Light availability influences the invasion of Teline monspessulana (L.) K. Koch in a temperate fragmented forest in Central Chile
iForest - Biogeosciences and Forestry, Volume 15, Issue 5, Pages 411-416 (2022)
doi: https://doi.org/10.3832/ifor4026-015
Published: Oct 19, 2022 - Copyright © 2022 SISEF
Research Articles
Abstract
The Maulino forest is a temperate ecosystem of the Mediterranean zone of Chile classified as one of the 34 biodiversity hot-spots of the world; however, there is still limited information about the ecological factors that make this native forest prone to be invaded. We assess to what extent forest attributes such as light availability and native species diversity control the invasion process of Teline monspessulana (L.) K. Koch, an aggressive weed, into the Maulino forest, an endemic forest ecosystem of Central Chile. We examined whether the seedling density of this exotic plant is related to forest attributes such as cover, incoming photosynthetically active radiation, litter depth, and native species density and richness. We found that a decrease of light availability reduces T. monspessulana invasion. No relationships were observed between native species diversity and the abundance of T. monspessulana plants. Increasing the forest cover will recover forest structure but at the same time, it will prevent the invasion of T. monspessulana and other exotic plants with similar regeneration niche requirements.
Keywords
Invasibility, Invasiveness, French Broom, Genista monspessulana, Forest Cover, Native Species Diversity
Introduction
Which features makes an ecosystem to be invaded? This question has been central in studies of invasion ecology and has oriented most of research to elucidate why some natural ecosystems are more or less prone to be invaded ([34]). The concept of invasibility is defined as “the susceptibility of biological communities to colonization and dominance by introduced organisms” ([29], [4]). It basically refers to some intrinsic attributes of natural communities which defines the likelihood to be invaded ([21]). A set of diverse hypotheses has been proposed to explain invasibility ([12]). Firstly, the diversity-invasibility hypothesis ([11]) proposes that alien species will be less successful in more diverse native communities, because resources become limiting for the new colonizers, which in turn reduce their fitness ([22], [12]). Secondly, the Darwin naturalization hypothesis proposed that alien species will be more successful in a native community if they are phylogenetically distant from residents ([8]), in that phylogenetic distance reflects niche differentiation which in turn implicate a reduction of interspecific competition ([48]). Thirdly, the disturbance-invasibility hypothesis, states that disturbance regime enhances the likelihood of natural communities to be invaded because disturbance provide new conditions and resources for alien species that are unsuitable or cannot be exploited by resident species ([9]).
In general, undisturbed forests are considered resistant to plant invasion; however, they increase their invasibility when disturbances occur ([43]); for instance, deforestation and fragmentation induce new abiotic condition such as decreases of soil humidity and increases of light availability ([25], [26]), which are favorable for colonizer, shade-intolerant exotic plants ([27], [47]). On the other hand, the edge effects (an indirect consequence of deforestation and fragmentation) significantly increase forest invasibility as most alien plants which occur in the semi-natural matrix colonize edge microhabitats and eventually become successful toward the interior of forests ([42], [35]).
The knowledge of forest invasibility, besides to gain an understanding about their potential to persist faced to species invasion, can be converted as a management tool to prevent or control exotic species invasion in native forests ([18]). The Maulino forest is a temperate ecosystem of the Mediterranean zone in central Chile ([41]). Due to its high endemism and intense anthropogenic disturbances, it is classified as one of the 34 biodiversity hot-spots of the world ([33], [37]). This forest harbors ecosystems and several plant species listed in the IUCN Red List as Endangered (e.g., Gomortega keule [Molina] Baill., Pitavia punctata [Ruiz & Pav.] Molina, Nothofagus alessandri [Espinosa]), Vulnerable (e.g., Nothofagus glauca [Phil.] Krasser), and of Least Concern (e.g., Nothofagus pumilio [Poepp. & Endl.] Krasser - [5]). During the last centuries, this forest has suffered dramatic deforestation and fragmentation, which has resulted in a significant reduction of its surface ([6]), and concomitantly, a reduction of species diversity ([10]). In the period between 1975 and 2000 the Maulino forest experienced a reduction in natural forest area of about 67% in the study area ([10]). Lastly, mega-fires which occurred during the year 2017 have contributed with an additional reduction of forest surface, consuming nearly 184.000 ha of native vegetation in the area of the Maulino forest ([46]). Currently, Pinus radiata D. Don, Acacia dealbata Link. and Teline monspessulana (L.) K. Koch are listed as the most important woody alien plants that are colonizing this native forest ([2], [20]).
T. monspessulana (French broom) is the most widely distributed invasive species of the Fabaceae family in central Chile ([16]). The species has successfully invaded Australia, New Zealand, and USA ([1]). In Chile, it has a wide distribution reaching a latitudinal gradient close to 1000 km, concentrating on territories with Mediterranean and temperate climate ([31]). The species is frequently found within clearings or at the edges of P. radiata and Eucalyptus globulus Labill plantations, as well as in open degraded areas or along roads, thus forming extensive and dense linear shrublands ([16], [38], [19]). T. monspessulana is considered an aggressive weed ([31]) which can compete with native species and promote the conditions to increase wildfire occurrence ([38]). The species has high reproduction capacity both by vegetative regeneration and by the formation of an abundant and persistent seed bank, which persists in the soil for long periods of time, increasing the possibility to establish after fires occurrences ([16], [15]).
The rapid spread of T. monspessulana across landscapes has converted this species in a threat for the integrity of the Maulino forest, which has increased its invasibility due to the intense degradation by forestry, wildfires and the tree extraction for firewood and charcoal production. The reduction of canopy cover in turn, has increased light availability as well as a decrease in soil and air humidity ([14], [32]). The increase in light availability (measured as the photosynthetically active radiation, PAR) promotes the regeneration of shade-intolerant exotic species, which outcompete native shade-tolerant species. In this study we aimed to test the disturbance-invasibility hypothesis, examining whether the reduction of canopy cover, and the consequent increase of light availability, may affect T. monspessulana regeneration. Given that this species is a colonizer, shade-intolerant alien plant, we predict a negative relationship between T. monspessulana seedling density and canopy cover. We also aimed to test the diversity-invasibility hypothesis, examining the relationship between native species diversity and T. monspessulana regeneration. If there is some biotic resistance, we expect a negative relationship between T. monspessulana seedling density and native species diversity.
Materials and methods
Study site
The study area was located within native remnants of the Maulino forest nearby the city of Cauquenes, Maule region, central Chile (35° 58′ 12″ W, 72° 21′ 00″ S). The Maulino forest is a mesic forest type located in one of the global biodiversity hotspots ([36]) and dominated by the long-lived and broadleaved caducifolious species N. alessandrii and N. glauca. We worked on eight fragments dominated by the species N. glauca ([19]) and surrounded by a matrix of forest plantations with the exotic P. radiata.
Sample design
Tab. 1 - Main characteristics of the eight forest fragments in the Maulino forest, Central Chile. Values are means ± standard deviation.
| Fragment ID |
Fragment size (ha) |
No. plots (100 m2 each) |
T. monspessulana plants |
Native plants |
PAR (μmol photons m-2 s-1) |
Shannon index |
Species richness |
|---|---|---|---|---|---|---|---|
| 1 | 11 | 11 | 2.36 ± 1.18 | 190 ± 25 | 6 ± 0.9 | 0.21 ± 0.02 | 14.4 ± 0.7 |
| 2 | 19 | 15 | 3.20 ± 1.48 | 250 ± 28 | 730 ± 220 | 0.17 ± 0.01 | 15.0 ± 1.1 |
| 3 | 3 | 3 | 0.00 ± 0.00 | 380 ± 140 | 2116 ± 225 | 0.32 ± 0.00 | 13.6 ± 3.6 |
| 4 | 20 | 16 | 3.06 ± 2.61 | 208 ± 21 | 972 ± 202 | 0.17 ± 0.01 | 15.9 ± 0.8 |
| 5 | 7 | 7 | 2.00 ± 1.36 | 186 ± 35 | 1442 ± 378 | 0.26 ± 0.02 | 16.5 ± 1.3 |
| 6 | 70 | 35 | 18.23 ± 3.89 | 105 ± 10 | 926 ± 124 | 0.09 ± 0.00 | 9.9 ± 0.6 |
| 7 | 53 | 30 | 2.77 ± 2.65 | 144 ± 13 | 739 ± 117 | 0.11 ± 0.00 | 11.5 ± 0.5 |
| 8 | 152 | 45 | 0.78 ± 0.35 | 120 ± 11 | 626 ± 66 | 0.08 ± 0.00 | 13.6 ± 0.5 |
| Total | 332 | 162 | - | - | - | - | - |
During the summer period of 2011 we disposed 162 sampling plots, 100 m2 each, distributed across eight forest fragments of the Maulino forest. The size of the eight fragments ranged from 3 to 152 ha; except for fragment no. 8, the spatial distribution of each plot was chosen to best reflect the structure and composition of about 1-2 ha of the parent forest fragments (Tab. 1). Within each plot, we counted the total number of T. monspessulana individuals to estimate their density. The seedlings (up to 50 cm in height) and the reproductive status of the individuals was determined, recording as reproductive all those carrying fruits. Additionally, we assessed canopy cover (%), litter depth (cm), and photosynthetically active radiation (PAR, μmol photons m-2 s-1). We assessed the canopy cover using a camera 10.1 mega pixel (Sony Corp., Tokyo, Japan), disposed on tripod at 1.20 m above the ground. We estimated the canopy cover (in percentage), with the photographs using the software SigmaScan® (SPSS Inc., Chicago, IL, USA). We measured PAR radiation (average of 5 readings per plot) using a radiometer (model LI-250® Light Meter, LI-COR Biosciences, Lincoln, NE, USA) at the ground level. We assessed litter depth (cm) disposing a ruler 30 cm-long in five randomly selected points within sampling plots, thus obtaining an average for each plot. Within plots we also counted the number of different native species, and the total density of native plants. We estimated the Shannon-Wiener diversity index (H’) following Magurran ([30]) as follows (eqn. 1):
where n is the number of individuals in a plot, and N is the total number of individuals in the fragment where that plot belongs.
Statistical analyses
The different factors measured to check whether they affect T. monspessulana seedling density (i.e., canopy cover, litter, PAR, plant diversity, and richness of native plant species) were highly correlated with each other. For that reason, we decided to use a Principal Component Analysis (PCA) to reduce multicollinearity and capture the information in orthogonal axes. We identified the ecological factors most contributing to the PC axes, and then we conducted a Generalized Linear Mixed Model (GLMM) with T. monspessulana seedling density as the dependent variable. The first two ordination axes were considered as the fixed factors, whereas the eight fragments were considered as random factors. We used a likelihood ratio test to compare the full model (with fixed and random factors) with the reduced model (with only fixed factors). We found that the full model (AIC = 606, BIC = 621) had a better performance (p-value = 0.0003) than the reduced model (AIC = 616, BIC = 628). Then, we used a GLMM with a negative binomial distribution (link function: log x). This analysis was performed using SPSS® v. 18 (SPSS Inc., Chicago, IL, USA). As an alternative approach to assess the importance of factors controlling the abundance of T. monspessulana plants, we employed a Path analysis aimed to evaluate the direct and indirect contributions of the independent variables (i.e., canopy cover, litter, PAR, species richness, and plant diversity) on density of T. monspessulana plants. This analysis was performed using INFOSTAT v. 2018 (Group Infostat, Universidad Nacional de Córdoba, Argentina). All the analyses were carried out on a plot-level basis.
Results
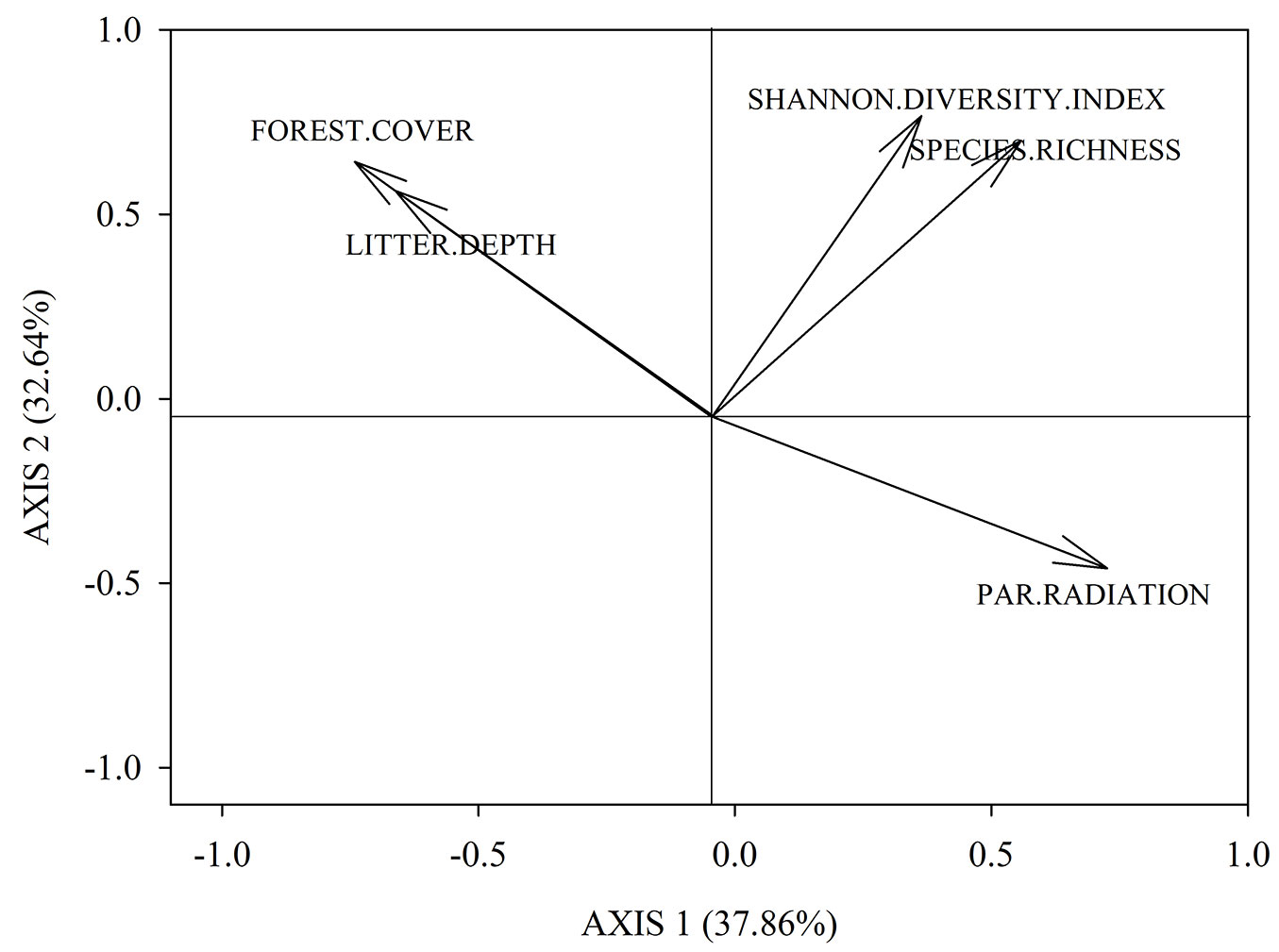
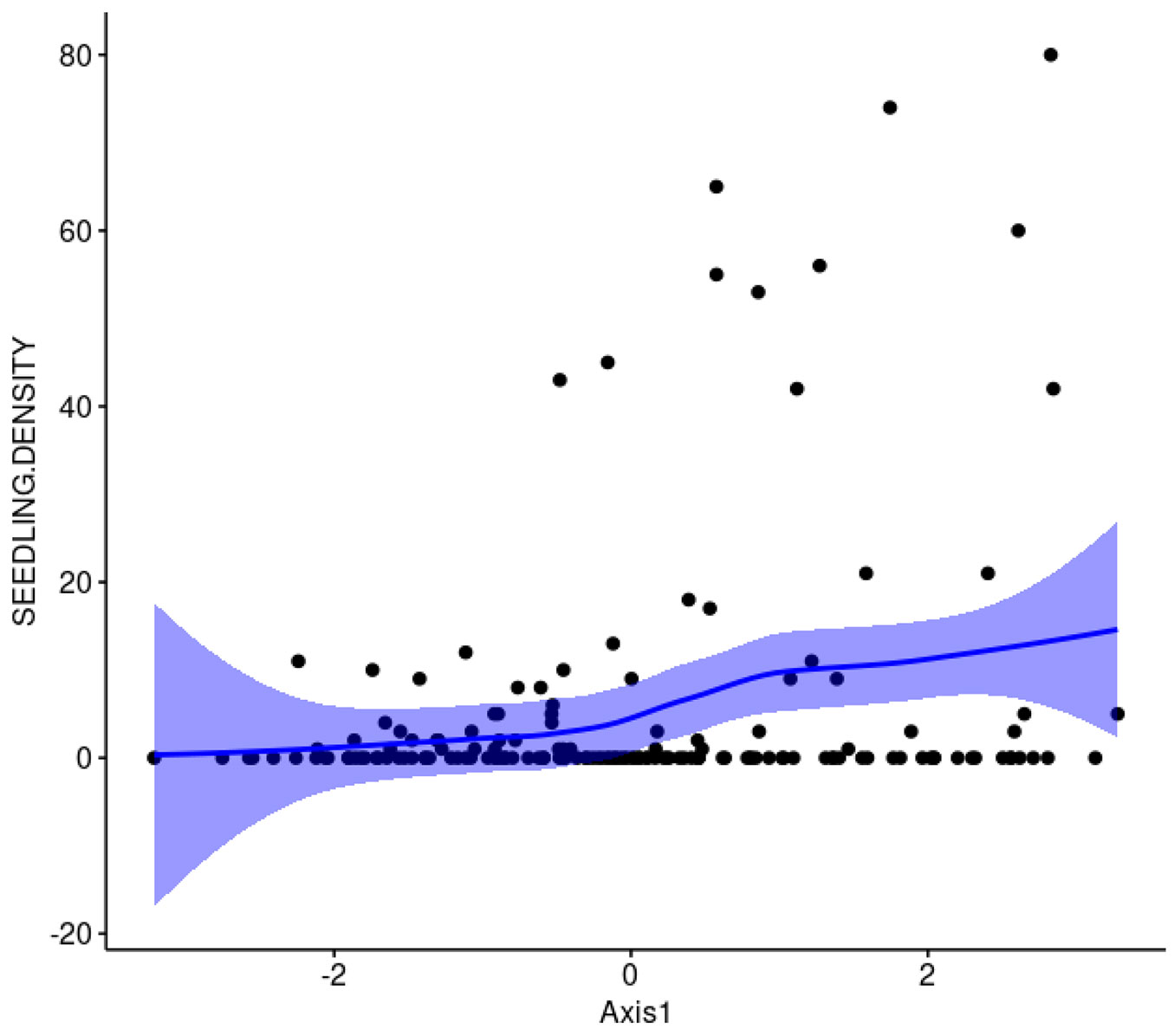
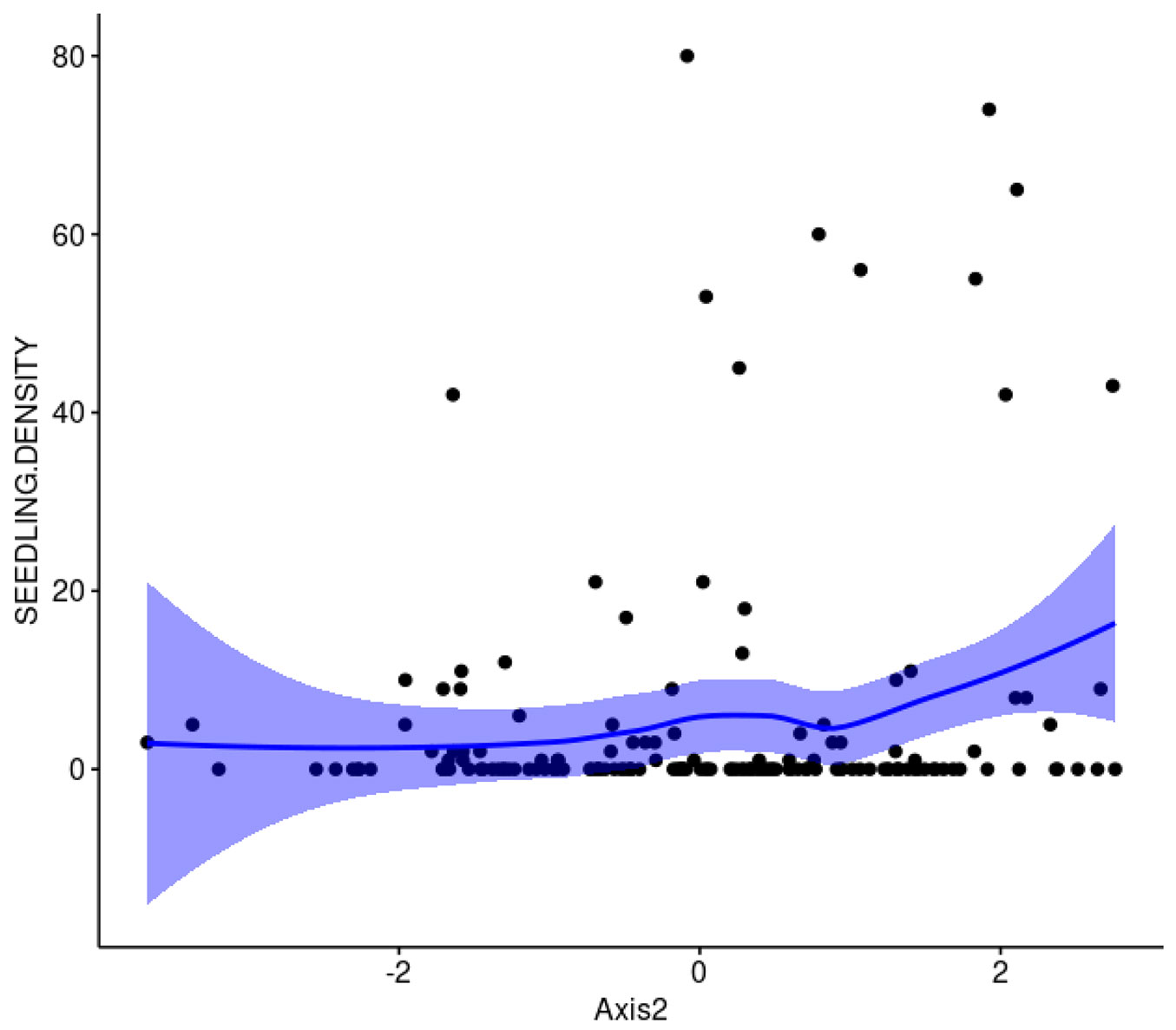
The number of T. monspessulana plants recorded in the eight plots was 893, of which 94.5% were seedlings. The results of the PCA indicates that the first two axes accounted for 70.5% of the total variance in the dataset (Fig. 1). The first axis correlated negatively with canopy cover (r = -0.73, 27.8% of contribution), and litter depth (r = -0.67, 23.4% contribution), and positively with PAR radiation (r = 0.63, 21.3% contribution); thus, axis 1 captured indirectly light availability. The second axis correlated positively with Shannon-Wiener index (r = 0.81, 40.6% contribution) and species richness (r = 0.73, 32.4% contribution); thus, axis 2 captured native plants diversity (Fig. 1). Light availability (represented by axis 1) was positively and significantly correlated with T. monspessulana seedling density (P = 0.002, Fig. 2), whereas native plants diversity (represented by axis 2) was not related with T. monspessulana seedling density (p = 0.324, Fig. 3).
Fig. 1 - Principal Component Analysis (PCA) showing the influence of different factors analyzed in our study in a bi-plane formed by axis 1 and axis 2.
Fig. 2 - The relationship between PC axis 1 and the density of T. monspessulana within Maulino forest fragments, Central Chile. Confidence Intervals were obtained using LOESS procedure. Axis 1 reflects light availability. Positive values represent higher values of light availability.
Fig. 3 - The relationship between PC axis 2 and the density of T. monspessulana within Maulino forest fragments, Central Chile. Confidence Intervals were obtained using LOESS procedure. Axis 2 reflects native species diversity. Positive values represent high values of native diversity.
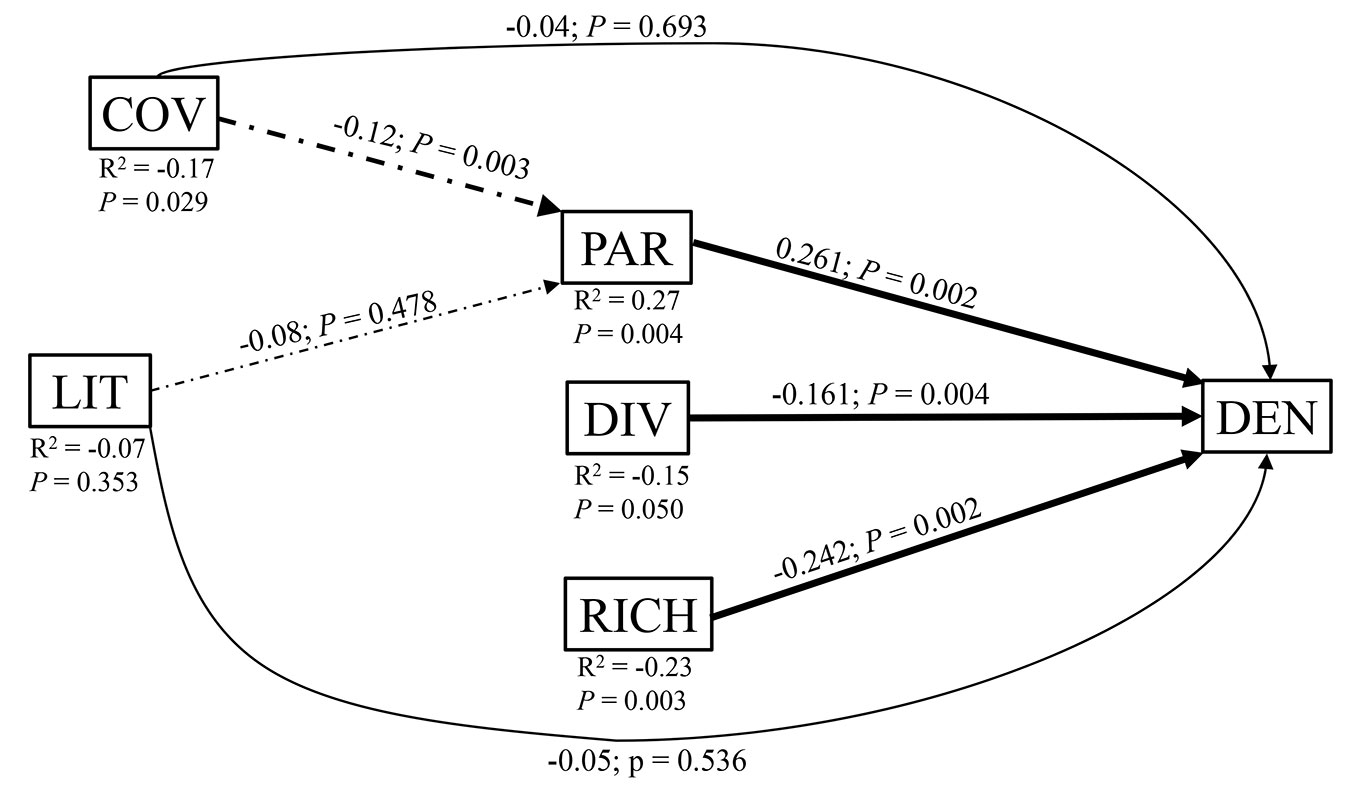
The Path analysis indicated that the variable with the greatest direct effect on density of T. monspessulana was PAR, followed by species richness and plant diversity (Tab. 2). There was also a significant indirect correlation between density of T. monspessulana with canopy cover (r = -0.17) which is mainly determined by PAR as an indirect effect. Litter depth was not correlated with density of T. monspessulana, despite this variable has an important contribution to PC axis 1, thus the effects of litter must be interpreted with caution (Fig. 4).
Tab. 2 - Path analysis of the relationships between density of T. monspessulana plants and the various independent variables in each plot. (COV): forest cover; (LIT): litter depth; (PAR): photosynthetically active radiation; (DIV): plant diversity; (RICH): richness of native plant species; (ns): non- significant; (**): p < 0.01; (*): p < 0.05. The sum of all direct and indirect effects yields the total correlation between a causative variable and the dependent variable.
| Factors | Direct effect |
Indirect effect | Total correlation (r) |
||||
|---|---|---|---|---|---|---|---|
| COV | LIT | PAR | DIV | RICH | |||
| COV | -0.04 ns | - | -0.02 | -0.12 | -0.01 | 0.02 | -0.17** |
| LIT | -0.05 ns | -0.02 | - | -0.08 | 0.03 | 0.05 | -0.07 ns |
| PAR | 0.26** | -0.02 | -0.02 | - | 0.01 | 0.01 | 0.27** |
| DIV | -0.16** | -0.00 | 0.01 | 0.01 | - | -0.02 | -0.15* |
| RICH | -0.24** | 0.00 | 0.01 | 0.02 | -0.01 | - | -0.23** |
Fig. 4 - Path analysis diagram of the relationships between density (DEN) of T. monspessulana plants with COV, LIT, PAR, DIV, and RICH. Thick arrows indicate significant direct effects (solid lines) and indirect effects (dashed lines). Over the arrows it is represented the path coefficient and its statistical significance. Below each causative variable it is represented the total correlation (R2) and significance between that variable with DEN.
Discussion
In this study we have demonstrated that the most important forest feature that determine T. monspessulana invasion into forest fragments of the Maulino forest in Central Chile is the light availability, which is associated to canopy cover and PAR. It seems that the more closed the forest, the lower the light availability and the less invasion of T. monspessulana. This basic result has tremendous implications for the conservation and management of this important ecosystem.
Currently, native forests are submitted to a variety of anthropogenic disturbances such as fire, deforestation and fragmentation. Given that most exotic species are shade-intolerant ([13]), these disturbances act favoring the arrival and establishment of exotic plants ([39], [24]). This is the case of T. monspessulana which rarely inhabits environments with low light availability. Light availability and high temperature at the ground level triggers a massive germination from seed bank, forming the so-called “seedling gardens” with thousands of individuals per m2 ([15], [45]). Based on our results, we argue that unsuitable microsites for invasion of T. monspessulana are forests with closed canopy ([7]). However, in our study, fragment 3 had high light availability (average PAR of 2.116 μmol photons m-2 s-1), but absence of T. monspessulana plants. This can be explained by the presence of a propagule source in the near area, but this hypothesis needs further research.
A typical trait of leguminous plants (such as T. monspessulana) is the profuse seed bank that remains dormant in the ground for a long period of time ([3]). Given that just one plant can produce thousands of seeds during one season ([15], [17], [23]), it is highly probable that T. monspessulana be a successful invader only by a “mass effect”, i.e., a high seed rain that forms a persistent seed bank that remains latent during long periods of time until light conditions are optimal for germination. In fact, in the absence of biotic and abiotic limitations, plants that maintain a large seed bank will be highly invasive, following the propagule pressure hypothesis, i.e., a large number of individuals arriving per unit of time increases invasiveness ([28], [44]). However, there can be abiotic filters (such as light availability) to counterbalance this potentiality, thus arresting plant establishment ([4]).
In our study, we detected no significant effects of the native plant community (axis 2 of the PCA) on T. monspessulana density. This suggests no biotic resistance (in terms of interspecific competition) of the resident community to plant invasion. This result is of major importance because it suggests that biotic filters against the colonization and establishment of T. monspessulana do not exist in the Maulino forest. This result significantly differs from other similar studies in forest ecosystems ([40], [24]). However, the Path Analysis indicated a significant though low effect of diversity and richness in our study. Fragment 3 (the smallest fragment with the highest average PAR) had the highest density of native plants (average of 380 plants), but absence of T. monspessulana plants. On the contrary, fragment 6 had the highest abundance of T. monspessulana plants but the lowest density of native plants (average of 105 plants). This suggests that further research is needed to elucidate other microsite conditions controlling the invasion of T. monspessulana (e.g., soil humidity and temperature, number of reproductive individuals, presence of reproductive individuals, seed viability, etc). From the conservation point of view, our results suggest that the invasiveness of T. monspessulana in highly valuable ecosystems such as the Maulino forest could be reduced by means of restoration with native plants or by reducing forest degradation. Both actions maintain higher canopy cover which in turn will decrease light availability and will hamper the massive establishment of T. monspessulana.
Conclusion
Our results indicate that the successful establishment of the shade-intolerant T. monspessulana in the Maulino forest is driven by light availability. The lower the light availability, the less the density of T. monspessulana. This suggests that the disturbance-invasibility hypothesis seems more appropriate to explain the successful establishment of this species. The Maulino forest has experienced wildfires, deforestation, and reduction of canopy cover, creating the conditions of light availability suitable to the invasion by T. monspessulana. Thus, increasing or maintaining the canopy cover, together with an intensive control of T. monspessulana seems to be an appropriate action to minimize the invasion of this aggressive weed.
Acknowledgements
This study was supported by FONDECYT 1180193 to Ramiro O. Bustamante and “The structure of mutualist networks in fragmented forests” (PBCTACT34/2006), Chile. Other partial support come from project “Integrated conservation of two threatened Chilean endemic trees, Nothofagus alessandrii and Gomortega keule in the coastal cordillera of central Chile” (2020-2022), Foundation Franklinia, the Global Trees Campaign and BGCI.
References
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Facultad de Ciencias Agrarias y Forestales, Universidad Católica del Maule, Avenida San Miguel, 3605, Talca (Chile)
Estefany Goncalves 0000-0002-1066-4519
Ramiro Bustamante 0000-0001-6441-7006
Departamento de Ciencias Ecológicas, Facultad de Ciencias, Universidad de Chile, Santiago (Chile)
Instituto de Ecología y Biodiversidad (IEB), Facultad de Ciencias, Universidad de Chile, Santiago (Chile)
Corresponding author
Paper Info
Citation
Gómez P, Espinoza S, Cuadros N, Goncalves E, Bustamante R (2022). Light availability influences the invasion of Teline monspessulana (L.) K. Koch in a temperate fragmented forest in Central Chile. iForest 15: 411-416. - doi: 10.3832/ifor4026-015
Academic Editor
Rossella Guerrieri
Paper history
Received: Nov 22, 2021
Accepted: Aug 04, 2022
First online: Oct 19, 2022
Publication Date: Oct 31, 2022
Publication Time: 2.53 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2022
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 26042
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 22073
Abstract Page Views: 2167
PDF Downloads: 1361
Citation/Reference Downloads: 2
XML Downloads: 439
Web Metrics
Days since publication: 1231
Overall contacts: 26042
Avg. contacts per week: 148.09
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2022): 2
Average cites per year: 0.50
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Spread intensity and invasiveness of sycamore maple (Acer pseudoplatanus L.) in Lithuanian forests
vol. 8, pp. 693-699 (online: 19 March 2015)
Research Articles
Cryptogamic epiphytes and microhabitat diversity on non-native green ash (Fraxinus pennsylvanica Marsh., Oleaceae) in urban habitats
vol. 14, pp. 393-399 (online: 01 September 2021)
Research Articles
Scale dependency of the effects of landscape structure and stand age on species richness and aboveground biomass of tropical dry forests
vol. 16, pp. 234-242 (online: 23 August 2023)
Research Articles
Plant cover evolution and naturalisation of revegetated ski runs in an Apennine ski resort (Italy)
vol. 2, pp. 178-182 (online: 15 October 2009)
Research Articles
Can the dynamics of forest restoration reduce landscape fragmentation in the Atlantic forest?
vol. 18, pp. 61-68 (online: 04 April 2025)
Research Articles
Diversity and distribution patterns of medium to large mammals in a silvicultural landscape in south-eastern Brazil
vol. 11, pp. 802-808 (online: 14 December 2018)
Review Papers
Large-scale effects of forest management in Mediterranean landscapes of Europe
vol. 6, pp. 342-346 (online: 29 August 2013)
Research Articles
Bird composition and diversity in oak stands under variable coppice management in Northwestern Turkey
vol. 11, pp. 58-63 (online: 25 January 2018)
Research Articles
Is cork oak (Quercus suber L.) woodland loss driven by eucalyptus plantation? A case-study in southwestern Portugal
vol. 7, pp. 193-203 (online: 17 February 2014)
Research Articles
The estimation of canopy attributes from digital cover photography by two different image analysis methods
vol. 7, pp. 255-259 (online: 26 March 2014)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword