Endophytes in changing environments - do we need new concepts in forest management?
iForest - Biogeosciences and Forestry, Volume 6, Issue 3, Pages 109-112 (2013)
doi: https://doi.org/10.3832/ifor0932-006
Published: Mar 05, 2013 - Copyright © 2013 SISEF
Short Communications
Abstract
The occurrence of endophytic fungi, hosted by living tissues of forest trees seems to be a common phenomenon. Numerous studies show that these colonists are mostly symptomless or even live in a symbiotic relationship to the host plant. Our investigations on Douglas-fir and Rhabdocline needlecast show that Rhabdocline pseudotsugae (Sydow), which has been described exclusively as an obligatory needle pathogen up to now, is able to persist symptomless in different types of plant tissues and therefore an endophytic lifestyle has to be assumed. Whether this lifestyle is part of the infection strategy of the fungus is still unclear. However, examples of other wood associated fungi lead us to the hypothesis that environmental such as climate conditions are able to trigger the phenomenon of changing from a mutualist to a virulent parasite.
Keywords
Douglas-fir, Endophytes, Rhabdocline Needlecast, Climate Change
Introduction
It has been well described by Stone ([32]), Carroll & Carroll ([8]), McCutcheon et al. ([22]) and Sieber ([30]) that for Douglas-fir Rhabdocline parkeri (order Heliotiales, [19]) is the dominant needle endophyte with strict host specificity. This fungus infects single epidermal cells, which die due to the infection. The microorganism persists in the needle as a multicellular thallus without any further growth. In contrast to Rhabdocline parkeri, the closely related species Rhabdocline pseudotsugae, Rhabdocline oblonga, Rhabdocline obovata, Rhabdocline epiphylla and Rhabdocline weirii are fungi which induce Rhabdocline needlecast, one of the most economically important diseases of Douglas-fir ([10]), because it can cause substantial losses in Christmas tree plantations ([11]). In 1930 the pathogen of Rhabdocline needlecast was first described for Germany by v. Geyr ([16]). A detailed description of disease symptoms and life cycle can be found at Van Vloten ([37]), Stephan ([36]) and Butin ([5]). Rhabdocline pseudotsugae which is classified into the class of Ascomycota is a highly specialized parasite whose one-year life cycle is closely linked to the seasonal growth cycle of Douglas-fir needles ([37]). Small yellow-green spots are the first indication of the disease. They occur in autumn on this year’s needles. During winter, the spots become necrotic and brown. The characteristic orange-yellow to rust-colored fruiting bodies mature in May/June on the needle surface. High air humidity promotes the process of ascospore release and infection of the young sprouting needles. Finally the needles become brown and die ([36], [5]). A repeated infection causes a significant decrease in growth or even the death of the infected trees ([36]). Up to now it seems to be clear that ascospores infect the sprouting needles and afterward they directly penetrate through the needle epidermis into the host cells ([37], [21], [35], [36], [5]). The current state of knowledge on the morphology and life-cycle of the fungus is mainly based on macroscopic and microscopic investigations of characteristic fungal fruiting bodies, spores and needles showing symptoms of infection. The present study is the first investigation which verifies Rhabdocline pseudotsugae in different type of plant material other than needles. There are no previous investigations which show that the fungus shows an endophytic lifestyle. Our report is focused on the transmittance of the pathogen beside the regular distribution via ascospores, placed in the context of the idea that Rhabdocline pseudotsugae behaves in part as a latent pathogen and that this way of life can become problematic under certain host-related stress conditions.
Material and methods
Material and methods are following Morgenstern & Krabel ([24]). In short: buds, needles, cambial meristem as well as embryos were sampled from a 20-year-old controlled cross-breeding population. Around 94% of the trees showed clear symptoms of infection by some type of pathogenic needle cast. Additionally apparently healthy looking trees from the Forest Botanical Garden Tharandt were used as a negative control (non-infected). Needles with fruiting bodies of Rhabdocline pseudotsugae were collected in May 2011 serving as positive control. Embryos were dissected from seeds which originated from a controlled cross-breeding of interior and coastal varieties of Douglas-fir.
Genomic DNA was isolated from all types of plant tissues described above. For isolation of genomic plant DNA an extraction protocol, modified after Doyle & Doyle ([12]) was used ([24]). For extraction of fungal DNA the DNeasy Plant Mini Kit (Qiagen GmbH, Hilden, Germany) was used. Based on studies by Catal ([9]), a nested PCR protocol was established for the detection of Rhabdocline pseudotsugae. Primers ITS1F, ITS4, RPP1 and RPP4 were used according to Catal ([9]). PCR conditions are described in detail by Morgenstern & Krabel ([24]).
For the examination of embryos the Phire Plant Direct PCR Kit (Finnzymes, Part of Thermo Fisher Scientific, Espoo, Finland) was used. PCR was carried out with primer pair ITS1F/ITS4 according to the instructions of the manufacturer in a total volume of 20 µl.
Fragment analysis of PCR products was carried out with the ALFexpress II sequencer (Amersham Pharmacia Biotech Europe GmbH, Freiburg, Germany) in a polyacrylamide gel (ReproGel High Resolution, GE Healthcare Europe GmbH, Munich, Germany).
Results and discussion
General aspects of an endophytic lifestyle
Hawksworth et al. ([18]) suggested that before using the term endophyte one should clearly define it. We will follow the definition of Saikkonen et al. ([28], [29]) which defines endophythes as microorganisms which live the entire or at least parts of their life cycle asymptomatically inside plant tissues or inside plant cells. This includes latent pathogens as well as endophytic living bacterias ([38]) and endophytic fungi. Endophytic fungi have been found in all woody plants which have been investigated for endophytes ([28]). A comprehensive overview of the actual knowledge about this subject related to forest trees is given by A. M. Pirttilä and A. C. Frank in the book “Endophytes of forest trees” which has been published in 2011. Other excellent reviews focusing on endophytic fungi in woody plants are those e.g., from Carrol ([6], [7]), Petrini ([26]), Schulz & Boyle ([31]), Stone & Petrini ([33]) and Stone et al. ([34]).
Although the endophyte-plant interactions are products of evolutionary development ([30]), the lifestyle (mutualism, commensalism, parasitism) of a microorganism, which is living inside a plant is not always obvious and the question may rise for what is such a relationship good for? A more or less mutualistic relationship occurs in the case the endophyte provides the host plant with some ecological advantages. On the other hand, some apathogenic endophytes may become virulent ([4], [23]) under certain environmental conditions or by mutation ([13]). In general, it is suspected that the endophyte-plant interaction is as prevalent as the mycorrhiza-plant interaction, with advantages for both partners ([7], [30]). By the production of specific metabolites and biologically active chemicals which strengthen the plant, it reduces the level of herbivore damage, e.g., gall insects. A successful interaction can also be characterized by an increased resistance of the host against pathogens by “controlling” these pathogens (e.g., white pine blister rust in Pinus monticola - [15]). For the microorganisms the advantage may be the persistence in a host over a longer period and the dispersal with the next host generation up to the moment the host’s living conditions change ([28]). Such changes of environmental conditions mostly go in parallel with changes of the milieu inside the plant tissue (when the plant is stressed, e.g., caused by wounding or decreased vitality of the host plant) and causes an adaptation reaction of the endophytic microorganism ([28]). Examples are xylem fungi such as Fomes fomentarius ([3]) and Nectria coccinea ([30]), which are known to adopt a non-pathogenic behavior under limited oxygen ratio and/or nutrient, while they may switch to parasitism when the above conditions change (e.g., by wounding - [25]). In this case the host is latent infected by “non-active pathogens” until internal cell conditions change and the endophyte becomes virulent. The latent infection hypothesis is proposed by Boddy & Rayner ([2]). This hypothesis is closely connected to the assumption that a certain density of tissue colonization is required for changing into a pathogenic behavior. Sieber ([30]) reports that as soon as colonization density of the endophyte reaches a certain threshold the plant organ (e.g., needles) may die. This threshold can be reached very soon in case the living conditions of the host become unfavorable, e.g., by a lack of light in dense stands.
As stated by Sieber ([30]), depending on the selection pressure, it may also be the case that a pathogenic endophyte may change into non-pathogenic and back again.
Hints for an endophytic lifestyle of Rhabdocline pseudotsugae and its consequences
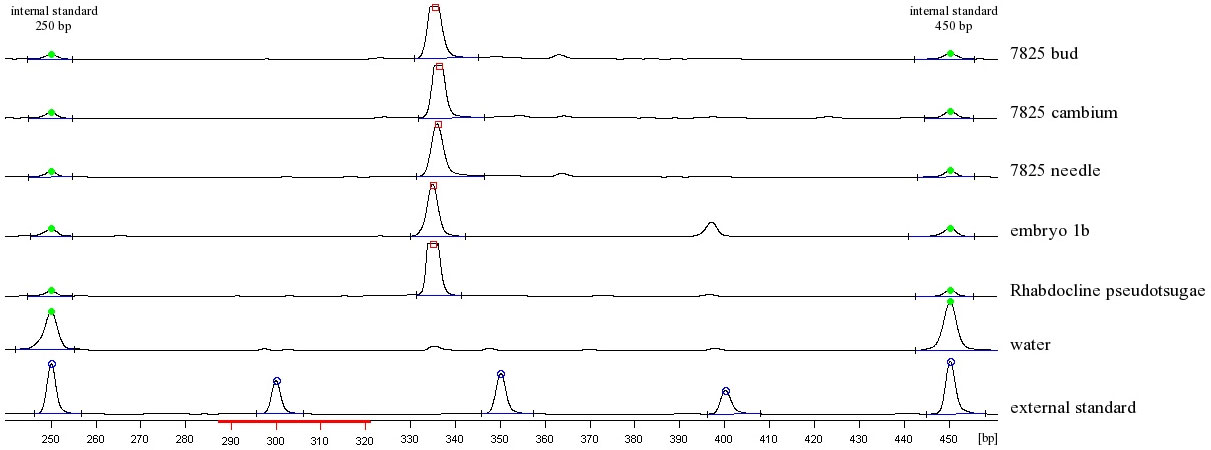
Our investigations on Rhabdocline needlecast clearly confirm that the Ascomycete Rhabdocline pseudotsugae known as a pathogen can be identified inside infected Douglas-fir needle material as well as in different types of plant tissues like buds, cambium and embryos (Fig. 1). This result is surprising insofar as the fungus has been classified exclusively as a needle parasite up to now. As already Petrini et al. ([27]) stated that far too little is known about endophytes of woody plants to assume that they are all non-systemic, our findings are not suited to explain whether the fungal material is distributed systemically via needles to cambial tissue, buds and seeds’ embryos, or whether these tissues are locally infected but do not show any symptoms of infection. To our opinion a systemic transmittance seems to be more probable than a non-systemic one, because infection and symptoms of a disease of other tissues than needle material by Rhabdocline pseudotsugae has never been described before.
Fig. 1 - Fragment analysis at the ALFexpress II sequencer. Polyacrylamide gel shows Rhabdocline pseudotsugae specific bands (335bp) in infected plant tissues as well as in the positive control (fruiting body of Rhabdocline pseudotsugae). The negative control is a water sample.
Nevertheless a vertically and maternally distribution of fungal material via seeds can imply an additional source of infection beside the ascospores which seems to infect only needles. Latent infections of plant material by pathogens have been recognized for a long time ([14]) and they may become virulent under environmental stress ([4]). For Douglas-fir such stress situations are realized in dense stands with reduced air circulation and high humidity. This is the case even in young stands around 10 years of age or in older stands when crown contact starts to be intense. But the question remains open whether the fungus is really able to persist symptomless in a young plant for more than one decade. The ecological significance of such a strategy is obvious. The persistence in Douglas-fir seeds and later on in the young plant helps the pathogen to be transmitted over larger distances and to colonize new areas independently from its sexual reproduction.
Host specificity of Rhabdocline pseudotsugae
It has been described that Rhabdocline pseudotsugae prefers to infect Douglas-firs belonging to the intermountain (glauca or “blue”) variety and that genotypes belonging to the coastal (viridis or “green”) variety are less susceptible ([11]). But Chastagner ([11]) also reports on the one hand about observations that Rhabdocline needlecast can cause extensive damage to susceptible genotypes of the viridis variety, and on the other hand that there is considerable genetic variation in susceptibility to the disease of glauca provenances. Our results show that, among a total of 120 seeds from 6 German viridis provenances, 10 to 42% genotypes per provenance show infection.
Based on the above results and those reported in Tab. 1, the equivalences viridis = resistant and glauca = susceptible have to be reconsidered.
Tab. 1 - Screening of different type of plant material (varieties and tissue).
| Material (number of genotypes x sampling location) |
Variety | Number of samples infected with Rhabdocline pseudotsugae |
|---|---|---|
| 3 x needles, cambium, buds | viridis x glauca | 1 x needles, cambium positive; buds negative 1 x needles and cambium positive, buds negative 1 x needles, cambium, buds positive |
| 11 x needles | viridis | 11 x negative |
| 4 x needles | glauca | 4 x negative |
| 8 x embryo | glauca x viridis | 3 x positive, 5 x negative |
| 15 x embryo | viridis x glauca | 5 x positive, 10 x negative |
Previous studies reported that natural grass populations are usually a mosaic of uninfected and infected plants ([28]), which implies that the host genotype is the crucial factor for susceptibility to the pathogen. This might be also true for Rhabdocline pseudotsugae and has to be tested in further investigations.
Conclusions
Up to now most approaches for pathogen characterization were based on visual detection in tissues of its typical structures (e.g., ascospores) using a microscope. Molecular methods based on the detection of fungi DNA in host tissues are a very sensitive technique available since a few years.
Our findings raise the question whether previous studies failed to detect the fungus in other plant tissues (independently from the host variety) due to a lack of methodological sensitivity, or whether our findings are due to a type of the fungus’ endophytic lifestyle, and therefore a plant-parasite interaction different from what is reported in previous investigations. In the latter case, we have to ask whether Rhabdocline pseudotsugae is only one example of a larger group of organisms causing “novel diseases” and emerging due to climatic changes. The role of environmental factors (like mild winter temperatures, drought periods alternating with high precipitation during the vegetation period etc.) has not been investigated up to now in this context. Several authors have tried to explain the infection pathway and history of the so called “novel” serious diseases, like ash dieback ([20]), bleeding cancer in Horse chestnut (Aesculus hippocastanum - [17]) or Cryptostroma corticale the sooty bark disease of sycamore (Acer pseudoplatanus - [1]). They hypothesized that climatic changes might induce stress reactions in the host plants which may favor the spread of aggressive pathogens. On the other hand, Chalara fraxinea, the fungus which seems to be responsible for the ash dieback, is known as a saprophyte and not as an aggressive parasite. Although the disease is spreading very fast all over Europe, the infection path is still unclear ([39]).
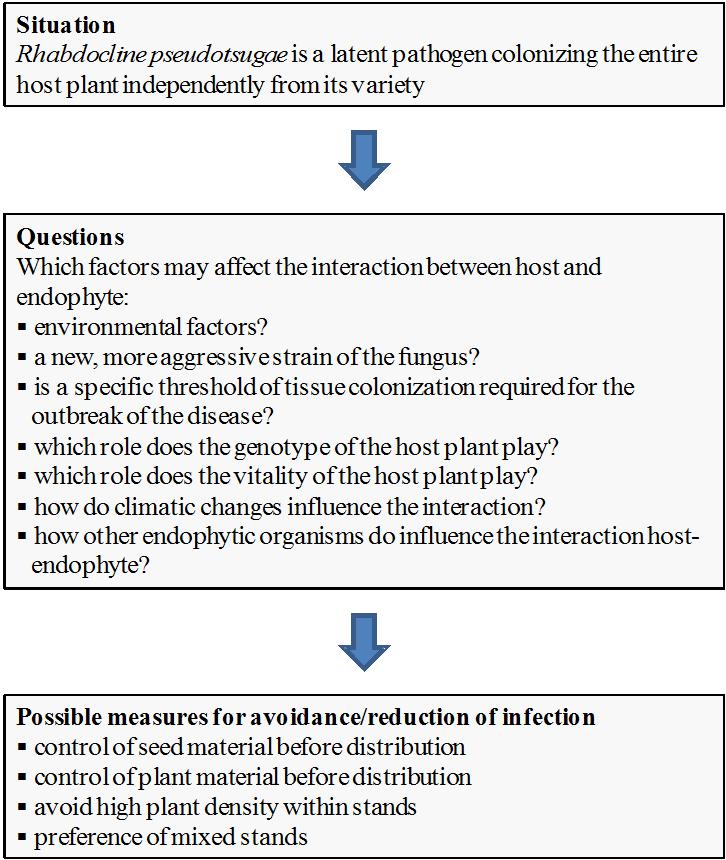
Nevertheless, the results reported in this study raise new concerns on the possible consequences of such a type of plant infection, and provide some suggestions to avoid infection of Douglas-fir tissues with Rhabdocline pseudotsugae (see Fig. 2).
Fig. 2 - Current situation, questions and possible managing measures for avoidance/reduction of the infection by Rhabdocline pseudotsugae in Douglas-fir.
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
K Morgenstern
Molecular Physiology of Woody Plants Group, Dresden University of Technology, Pienner Str. 7, D-01737 Tharandt (Germany)
Institute of Silviculture and Forest Protection, Dresden University of Technology, Pienner Str. 8, D-01737 Tharandt (Germany)
Corresponding author
Paper Info
Citation
Krabel D, Morgenstern K, Herzog S (2013). Endophytes in changing environments - do we need new concepts in forest management?. iForest 6: 109-112. - doi: 10.3832/ifor0932-006
Academic Editor
Marco Borghetti
Paper history
Received: Dec 17, 2012
Accepted: Feb 05, 2013
First online: Mar 05, 2013
Publication Date: Jun 01, 2013
Publication Time: 0.93 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2013
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 57848
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 47812
Abstract Page Views: 3392
PDF Downloads: 4995
Citation/Reference Downloads: 21
XML Downloads: 1628
Web Metrics
Days since publication: 4723
Overall contacts: 57848
Avg. contacts per week: 85.74
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2013): 9
Average cites per year: 0.69
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
The complexity of mycobiota associated with chestnut galls induced by Dryocosmus kuriphilus in Galicia (Northwestern Spain)
vol. 17, pp. 378-385 (online: 14 December 2024)
Research Articles
Seeing, believing, acting: climate change attitudes and adaptation of Hungarian forest managers
vol. 15, pp. 509-518 (online: 14 December 2022)
Research Articles
Potential impacts of regional climate change on site productivity of Larix olgensis plantations in northeast China
vol. 8, pp. 642-651 (online: 02 March 2015)
Research Articles
Measured and simulated tree and stand water use of Douglas-fir along a climatic gradient across Germany
vol. 18, pp. 309-318 (online: 30 October 2025)
Research Articles
Assessment of presence and distribution of Armillaria and Heterobasidion root rot fungi in the forest of Vallombrosa (Apennines Mountains, Italy) after severe windstorm damage
vol. 12, pp. 118-124 (online: 11 February 2019)
Research Articles
Predicting the effect of climate change on tree species abundance and distribution at a regional scale
vol. 1, pp. 132-139 (online: 27 August 2008)
Research Articles
Effects of planting density on the distribution of biomass in a douglas-fir plantation in southern Italy
vol. 8, pp. 368-376 (online: 09 September 2014)
Research Articles
Impact of climate change on radial growth of Siberian spruce and Scots pine in North-western Russia
vol. 1, pp. 13-21 (online: 28 February 2008)
Review Papers
Impacts of climate change on the establishment, distribution, growth and mortality of Swiss stone pine (Pinus cembra L.)
vol. 3, pp. 82-85 (online: 15 July 2010)
Research Articles
An assessment of climate change impacts on the tropical forests of Central America using the Holdridge Life Zone (HLZ) land classification system
vol. 6, pp. 183-189 (online: 08 May 2013)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword