown with interlocking grain and is often mistaken for cherry wood, given rise to the species also being called redgum. The red heartwood was often used in furniture, millwork, doors and paneling, and was once a valuable resource as it was sold in European markets as satin walnut ([11]). Dwindling supplies of the old-growth red heartwood, however, led to an increase in sapwood use. Today, most sweetgum lumber is from the sapwood which is white in color with a pinkish tint ([11]). Sapwood is used in making low end furniture and furniture frames. However, development of composite wood for this purpose has reduced the demand for sweetgum lumber. Due to its high shrinkage coefficient and its tendency to warp due to its interlocked grain, sweetgum wood is one of the lowest valued hardwoods available ([11]). Still, sweetgum has wood properties that make it amenable to modern wood processing techniques. The species is easily peeled into veneers for use in plywood which can have at least equal strength compared to similar processed southern pine plywood ([3]). Recent lamination developments in structural lumber have opened other uses for sweetgum which can be oriented to create beams of considerable strength and light weight for construction of bridges and ground flotation mats on which heavy equipment is placed to prevent soil disturbance ([65], [66]).
Biomass
Biomass is a term for organic material from plants and may be a key source of renewable energy, possibly replacing or reducing our demand for supplies of fossil fuels ([75]). The ability of sweetgum to rapidly grow on a wide range of soils, as well as its ability to re-sprout prolifically, make sweetgum trees a suitable biomass source to be used for bioenergy production including cellulosic ethanol, wood pellets and bio-oil ([81]). This rapid proliferation of products could be tied with recent advances in development of specific varieties produced through tissue culture that are promising for short-rotation biomass ([47]). The cultures would be grown for just a few years and then harvested and chipped before refined into a specific product (Fig. 4). However, hardwood plantations of sweetgum may not be economically viable. In 2006, estimated costs for production of a sweetgum plantation throughout the first 12 years range from 778 to 1742 US$ per hectare including costs associated with site preparation, planting, herbicide and pesticide treatments and fertilization. For a low producing plantation (5 Mg per hectare per year) that could result in costs of 31 to 64 US$ per Mg. Higher producing sweetgum plantations could expect costs of 13 to 27 US$ per Mg compared to rival species eastern cottonwood and pine which could cost 14-40 and 6-18 US$, respectively, in highly productive plantations ([33]). When costs of logging and transportation are factored in, only high producing plantations could expect to yield a profit given a target delivery price of 55 US$ per Mg ([19], [33]).
Fig. 4 - An industrial chipper is being used by a logging crew to produce chips from sweetgum and other hardwoods in a pine plantation. Chips are able to be used as a source of biomass fuel.
Cellulosic ethanol
The generation of ethanol from cellulosic biomass offers many benefits. Ethanol is cleaner burning that traditional fuels, thus reducing pollution and greenhouse emissions which may lead to global warming ([84]). Perhaps the biggest benefit of cellulosic ethanol is the utilization of abundant, low-cost feedstocks which may otherwise be considered waste. Cellulosic ethanol is generated from enzymatic hydrolysis of cellulose to form glucose which is then fermented to produce ethanol. However, due to the lignin content, which protects much of the cellulose from enzymatic action, a pretreatment step before hydrolysis is usually necessary to increases yields. Common pretreatment steps include treatment with dilute acid or base, hot water, an organic solvent (organoslov) or by steam explosion ([85], [86]).
Wyman et al. ([84]) studied the efficiency of simultaneous saccharification and fermentation (SSF) method in which biomass, hydrolysis enzymes and fermentation yeast are added together in one vessel. Greater ethanol yields are often seen with SSF because sugars, which are inhibitory to the conversion process, are rapidly converted to ethanol. Four woody crops (aspen, two hybrids poplars and sweetgum), three herbaceous crops (switchgrass, weeping love grass and a legume Sericea lespedeza) and three agricultural residues (corn cobs, corn stover and wheat straw) were studied. The biomass was pretreated in dilute acid and woody crops were ground before they were placed in the fermentation flask containing supplemented media along with cellulase, β-glucosidase (to reduce accumulation of the inhibitor cellobiose) and the yeast S. cerevisiae alone or in a mixed culture with B. clausenii and incubated at 37 °C for 8 days. With S. cerevisiae alone, the agricultural residues, corn cobs, corn stover and wheat straw produced the highest cellulose to ethanol conversion of 94, 92 and 90%, respectively. The herbaceous crops, weeping love grass and switch grass, produced 89% and 84%, respectively, while Sericea lespedeza showed poor yields of 52%. The woody crops Populus maximowiczii x nigra, Populus trichocarpa × deltoides, Populus tremuloides (aspen) and sweetgum produced 90, 82, 94, and 86% yields, respectively. Results were similar with mixed yeast cultures at high enzyme and β-glucosidase concentrations. Using a similar protocol of pentose fermentation, the costs of hardwood produced ethanol was estimated to be about 0.61 US$ L-1 with the main costs attributable to raw materials and capital ([63]).
McConnell & Shi ([45]) evaluated the partial hydrolysis of dilute sulfuric acid, dilute sodium hydroxide and deionized water on holocellulose content (total polysaccharides fraction from wood) from sweetgum, red oak and yellow-poplar (Liriodendron tulipifera). All treatments reduced holocellulose levels in all species; however, sulfuric acid treatment showed the greatest reduction in holocellulose levels in sweetgum and red oak, while sodium hydroxide showed the greatest reduction of holocellulose levels in yellow-poplar. In addition, partial hydrolysis with dilute sulfuric acid of sweetgum and red oak produced residual properties in the wood which may make them suitable for use in wood composites adding additional value to the lignocellulosic ethanol production process ([45]).
Sannigrahi et al. ([62]) used a two-step ozone pretreatment to increase polysaccharide release and hence increase ethanol production from sweetgum and miscanthus. In the first step, the biomass was treated with ozone gas to generate acidic components. The biomass was then equilibrated in water and subject to autohydrolysis at high temperature and pressure to increase solubilization of celluloses. The resultant cellulose was converted glucose at a conversion rate of 68% for both sweetgum and miscanthus. The glucose was finally fermented to produce ethanol with yields of 14.0 g ethanol per 100 g biomass for sweetgum and miscanthus was 11.1 g ethanol per 100 g biomass. In addition, ozone pretreatment is relatively inexpensive and generates an environmentally friendly waste stream and is thus a promising pretreatment strategy ([62]).
Bio-oil
Bio-oil is generated from the fast pyrolysis of biomass and has the potential to be used as liquid fuel ([7]). Sweetgum, switchgrass and corn stover were all evaluated for their ability to produce bio-oil after various pretreatments. Sweetgum, with particle sizes from 0.68 to 1.532 mm produced 52 wt% of bio-oil from fast pyrolysis in which the biomass was rapidly heated from 350-600 °C in the absence of oxygen. In comparison, similar sized switchgrass produced 33 wt% while corn stover produced 35 wt%. Steam explosion pre-treatment lower bio-oil yield for all three biomass compound pre-treatment with 1% sulfuric acid increase yields to 56%, 46% and 51% for sweetgum, switchgrass and corn stover, respectively. The reduced yield seen with steam explosion was thought to be due to the reduction of hemicellulose content after treatment, whereas pre-treatment with 1% acid increase yield due to reduced ash content which is thought to limit bio-oil production. The high rate of bio-oil production, along with the low cost of acid pretreatment and availability of sweetgum trees, make it feasible that sweetgum can be a source of biomass for bio-oil production ([75]).
Economic assessment
In the current era, most timber is sold by tonnage, segregated only by hardwood and pine with the exception of trees targeted for very specific, high-value uses (e.g., black walnut for high quality furniture and paneling or sycamore/cottonwood plantations used for specialty paper). In this common scenario of timber sales, logged hardwoods are combined and weighed in mass as either sawtimber or pulpwood, depending on the size of the tree (≥30.5 and <30.5 cm diameter at breast height, respectively). Over an 11 state region stretching from Texas in the west to the Atlantic and as north as Virginia, the prices of mixed hardwood pulpwood surpassed pine pulpwood in 2010 (in many cases the highest stumpage prices ever received for such products) with an average 1st quarter 2010 price of 11.05 US$ tonne-1. Hardwood sawtimber has historically garnered higher prices with an average of 20.40 US$ tonne-1 ([72]). Since then, prices fell slightly and have slowly rebounded to near 2010 levels in early 2014 with pulpwood averaging 9.15 US$ tonne-1 and sawtimber averaging 24.77 US$ tonne-1 (⇒ http://www.timbermart-south.com/ - [73]). As it is so often lumped together with other hardwoods, sweetgum has garnered little specific study even from an economic prospective as prices have risen. For instance, the hardwood pricing publication Harwood Review Global (⇒ http://⇒ www. hardwoodreview.com) has costs broken down for a variety of species including cottonwood, cherry, hard maple, hickory, red oak, white oak, and walnut. However, sweetgum is noticeably lacking.
Conclusion
Sweetgum is a unique species in North America and has been separated from it relatives for many thousands of years. In its current ecosystem spanning over much of the eastern United States and into Central America, the species thrives. The species rapid growth rate and adaptability on many sites poises both promise and problems for forest management. When hardwood markets were weak, the species was considered by many a weed among the desirable species. More recently with the increased market for hardwood lumber and newer markets such as various bio-based energy products, renewed interest is upon sweetgum. Instead of spending capital to rid sites of the native species, finding new methods to potentially harness value from the prolific species is now attractive.
The fast growth rate and developed culture systems make this species a prime candidate for use alone or with species mixtures for plantations. Systems of propagation have been developed that allow growers to totally capture any genetic trait deemed desirable and potentially mass-produce for planting. After planting, sweetgum competes well with other species and, with some treatment such as fertilization or irrigation, accumulates large quantities of biomass. Other exciting aspects of this species are the medicinal properties that have been recently outlined by Lingbeck et al. ([41]). Efficiently using the tree for both more traditional markets and integrating into developing markets such as biofuel and pharmaceuticals could propel the species to a more desirable status among the forest managers.
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
School of Forestry, Louisiana Tech University, Ruston, Louisiana 71270 (USA)
Sea Star International LLC, Fayetteville, Arkansas 72701 (USA)
Corliss A O’Bryan
Departments of Food Science and Center for Food Safety, Fayetteville, Arkansas 72704 (USA)
Departments of Biological and Agricultural Engineering, University of Arkansas, Fayetteville, Arkansas 72704 (USA)
Corresponding author
Paper Info
Citation
Adams JP, Lingbeck JM, Crandall PG, Martin EM, O’Bryan CA (2015). Sweetgum: a new look. iForest 8: 719-727. - doi: 10.3832/ifor1462-008
Academic Editor
Gianfranco Minotta
Paper history
Received: Oct 02, 2014
Accepted: Apr 21, 2015
First online: Jun 23, 2015
Publication Date: Dec 01, 2015
Publication Time: 2.10 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2015
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 59986
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 49872
Abstract Page Views: 3557
PDF Downloads: 4896
Citation/Reference Downloads: 60
XML Downloads: 1601
Web Metrics
Days since publication: 3906
Overall contacts: 59986
Avg. contacts per week: 107.50
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2015): 9
Average cites per year: 0.82
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Effect of stand density on longitudinal variation of wood and bark growth in fast-growing Eucalyptus plantations
vol. 12, pp. 527-532 (online: 12 December 2019)
Research Articles
NIR-based models for estimating selected physical and chemical wood properties from fast-growing plantations
vol. 15, pp. 372-380 (online: 05 October 2022)
Research Articles
Biomass production of Populus nigra L. clones grown in short rotation coppice systems in three different environments over four rotations
vol. 7, pp. 233-239 (online: 10 March 2014)
Research Articles
Improving dimensional stability of Populus cathayana wood by suberin monomers with heat treatment
vol. 14, pp. 313-319 (online: 01 July 2021)
Research Articles
Wood production and nutritional status of Pinus taeda L. in response to fertilization and liming: a meta-analysis of the Americas
vol. 16, pp. 195-201 (online: 25 July 2023)
Review Papers
Breeding and improvement of black locust (Robinia pseudoacacia L.) with a special focus on Hungary: a review
vol. 16, pp. 290-298 (online: 28 October 2023)
Research Articles
Interaction between planting spacing and wood properties of Eucalyptus clones grown in short rotation
vol. 14, pp. 12-17 (online: 02 January 2021)
Research Articles
Exploring the potential behavior of consumers towards transgenic forest products: the Greek experience
vol. 8, pp. 707-713 (online: 13 January 2015)
Research Articles
Variation of wood and bark density and production in coppiced Eucalyptus globulus trees in a second rotation
vol. 9, pp. 270-275 (online: 08 September 2015)
Research Articles
Compositions of compounds extracted from thermo-treated wood using solvents of different polarities
vol. 10, pp. 824-828 (online: 25 September 2017)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword