Characterization of two poplar homologs of the GRAS/SCL gene, which encodes a transcription factor putatively associated with salt tolerance
iForest - Biogeosciences and Forestry, Volume 8, Issue 6, Pages 780-785 (2015)
doi: https://doi.org/10.3832/ifor1330-008
Published: May 19, 2015 - Copyright © 2015 SISEF
Research Articles
Collection/Special Issue: COST Action FP0905
Biosafety of forest transgenic trees and EU policy directives
Guest Editors: Cristina Vettori, Matthias Fladung
Abstract
To cope with soil salinity, which is one of the most severe forms of abiotic stress, efforts are being undertaken to enhance the salt tolerance of economically important poplar clones in the Vojvodina region of Serbia. One approach is to screen nucleotide diversity in candidate genes (CG) in several poplar clones of high economic importance to Serbia to search for associations with salt stress tolerance. As plant-specific GRAS/SCL transcription factors (TFs) play diverse roles in abiotic stress resistance, two poplar homologs of GRAS/SCL TFs were chosen to differentiate the species background with respect to salt tolerance. A BLAST search of the Populus trichocarpa genome using the P. euphratica gene GRAS/SCL TF_GH611858 sequence identified two putative orthologs, Scaf_5 and Scaf_7, with identities of 100% and 94%, respectively. Primers were designed in identical sequences of Scaf_5 and Scaf_7 to amplify fragments of GRAS/SCL TF orthologs in four poplar clones that are economically important to Serbia. The primers spanned regions where, at least in P. trichocarpa, single nucleotide polymorphisms (SNPs) are present, thereby increasing the probability of distinguishing Scaf_5 and Scaf_7 orthologs in the four clones. Alignments and analyses of the gene fragments revealed that both orthologs were representative of the genetic diversity between different poplar clones, and the identified SNP markers differentiated the four poplar clones with respect to salt tolerance.
Keywords
Candidate Gene, Nucleotide Polymorphism, Tree Genomics, Poplar
Introduction
Populus deltoides Marsh. (cottonwood) of the section Aigeiros is one of the most important species for interspecific poplar breeding programs worldwide ([22], [17]). Clones of P. deltoides species (B229, PE19/66, 182/81) and P. × euramericana hybrid clone (M1) belonging to the Aigeiros section are of high economic importance and widely used in the forest sector in Serbia. These clones have long been cultivated in nurseries and plantations of the Institute of Lowland Forestry and Environment (ILFE) in Novi Sad, Serbia. Until now, four out of the 16 new poplar cultivars registered at the ILFE have been introduced into newly established plantations. So far, Serbian forest breeding programs at ILFE are based on a conventional clonal identification system, which relies on morphological and phenological characterizations ([13]).
Molecular markers such as AFLPs (dominant amplified fragment length polymorphisms) and SSRs (highly polymorphic, consistent and co-dominant simple sequence repeats), allow to obtain unique genetic profiles of individuals and are therefore excellent tools for clone identification ([5], [16], [21], [18]). Using these markers, newly selected Populus deltoides and P. × euramericana hybrid clones were genetically differentiated, and their genetic relationships within the population and with the most relevant clones were obtained ([8], [9], [14]).
Plant productivity is greatly affected by various environmental stresses. Soil salinity is one of the most severe abiotic stress factors, causing increasing agricultural and environmental problems worldwide ([4]). According to Ivanišević et al. ([11]), salt-affected soils currently comprise about 5.5% of the arable land in the Vojvodina region (Serbia). Moreover, soil salinization in Vojvodina may be further increased in the next years as a consequence of the climate change. This calls for a deeper knowledge of the genetic mechanisms underlying the response of forest species to abiotic stress.
In the past decades, a number of genes encoding different structural proteins have been studied with the aim of developing a range of abiotic stress-tolerant plants. Currently, the scientific community is focusing on the use of regulatory genes, such as transcription factors (TFs), as a more effective approach for the development of stress-tolerant plants ([19]). According to Gu et al. ([10]), the identification of a core set of stress-related transcripts by functional genomics studies are crucial for the screening of tolerant germplasms aimed at crop improvement.
Although numerous genetic studies on the adaptability of forest tree species have been published, little is known on the molecular basis of the adaptation process ([7]). Nucleotide diversity was screened in several candidate genes (CGs) putatively correlated with abiotic stress responses in several poplar clones. Plant-specific GRAS/SCL TFs are known to play diverse roles in abiotic stress resistance. According to Bolle ([2]), GRAS proteins are unique to plants, and GRAS homologs have been found in many higher plant species other than Arabidopsis. Although the Arabidopsis genome encodes at least 33 GRAS protein family members, only a few have been characterized in other plant species so far. In addition to their possible role in abiotic stress resistance, GRAS proteins also seem to play an important role in signal transduction, meristem maintenance and development.
Physh et al. ([15]) identified a number of Arabidopsis expressed sequence tags (ESTs) showing high similarity to the Arabidopsis SCARECROW (SCR) amino acid sequence, thereafter designated as SCARECROW-LIKE genes (SCL). Recently, Ma et al. ([12]) showed that the SCL poplar gene PeSCL7 is induced by drought and high salt stress, and encodes a member of the stress-responsive GRAS/SCL transcription factors (TFs). They concluded that this gene is potentially useful for engineering drought and salt tolerance in trees.
The aim of this paper was to identify homologs of the P. euphratica gene GRAS/ SCL TF_GH611858 in the P. trichocarpa genome, and to estimate their nucleotide diversity in different salt-tolerant poplar clones, as well as any possible association with salt tolerance.
Materials and methods
Plant material, DNA extraction and molecular biology analyses
Four different poplar clones (three P. deltoides and one P. × euramericana - Tab. 1) were analyzed in this study. Frozen leaf material was ground under liquid nitrogen using the Retsch Bead Mill (Retsch, Duesseldorf, Germany). Genomic DNA extraction was performed using the Plant DNeasy® Minikit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. PCR was performed using annealing temperatures specific for each primer combinations. PCR-amplified products were purified, analysed by agarose gel electrophoresis and sequenced. The sequence of primer combinations used in the PCRs is shown in Tab. 2.
Tab. 1 - General information on the four salt-tolerant poplar clones investigated in this study.
| No | Clone collection No. | Species |
|---|---|---|
| 1 | B229 | P. deltoides |
| 2 | PE19/66 | P. deltoides |
| 3 | M1 | P. × euramericana |
| 4 | 182/81 | P. deltoides |
Tab. 2 - List of primer sequences and primer combinations used in this study.
| Homolog | Sequence number | Primer sequence | Primer combinations |
|---|---|---|---|
| Scaf_7 | F833 | 5’-CAC CAC CAT AAC CAA CAG CAA-3’ | - |
| R835 | 5’-GTG TAC ATC CCA AAA TCA GTA C-3’ | F833/R835 | |
| F836 | 5’-GTA CTG ATT TTG GGA TGT ACA C-3’ | F836/R838 | |
| R838 | 5’-AGT TGC CTC AAG AAT TGC TTG-3’ | F839/R840 | |
| F839 | 5’-CAA GCA ATT CTT GAG GCA ACT-3’ | - | |
| R840 | 5’-CAA ATC ACT GTA ATT GCA ATA CC-3’ | - | |
| Scaf_5 | F854 | 5’-ACC ACC CCC AGC AAC AAT TT-3’ | - |
| R855 | 5’-CGG TAA ATG CCG AAA-3’ | F854/R855 | |
| F856 | 5’-ATG CTG ATT TCG GCA TTT ACG G-3’ | F856/R857 | |
| R857 | 5’-GGT CGC TTC AAG AAT TGC TTG-3’ | F858/R859 | |
| F858 | 5’-CAA GCA ATT CTT GAA GCG ACC-3’ | - | |
| R859 | 5’-CAT AGT ACT GGA ATT GTA ATT CC-3’ | - |
All purified fragments were sequenced in both directions and analysed using the software package SFRMAO® (DNAStar Lasergene, Madison, WI, USA). DNA polymorphism patterns were recorded across all specimens within species, and the number of polymorphic sites was obtained. Nucleotide pairwise comparisons among clone sequences allowed to obtain three different polymorphism patterns: (i) those based on the number of fixed nucleotide substitution positions in all clones; (ii) those based on the occurrence of a polymorphic pattern position in one clone but not in others; and (iii) those polymorphic positions shared by all clones.
Candidate gene selection and primer design
The selected candidate gene, GRAS/SCL, encodes a TF with relevance to drought and salt tolerance, as demonstrated for poplar and other organisms ([1], [2], [12]). The complete sequence of the GRAS/SCL gene of P. euphratica (Accession No. GH611858) was compared to the P. trichocarpa genome by BLAST analysis (⇒ http://www.phytozome.net/poplar.php - [20]), and two putative homolog sequences were obtained. Exon/intron boundaries were defined through comparisons of genomic and cDNA sequences. Primers were designed according to the available sequences, and three primer combinations for each homolog were applied to cover all the exons sequences, with amplification products between 200 and 600 bp. In total, 48 fragments were sequenced in the four Populus clones investigated. The amplified sequences span only the exon region. The P. trichocarpa sequence was taken as reference in all sequence alignments.
Results
After BLAST search of the P. trichocarpa genome using the P. euphratica gene GRAS/ SCL TF_GH611858, two putative orthologous genes were found, Scaf_7 and Scaf_5, with identities of 100% and 94%, respectively. Scaf_7 is 1918 base pairs (bp) long, and the transcriptional start position is 1064 bp downstream with respect to that of P. euphratica GRAS/SCL TF_GH611858. Scaf_5 is 1780 bp long, and the transcriptional start position is 934 bp downstream with respect to that of P. euphratica GRAS/SCL TF_ GH611858. As already indicated by the sequence identities following the BLAST analysis, Scaf_7 was more similar to the P. euphratica gene than Scaf_5. Neither Scaf_7 nor Scaf_5 did reveal any indels or microsatellites. Primers were designed for regions shared by Scaf_5 and Scaf_7 (e.g., with identical sequences in both genes) to amplify fragments of GRAS/ SCL TF orthologs in the four poplar clones. However, the primers spanned regions where single nucleotide polymorphisms (SNPs) were present, at least in P. trichocarpa, thereby increasing the probability of distinguishing both orthologs in the four clones included in this study.
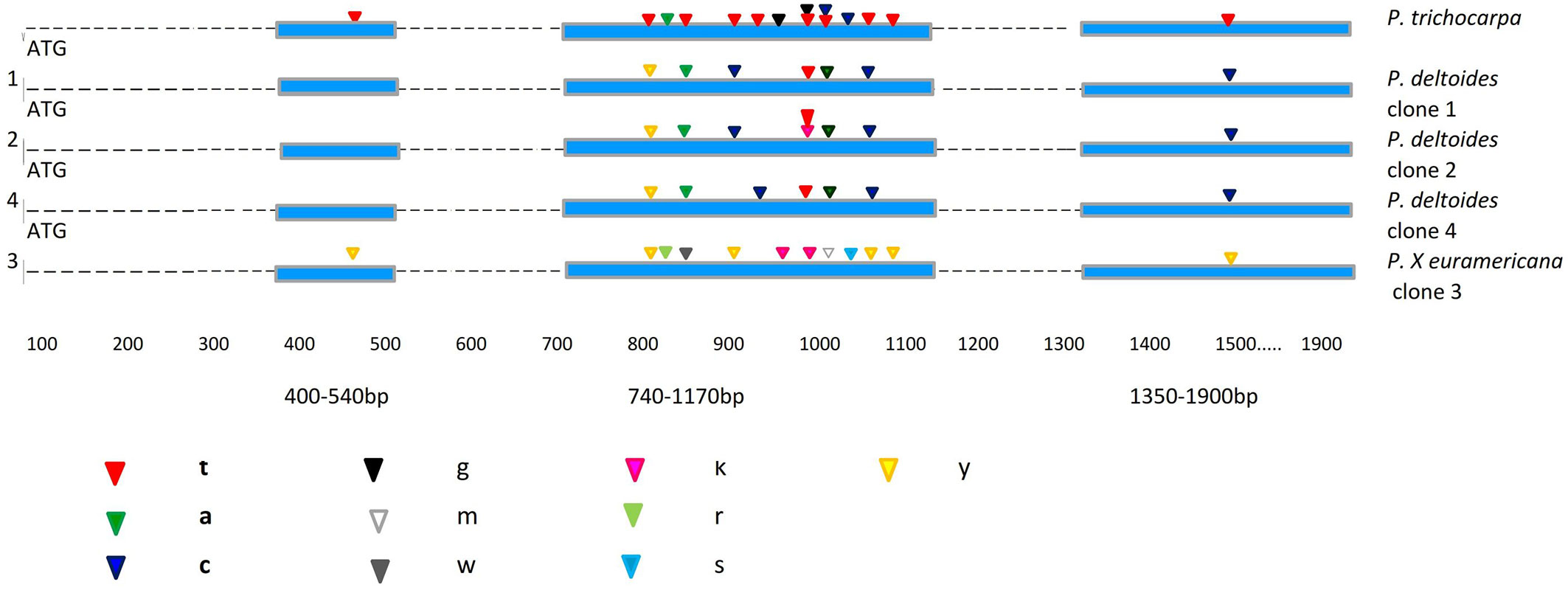
The alignment of all sequences of the gene fragments for both loci (Scaf_5 and Scaf_7) allowed to obtain a picture of the genetic diversity between the four poplar clones. Three fragments of different length were obtained for both loci. The sequences amplified from Scaf_7 were 400-540 bp, 740-1170 bp and 1350-1900 bp long, while the length of sequences from Scaf_5 were 150-390 bp, 520-860 bp and 1000-1530 bp. Those sequences covering exons regions in both loci were amplified and analysed (Tab. 3, Tab. 4, Fig. 1, Fig. 2). The number of nucleotide polymorphisms varied between each locus. For Scaf_7, 12 polymorphic positions were detected, while 19 positions were found for Scaf_5, each revealing a different degree of polymorphism. Out of the 12 polymorphic positions in Scaf_7, six were heterozygous, while the other six polymorphisms were non-specific. An A-T SNP was found at the 861 base position (starting from the codon of the P. trichocarpa Scaf_7 gene) in clones B229 and PE19/66 and 182/81, while a heterozygous polymorphism was found at the same base position in clone M1.
Tab. 3 - Nucleotide polymorphisms detected in the gene Scaf_7. The IUPAC code specification for DNA sequences is: (Y): C or T; (R) A or G; (W): A or T; (K): G or T; (S): G or C; (M): A or C. (1): Base position in the sequence beginning from the start codon of P. trichocarpa; (2): nucleotide observed at that position in the clone sequence; (3): nucleotide at the same position in the P. trichocarpa gene.
| Populus clone and primer pair used |
Base position (1) |
Observed Nucleotide (2) |
Reference Nucleotide (3) |
Type |
|---|---|---|---|---|
| 3-833 and 3-835 | 475 | Y | T | Heterozygous |
| 1-836 and 1-838 | 831 | Y | T | Heterozygous |
| 2-836 and 2-838 | 831 | Y | T | Heterozygous |
| 3-836 and 3-838 | 831 | Y | T | Heterozygous |
| 4-836 | 831 | Y | T | Heterozygous |
| 3-836 and 3-838 | 841 | R | A | Heterozygous |
| 3-836 and 3-838 | 861 | W | T | Heterozygous |
| 1-836 and 1-838 | 861 | A | T | SNP |
| 2-836 and 2-838 | 861 | A | T | SNP |
| 4-836 | 861 | A | T | SNP |
| 3-836 and 3-838 | 917 | Y | T | Heterozygous |
| 1-836 and 1-838 | 917 | C | T | Not specific |
| 2-836 and 2-838 | 917 | C | T | Not specific |
| 4-836 | 918 | C | T | Not specific |
| 3-836 and 3-838 | 986 | K | G | Heterozygous |
| 3-836 and 2-839 | 1005 | K | T | Heterozygous |
| 1-836 and 1-838 | 1005 | T | G | Not specific |
| 2-836 and 2-838 | 1005 | T | G | Not specific |
| 4-836 | 1005 | T | G | Not specific |
| 3-836 and 3-838 | 1006 | M | T | Heterozygous |
| 1-836 and 1-838 | 1006 | A | C | Not specific |
| 2-836 and 2-838 | 1006 | A | C | Not specific |
| 4-836 | 1006 | A | C | Not specific |
| 3-836 and 3-838 | 1012 | S | C | Heterozygous |
| 3-836 and 3-838 | 1017 | Y | T | Heterozygous |
| 1-836 and 1-838 | 1017 | C | T | Not specific |
| 2-836 and 2-838 | 1017 | C | T | Not specific |
| 4-836 | 1017 | C | T | Not specific |
| 3-836 and 3-838 | 1120 | Y | T | Heterozygous |
| 1-839 | 1533 | C | T | Not specific |
| 2-839 and 2-840 | 1533 | C | T | Not specific |
| 3-839 and 3-840 | 1533 | Y | T | Heterozygous |
| 4-839 and 4-840 | 1533 | C | T | Not specific |
Tab. 4 - Nucleotide polymorphisms detected in the gene Scaf_5. The IUPAC code specification for DNA sequences is: (Y): C or T; (R): A or G; (W): A or T; (K): G or T; (S): G or C; (M): A or C. (1): Base position in the sequence beginning from the start codon of P. trichocarpa; (2): nucleotide observed at that position in the clone sequence; (3): nucleotide at the same position in the P. trichocarpa gene.
| Clone sequences | Base position (1) |
Observed Nucleotide (2) |
Reference Nucleotide (3) |
Type |
|---|---|---|---|---|
| 3-854 and 3-855 | 184 | Y | T | Heterozygous |
| 1-854 and 1-855 | 287 | Y | C | Heterozygous |
| 2-854 and 2-855 | 287 | Y | C | Heterozygous |
| 3-856 | 600 | S | C | Heterozygous |
| 4-856 | 600 | S | C | Heterozygous |
| 3-856 | 796 | W | A | Heterozygous |
| 4-856 | 796 | W | A | Heterozygous |
| 3-856 | 797 | M | C | Heterozygous |
| 4-856 | 797 | M | C | Heterozygous |
| 3-856 | 814 | K | G | Heterozygous |
| 1-858 and 1-859 | 1003 | C | T | Not specific |
| 2-858 | 1003 | C | T | Not specific |
| 3-858 | 1003 | C | T | Not specific |
| 4-858 | 1003 | C | T | Not specific |
| 1-858 and 1-859 | 1009 | A | C | Not specific |
| 2-858 | 1009 | A | C | Not specific |
| 3-858 | 1009 | A | C | Not specific |
| 4-858 | 1009 | A | C | Not specific |
| 2-858 | 1049 | S | G | Heterozygous |
| 3-858 | 1049 | S | G | Heterozygous |
| 4-858 | 1049 | S | G | Heterozygous |
| 2-858 | 1050 | Y | C | Heterozygous |
| 3-858 | 1050 | Y | C | Heterozygous |
| 4-858 | 1050 | Y | C | Heterozygous |
| 1-858 and 1-859 | 1054 | A | G | Not specific |
| 2-858 | 1054 | A | G | Not specific |
| 3-858 | 1054 | A | G | Not specific |
| 4-858 | 1054 | A | G | Not specific |
| 1-858 and 1-859 | 1165 | Y | T | Heterozygous |
| 2-858 | 1165 | Y | T | Heterozygous |
| 3-858 | 1165 | Y | T | Heterozygous |
| 1-858 and 1-859 | 1267 | Y | T | Heterozygous |
| 2-858 | 1267 | Y | T | Heterozygous |
| 1-858 and 1-859 | 1286 | C | T | Not specific |
| 2-858 | 1286 | C | T | Not specific |
| 3-858 | 1286 | C | T | Not specific |
| 4-858 | 1286 | C | T | Not specific |
| 3-858 | 1303 | K | T | Heterozygous |
| 1-858 and 1-859 | 1327 | Y | C | Heterozygous |
| 2-858 | 1327 | Y | C | Heterozygous |
| 4-858 | 1327 | Y | C | Heterozygous |
| 1-858 and 1-859 | 1333 | R | A | Heterozygous |
| 2-858 | 1333 | R | A | Heterozygous |
| 3-858 | 1390 | Y | T | Heterozygous |
| 3-858 | 1486 | M | A | Heterozygous |
Fig. 1 - Localization of fixed nucleotide differences for the Scaf_7 locus between the four analysed poplar clones and P. trichocarpa as a reference sequence. Exons and introns are indicated by boxes and lines, respectively. The IUPAC code specification for DNA sequences is: (Y): C or T; (R): A or G; (W): A or T; (K): G or T; (S): G or C; (M): A or C.
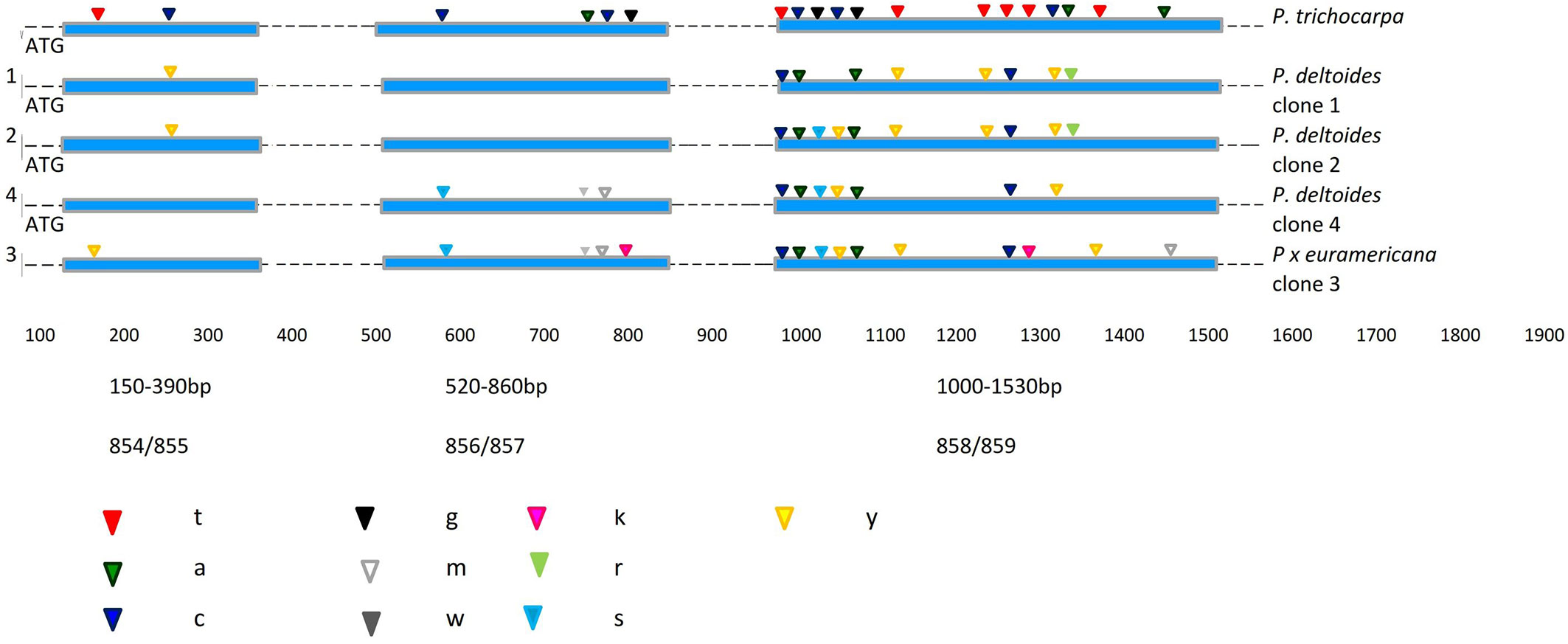
Fig. 2 - Localization of fixed nucleotide differences for the Scaf_5 locus between the four analysed poplar clones and P. trichocarpa as a reference sequence. Exons and introns are indicated by boxes and lines respectively. The IUPAC code specification for DNA sequences is: (Y): C or T; (R): A or G; (W): A or T; (K): G or T; (S): G or C; (M): A or C.
At the Scaf_5 locus, a different polymorphic pattern was found, as all the clones exhibited heterozygous positions and no SNPs were found. Out of 19 positions, 15 were heterozygous and four were non-specific polymorphisms.
Homologous regions in the four different Populus clones were sequenced and consensus sequences were designated for each clone. Out of 33 polymorphic positions of Scaf_7 locus, 15 were heterozygous (5%) and the majority (3.3%) belonged to the hybrid M1 clone. Most of the mutations were synonymous or silent. In sequences from P. deltoides clones, non-synonymous SNPs could be identified with a change of an amino acid leading to a possible effect on the protein structure. In the first fragment of Scaf_7, which is 400-540-bp long, only one heterozygous SNP was detected at position 475 in the M1 hybrid clone. This position may be used in the differentiation of Populus clones and in the characterization of the M1 hybrid. The second expressed sequence analysed had the majority of SNPs and heterozygous positions (Fig. 1). In this fragment (740-1170 bp) all three P. deltoides clones revealed a SNP at bp 861, while only the hybrid P. × euramericana was heterozygous. This fragment is highly polymorphic, with mostly heterozygous positions found in the hybrid clone. The third fragment (1350-1900 bp) has only one position (at bp 1533) shared by all the clones, as well as the hybrid. All three species have SNPs in that position, but only the hybrid is heterozygous. This finding, i.e., hybrids having more heterozygous SNPs than single species, is not surprising and is supported by several other studies ([7], [4]).
In the Scaf_5 locus, more than half of the 45 polymorphic positions were heterozygous (55.5%). Of these, 10 (22.2%) belonged to the hybrid M1 clone, assuming that less than half are truly heterozygous of all the polymorphic positions. Most mutations were found to be synonymous or silent, thereby the amino acid sequence of the relative proteins does not change. In the first fragment of Scaf_5 (150-390 bp) only one position (at bp 184) had a SNP showing a Y→T transition, which was found in the M1 hybrid clone, while a heterozygous Y→C substitution at bp 287 was shared by B229 and PE19/66 clones. Interestingly, the second expressed sequence analysed had the majority of heterozygous positions (at bp 600, 796, 797 and 814). The other two P. deltoides clones (B229 and PE19/66) did not show any polymorphisms.
The highest number of mutations per site was detected in the third fragment (1000-1530 bp) analysed. All four clones shared mutations at bp 1003, 1009, 1054 and 1286. In the first and second sequences of this locus, mainly heterozygous positions were found (Fig. 2). Therefore, the four poplar clones could be differentiated by “one-nucleotide SNPs”.
Discussion
The present study provides the first insights into nucleotide polymorphisms of the GRAS/SCL TF gene among Populus clones and species. The four poplar clones selected are of high economic importance to Serbia, as they are frequently used in plantations. All four clones are characterized by varying degrees of salt tolerance, with clone M1 being the most tolerant. Both Scaf_5 and Scaf_7 revealed high polymorphisms in the consensus sequences of different conserved parts of the exons. This high nucleotide diversity in homologous sequences of the exons of the GRAS/SCL TF could have consequences for abiotic stress-resistance of different poplar clones. Indeed, Galovic & Szabados (unpublished data) used quantitative PCR to measure gene expression, finding a significant induction of Scaf_7 during salt stress, while Scaf_5 was expressed at a lower level during salt stress.
Our results indicate a high genetic similarity of the investigated clones to P. trichocarpa. As the latter species belongs to the Tacamahaca section, a close genetic relationship between the two sections (Aigeiros and Tacamahaca) is likely, as supported by similar findings in the literature. For example, Cervera et al. ([3]) suggested close relationships between the Leucoides, Tacamahaca and Aigeiros sections according to phylogenetic analyses. Our results are also consistent with the findings of Fladung & Buschbom ([7]) and Dvornyk et al. ([6]), and further support a genetic similarity, to some extent, between the different investigated clones and P. trichocarpa. Moreover, these findings confirm the results reported by Cervera et al. ([3]), who found a close relationship between P. deltoides (section Aigeiros) and species of the Tacamahaca section. Furthermore, Galovic et al. ([8]), Galovic & Orlovic ([9]) and Orlovic et al. ([14]) also supported the findings of genetic similarities between two different species of the Aigeiros and Tacamahaca sections using AFLPs and SSR nuclear markers.
As a next step, the selected candidate GRAS/SCL TF genes and the identified SNP markers should be tested as diagnostic tools in a larger collection of salt-sensitive and salt-tolerant poplar lines, and their ability to increase salt tolerance should be verified using genetic engineering tools.
Conclusions
The GRAS/SCL gene is a promising candidate for improving salt stress tolerance in the poplar clones under investigation. Successful amplification of the GRAS/SCL TF gene and the resulting different SNP patterns detected allowed to detect differences in the genetic background of clones belonging to P. deltoides. The different fingerprinting patterns observed indicate that each clone could have a different potential for stress tolerance.
Acknowledgements
VG conceived the study and wrote the manuscript. SO supported VG with suggestions on the draft of the manuscript. MF supervised the laboratory work and corrected and edited the manuscript.
The authors thank the EU-COST Action FP0905 for its contribution to this work by financing the short-term scientific mission of Vladislava Galovic.
We would like to thank Katrin Groppe (Thünen-Institute of Forest Genetics, Grosshansdorf, Germany) and Sreten Vasic (Institute of Lowland Forestry and Environment, Novi Sad, Serbia) for excellent technical assistance. We also thank Karin Pfennig for her assistance with the sequence analysis. Thanks to Hasman Stefan for final text formatting and to Milica Gavrilovic and Dina Führmann for English language editing.
References
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Sasa Orlovic
University of Novi Sad, Institute of Lowland, Forestry and Environment, Antona Cehova 13, 21000 Novi Sad (Serbia)
Thuenen Institute of Forest Genetics, Sieker Landstr. 2, D-22927 Grosshansdorf (Germany)
Corresponding author
Paper Info
Citation
Galovic V, Orlovic S, Fladung M (2015). Characterization of two poplar homologs of the GRAS/SCL gene, which encodes a transcription factor putatively associated with salt tolerance. iForest 8: 780-785. - doi: 10.3832/ifor1330-008
Academic Editor
Marco Borghetti
Paper history
Received: Apr 29, 2014
Accepted: Feb 12, 2015
First online: May 19, 2015
Publication Date: Dec 01, 2015
Publication Time: 3.20 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2015
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 53148
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 44368
Abstract Page Views: 3531
PDF Downloads: 3819
Citation/Reference Downloads: 21
XML Downloads: 1409
Web Metrics
Days since publication: 3939
Overall contacts: 53148
Avg. contacts per week: 94.45
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2015): 5
Average cites per year: 0.45
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Adaptive response of Pinus monticola driven by positive selection upon resistance gene analogs (RGAs) of the TIR-NBS-LRR subfamily
vol. 10, pp. 237-241 (online: 02 February 2017)
Research Articles
Early flowering and genetic containment studies in transgenic poplar
vol. 5, pp. 138-146 (online: 13 June 2012)
Technical Advances
Gene flow in poplar - experiments, analysis and modeling to prevent transgene outcrossing
vol. 5, pp. 147-152 (online: 13 June 2012)
Review Papers
Fifteen years of forest tree biosafety research in Germany
vol. 5, pp. 126-130 (online: 13 June 2012)
Research Articles
Comparison of genetic parameters between optimal and marginal populations of oriental sweet gum on adaptive traits
vol. 11, pp. 510-516 (online: 18 July 2018)
Research Articles
Genetic variation and heritability estimates of Ulmus minor and Ulmus pumila hybrids for budburst, growth and tolerance to Ophiostoma novo-ulmi
vol. 8, pp. 422-430 (online: 15 December 2014)
Commentaries & Perspectives
The genetic consequences of habitat fragmentation: the case of forests
vol. 2, pp. 75-76 (online: 10 June 2009)
Research Articles
Seedling emergence capacity and morphological traits are under strong genetic control in the resin tree Pinus oocarpa
vol. 17, pp. 245-251 (online: 16 August 2024)
Research Articles
Genetic diversity of core vs. peripheral Norway spruce native populations at a local scale in Slovenia
vol. 11, pp. 104-110 (online: 31 January 2018)
Review Papers
Indicators of drought effects in Pinus sylvestris: genetic analyses to corroborate the results of empirical methods
vol. 3, pp. 89-91 (online: 15 July 2010)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword