Growing at the forest edges: how natural regeneration develops under fragmentation
iForest - Biogeosciences and Forestry, Volume 15, Issue 4, Pages 248-255 (2022)
doi: https://doi.org/10.3832/ifor3834-015
Published: Jul 19, 2022 - Copyright © 2022 SISEF
Research Articles
Abstract
Environmental changes caused by edge effects and matrix land use can interfere with plant community resilience and, consequently, alter forest succession. Here, we aimed to (i) investigate whether species composition, density and richness in a forest’s regeneration layer vary in its edge-to-interior gradient and (ii) analyze the relationship between regeneration and local abiotic variables. We conducted the study in the lowland rainforest of the Atlantic Forest biodiversity hotspot at the Córrego Grande Biological Reserve, Espírito Santo state, Brazil. We sampled the regeneration layer in two edge environments with different matrices (forest and road) and the fragmented interior to link vegetation structure with environmental variables. In each environment, we set up 12 plots of 5 × 10 m size and recorded, in each plot, the height and stem base diameter of all living individuals above 50 cm of height and below 2.5 cm of diameter at breast height (1.30 m height). We applied different multivariate analyses to assess the influence of environmental data, such as canopy openness and physical-chemical soil variables. The three environments shared 22 out of the 174 morphospecies recorded, and the forest-side edge had the lowest species richness among all environments. The environmental variables that better explained the distribution of species across the three environments were: canopy openness, soil penetration resistance, zinc, and calcium content. Our results revealed significant environmental differences among the forest edges and the forest interior of the study site, highlighting the relevant role of the forest surrounding matrix for the maintenance of protected remnants.
Keywords
Seedling, Forest Resilience, Biological Reserve, Environmental Variables
Introduction
The Brazilian Atlantic Forest is recognized as a highly biodiverse and endemic region ([39]). Combined with the predominantly fragmented distribution that makes it susceptible to human disturbance, these characteristics have placed the Atlantic Forest among the global biodiversity hotspots prioritized for conservation ([39]). The current state of fragmentation, which results from years of human activity in the region ([20]), exposes Atlantic Forest remnants to different impacts ([35]). Some permanent impacts faced by these forests are edge effects, which occur along fragmented boundaries ([32]).
Forest edge effects result from interactions between two adjacent environments separated by an abrupt transition zone ([38]). The environmental quality of forest edges is therefore related to the surrounding matrix of the fragment. Environmental changes induced by edge effects alter the biotic and abiotic variables ([9]) and can promote further biodiversity loss ([32]). The edges of a forest represent a boundary zone where the microclimate and species composition largely differ from those in the forest interior ([45]). Changes in the microclimate along forest edges, which include increased temperature, decreased humidity ([45]), and altered wind turbulence, may potentially change local species composition and structure ([27]), leading to high temporal species turnover ([32]). This turnover is related to the higher numbers of pioneer and early secondary species usually found along the edges ([46]) and on the regeneration layer of forest fragments ([40]).
Natural regeneration is a mechanism of slow ecological recovery (in terms of ecosystem structure, composition, and function) that follows an ecosystem disturbance ([15]). Through the recruitment of individuals and species, this process involves successive progress towards increasing functional and structural complexity in the natural community ([14]). Understanding the role of natural regeneration in the succession process is essential to predicting how different disturbances may influence the future plant diversity of a forest ([21]). Given the different environmental tolerance spectra exhibited by plant species ([18]), the type of surrounding matrix can be a determining factor in alleviating or worsening the adverse consequences of edge effects on the community ([25]). Furthermore, plant community structure in mature tropical forests is largely determined by the composition of the regeneration layer in the early stages of succession ([7]). Therefore, forest succession and diversity maintenance also depends on the regeneration layer ([3]).
Considering the importance of the regeneration layer for forest succession and the consequences of edge effects on forest communities, in this study, we aimed to investigate the influence of edge effects on the composition of the regeneration layer along two forest edges bounded by different surrounding matrices with the forest interior in an Atlantic lowland forest. We hypothesize that the matrices influence the edge of the patch differently. Therefore, it is expected that the properties of the different environments may shape the taxonomic diversity of the regeneration layer. To identify variation in the composition and structure of the forest regeneration layer, we asked: (i) do richness and diversity vary along forest edges with different surrounding matrices in the same remnant forest? (ii) What abiotic variables may shape species distribution in these contexts?
Materials and methods
Study area
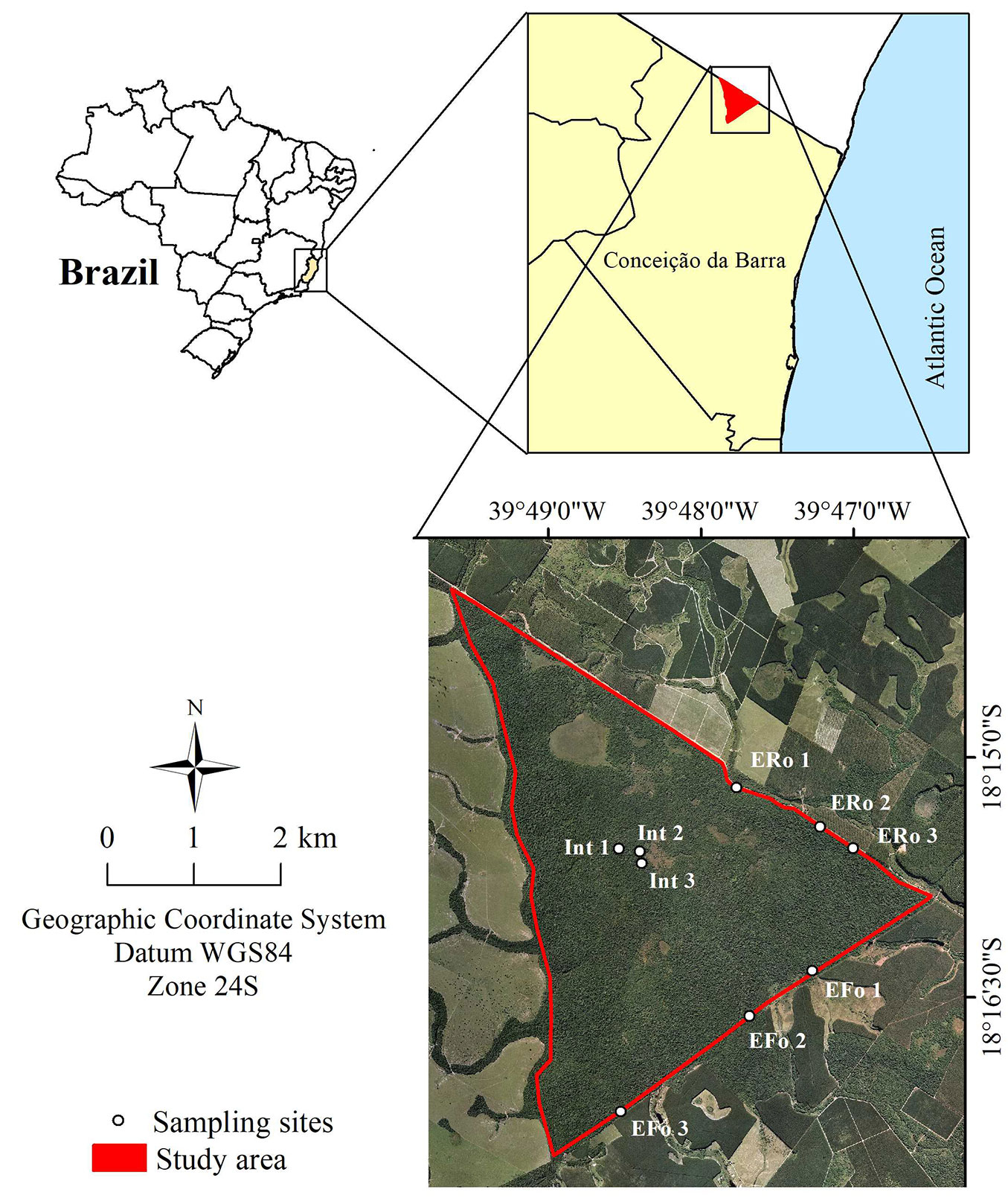
The study was conducted in the Córrego Grande Biological Reserve (CGBR), a protected site with an area of 1504.8 hectares in the municipality of Conceição da Barra, state of Espírito Santo, Brazil (18° 12′ - 18° 18′ S; 39° 45′ - 39° 50′ W - Fig. 1). The CGBR is a triangle-shaped forest fragment surrounded by cattle pasture, eucalyptus, and agricultural plantations ([47]). A non-paved state road also encircled the site, with 8 km of extension that separates the Brazilian states of Espírito Santo and Bahia. The road, locally known as “Picadão da Bahia”, is one of the main distribution outlets of the region’s eucalyptus production. Before the establishment of this biological reserve in the late 1980s, the study site underwent periods of selective logging for timber production, as well as hunting and forest fires. The last fire recorded on the site occurred in the late 1990s ([19]).
Fig. 1 - Geographic location of the Reserva Biológica do Córrego Grande in the municipality of Conceição da Barra, state of Espírito Santo, Brazil. The sampling sites (white circle) are roadside edge (ERo), forest-side edge (EFo), and fragment’s interior (Int).
This Atlantic Forest remnant, located 55 m a.s.l. ([19]), is classified as Lowland Dense Moist Forest (i.e., lowland Atlantic rainforest - [24]) and nationally known as a tabuleiro forest. The occurrence range of tabuleiro forests is usually associated with nutrient-poor soils across alluvial flatlands (between 20 and 200 m a.s.l.) near the Brazilian Atlantic coast. The region’s climate falls within Köppen’s Af category (tropical rainforest climate), with a mean annual temperature above 23 °C and annual precipitation above 1200 mm ([1]).
Vegetation sampling
To sample the natural regeneration layer, we established 36 permanent plots of 50 m2 (5 × 10 m, totaling 1800 m2) distributed across three environments: forest-side edge (EFo), roadside edge (ERo), and fragment interior (Int). The sampling of each environment (EFo, ERo, and Int) was performed along three transects, in each of which we located four plots, distanced 10 m from each other and distributed along the edge-to-interior direction (12 plots per environment). We adopted two inclusion criteria to sample the regeneration layer: minimum height of 50 cm and maximum diameter at breast height (at 1.30 m height) of 2.5 cm. We measured the height and stem base diameter of all trees and shrubs that met these criteria.
When species identification was not possible in the field, we collected plant material for later comparisons with the specialized literature, regional herbaria (Herbário Capixaba, CAP and Reserva Natural Vale, CRVD), and virtual herbarium collections (Reflora Virtual Herbarium - ⇒ http://reflora.jbrj.gov.br/reflora/herbarioVirtual/; and Jabot - ⇒ http://jabot.jbrj.gov.br/v2/consulta.php). Species names were standardized according to the Flora do Brasil 2020 species list (⇒ http://floradobrasil.jbrj.gov.br/), and family classification followed Angiosperm Phylogeny Group IV ([5]).
Environmental sampling
To assess canopy openness (CO) in each environment, we took hemispherical canopy photographs at the center of each plot, using a fisheye lens (180° of angle view) attached to a smartphone and supported by a tripod (1.30 m height - [51]). The camera was set up following the magnetic north direction, and photographs were taken near the beginning (early morning) or the end (late afternoon) of each collection day, under lighting conditions as uniform as possible. We collected a total of 72 canopy photographs, 36 for each month: April 2018, at the end of the rainy season, and September 2018, at the end of the dry season. To process these data, we used the Gap Light Analysis Mobile app (⇒ https://www.sci.muni.cz/botany/glama/). This app differentiates between white pixels (light that penetrates the canopy) and black pixels (vegetation) and calculates the percentage of canopy openness based on the white/black pixel count ([51]).
To characterize the soil, we collected soil samples near the plots used in this study, which were installed to perform a simultaneous tree inventory. Soil samples were systematically collected from five points at 0-20 cm of depth and later homogenized into one composite sample per plot. All 36 soil samples were analyzed at the Laboratory of Fertiliser, Water, Mineral, Residual, Soil and Plant Analyses (LAFARSOL) of the Federal University of Espírito Santo, Brazil. The following physical-chemical attributes were obtained under the methodology proposed by EMBRAPA ([23]): C, P, K, Al, S, Ca, Na, Mg, Fe, Cu, B, Mn, Zn, exchangeable acidity (HAl), pH (H2O), organic matter (OM), sum of exchangeable bases (SB), aluminum saturation (m), Cation Exchange Capacity (CTC), base saturation (V), sodium saturation index (ISNa), and effective cation exchange capacity (CTC1), sand content, silt content, clay content, soil humidity, and soil bulk density.
To analyze soil penetration resistance (PR) in each plot, we used an impact penetrometer. At five points within each plot (one at each plot corner and one at the plot center), we recorded the number of hammer drops taken to reach 20 cm of soil depth. We used Stolf ([50]) equation to determine soil penetration resistance.
Data analysis
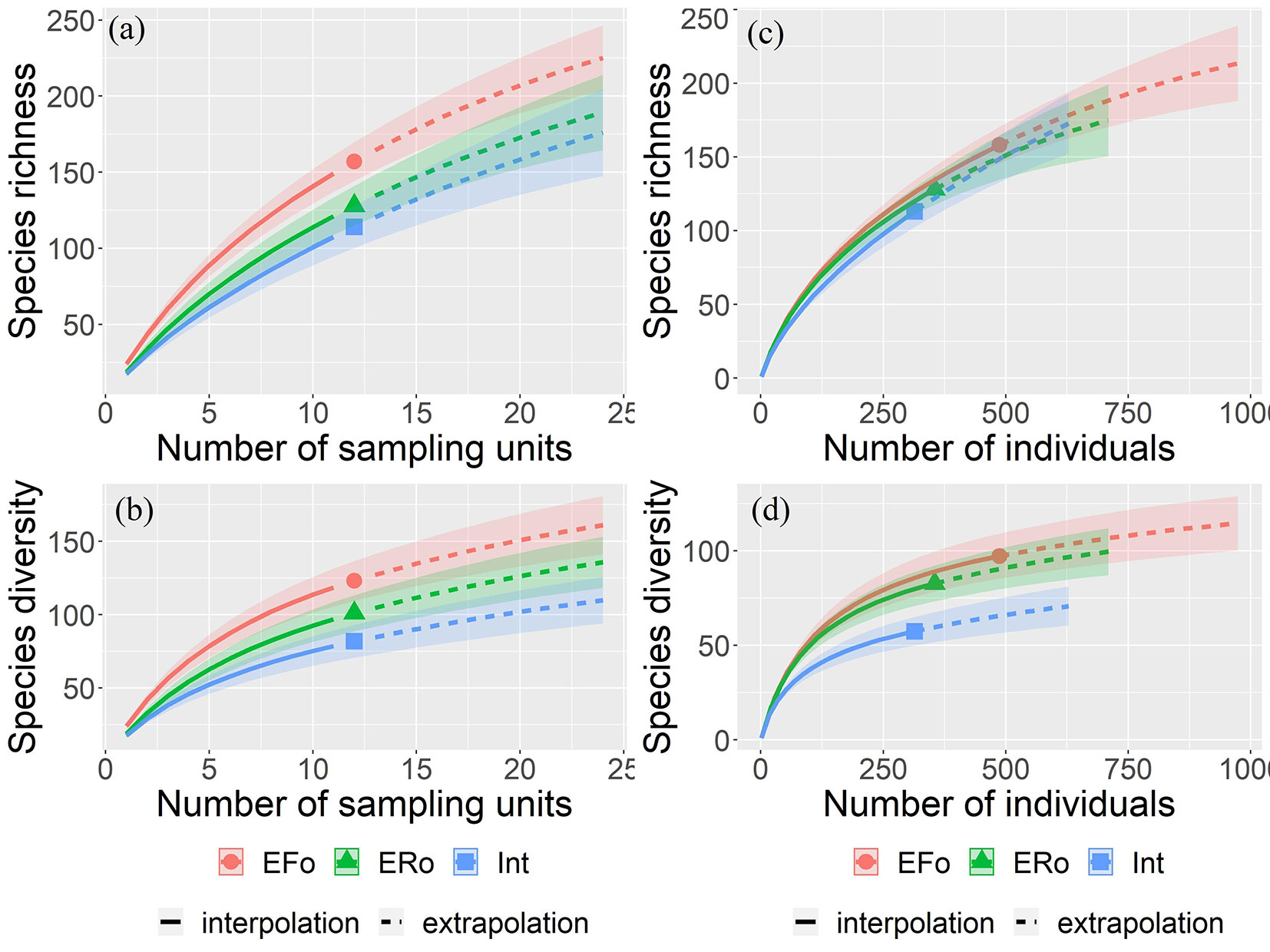
We calculated parameters based on Mueller-Dombois & Ellenberg ([37]) for the three environments studied. Likewise, we estimated species richness and diversity in the three environments by plotting rarefaction and extrapolation curves that considered the number of sampled individuals and the number of sample units. We estimated species richness using the first Hill number (species richness, q = 0) and species diversity using the exponential of Shannon entropy (diversity, q = 1 - [13]). We based extrapolations on abundance data, assuming between two and three times the sample size for each environment ([17]). Furthermore, we used the package iNEXT ([29]) to calculate rarefaction and extrapolation curves based on the number of sampled individuals and the number of sample units. We estimated 95% confidence intervals through 100 bootstrap replications. Non-overlapping confidence intervals indicated significantly different species numbers with p<0.05 ([17]).
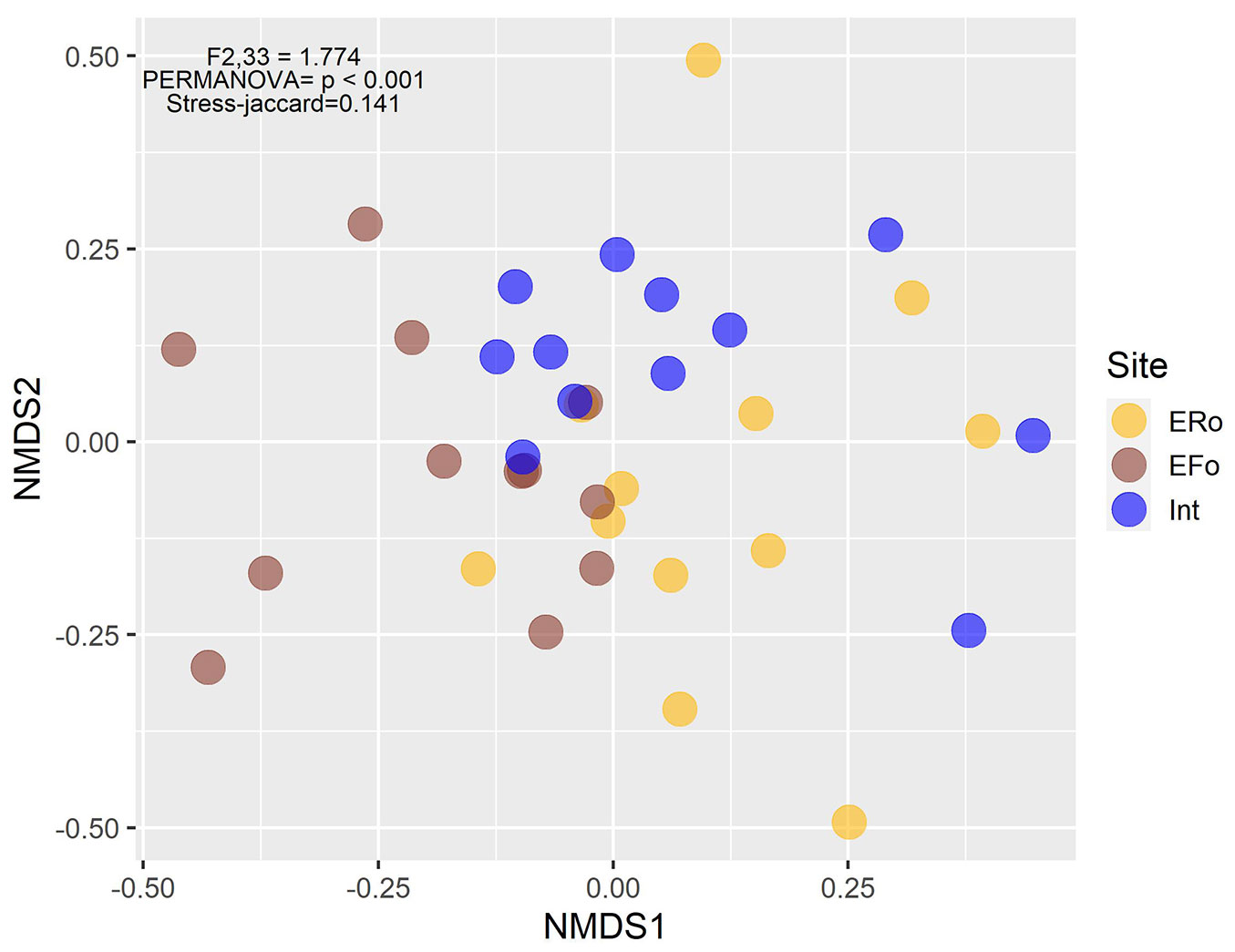
We performed a non-metric multidimensional scaling (NMDS) using Jaccard distances to compare the floristic composition among the three environments ([16]). For such, we used the “metaMDS” function of the “vegan” package ([41]). The NMDS was generated with four dimensions (k=4), but only the first two axes were used. To verify differences in species composition, we performed a permutational multivariate analysis of variance (PERMANOVA, 9999 permutations) using the “adonis” routine available within the “vegan” package ([41]).
We used a Principal Component Analysis (PCA) to verify the possibility of a soil gradient between the environments. We separated the analyses into two groups: soil fertility and soil physical data. To perform the PCA, we standardized the data to the correlation matrix using the “FactoMineR” package ([33]). Soil fertility, soil texture, canopy openness, and species composition were used to perform a canonical correspondence analysis (CCA) to investigate potential relationships between biotic and abiotic variables. The ordination of the data in the CCA allows one to observe the similarity or dissimilarity between species and between sites. To select the variables that best explain species distribution, we used the “ordistep” function from the “vegan” package ([41]). We used only variables with a variance inflation factor (VIF) less than 10. We assessed the significance of each abiotic variable through Monte Carlo randomizations (999 permutations). To illustrate the CCA results, we use the “autoplot” function from the “ggplot2” package ([53]). We performed all analyses in the R environment.
Results
We sampled in the regeneration layer 1156 individuals assigned to 174 morphospecies, 63 genera, and 20 families. In 0.18 ha of sampled area, we found that the three environments share 22 species, whereas 76 species were recorded exclusively at the forest-side edge, 52 species in the fragment’s interior, and 50 species at the roadside edge. Myrtaceae was the most abundant family and the most species-rich family for the roadside edge (53 individuals/13 spp.) and the forest-side edge (78 ind./21 spp). In the interior, the most abundant family and the most species-rich was Fabaceae (42 ind./11 spp.). The three most abundant species in each area were: at the roadside edge, Adenocalymma sp.1 (19 individuals), Eugenia astringens (16), and Cordia taguahyensis (13); at the forest-side edge, Cordia taguahyensis. (28), Psychotria sp.2 (21) e Myrcia amazonica (15); and in the fragment’s interior, Protium heptaphyllum (29), Pouteria bangii (24) e Pogonophora schomburgkiana (23 - Tab. 1). The list containing full species names and phytosociological results can be found in the Supplementary material (Tab. S1).
Tab. 1 - The ten species with the highest importance values in the three environments. Data were obtained through a phytosociological analysis. Number of individuals (NI), relative dominance (RDo), relative frequency (RF) and relative density (RD). Percent importance values (IV, %) for each of the three environments are also shown.
| Habitat | Species | Family | NI | RDo | RF | RD | IV% |
|---|---|---|---|---|---|---|---|
| Roadside edge | Eugenia astringens | Myrtaceae | 16 | 4.44 | 3.26 | 4.49 | 4.06 |
| Adenocalymma sp.1 | Bignoniaceae | 19 | 3.89 | 2.79 | 5.34 | 4.01 | |
| Cordia taguahyensis | Boraginaceae | 13 | 5.01 | 1.86 | 3.65 | 3.51 | |
| Ecclinusa ramiflora | Sapotaceae | 12 | 3.77 | 1.40 | 3.37 | 2.84 | |
| Acanthocladus pulcherrimus | Polygalaceae | 9 | 4.30 | 0.93 | 2.53 | 2.59 | |
| Swartzia apetala var. apetala | Fabaceae | 8 | 2.74 | 2.33 | 2.25 | 2.44 | |
| Myrcia splendens | Myrtaceae | 10 | 1.82 | 2.33 | 2.81 | 2.32 | |
| Eugenia inversa | Myrtaceae | 12 | 1.37 | 1.86 | 3.37 | 2.20 | |
| Licania kunthiana | Chrysobalanaceae | 8 | 2.01 | 1.86 | 2.25 | 2.04 | |
| Eschweilera ovata | Lecythidaceae | 10 | 1.74 | 1.40 | 2.81 | 1.98 | |
| Forest-side edge | Cordia taguahyensis | Boraginaceae | 28 | 3.57 | 4.17 | 5.76 | 4.50 |
| Helicostylis tomentosa | Moraceae | 14 | 3.24 | 1.74 | 2.88 | 2.62 | |
| Psychotria sp.2 | Rubiaceae | 21 | 1.93 | 1.39 | 4.32 | 2.55 | |
| Guapira venosa | Nyctagenaceae | 9 | 4.62 | 1.04 | 1.85 | 2.51 | |
| Sorocea guilleminiana | Moraceae | 13 | 2.71 | 1.74 | 2.67 | 2.38 | |
| Myrcia amazonica | Myrtaceae | 15 | 2.04 | 1.74 | 3.09 | 2.29 | |
| Protium heptaphyllum | Burseraceae | 14 | 2.26 | 1.39 | 2.88 | 2.18 | |
| Pausandra morisiana | Euphorbiaceae | 8 | 3.46 | 1.04 | 1.65 | 2.05 | |
| Ecclinusa ramiflora | Sapotaceae | 8 | 2.50 | 1.74 | 1.65 | 1.96 | |
| Dialium guianense | Fabaceae | 9 | 2.81 | 1.04 | 1.85 | 1.90 | |
| Fragment’s interior | Dialium guianense | Fabaceae | 5 | 15.35 | 1.99 | 1.59 | 6.31 |
| Pogonophora schomburgkiana | Peraceae | 23 | 7.89 | 3.48 | 7.32 | 6.23 | |
| Protium heptaphyllum | Burseraceae | 29 | 3.08 | 5.47 | 9.24 | 5.93 | |
| Cordia taguahyensis | Boraginaceae | 17 | 7.66 | 3.48 | 5.41 | 5.52 | |
| Clitoria sp.1 | Fabaceae | 21 | 3.08 | 4.98 | 6.69 | 4.92 | |
| Pouteria bangii | Sapotaceae | 24 | 3.44 | 2.49 | 7.64 | 4.52 | |
| Solanum sooretamum | Solanaceae | 10 | 3.66 | 2.49 | 3.18 | 3.11 | |
| Eschweilera ovata | Lecythidaceae | 6 | 2.97 | 2.49 | 1.91 | 2.46 | |
| Pausandra morisiana | Euphorbiaceae | 5 | 3.31 | 1.99 | 1.59 | 2.30 | |
| Ocotea glauca | Lauraceae | 4 | 2.51 | 1.99 | 1.27 | 1.92 |
We found significantly higher species richness along the forest-side edge in comparison with the roadside edge and fragment interior, taking the number of sampling units into account (Fig. 2a). Species diversity was also significantly higher in the forest-side edge in comparison with the fragment’s interior, considering both the number of sampling units and the number of individuals (Fig. 2b, Fig. 2d). The NMDS revealed that species composition differs between sites, at least between two different environments (PERMANOVA: F[2, 33] = 1.774, p < 0.001 - Fig. 3).
Fig. 2 - Rarefaction curves (solid lines) based on the number of sampling units (a, b) and the number of individuals (c, d); extrapolation curves (dashed lines) of species richness (a, c) and species diversity (b, d) of the regeneration layer in the three environments. The rarefaction and extrapolation curves represent mean values, and the colored bands the 95% confidence intervals.
Fig. 3 - Results of the non-metric multidimensional scaling (NMDS) based on species composition of the three environments. (ERo): roadside edge; (EFo): forest-side edge; (Int): fragment interior
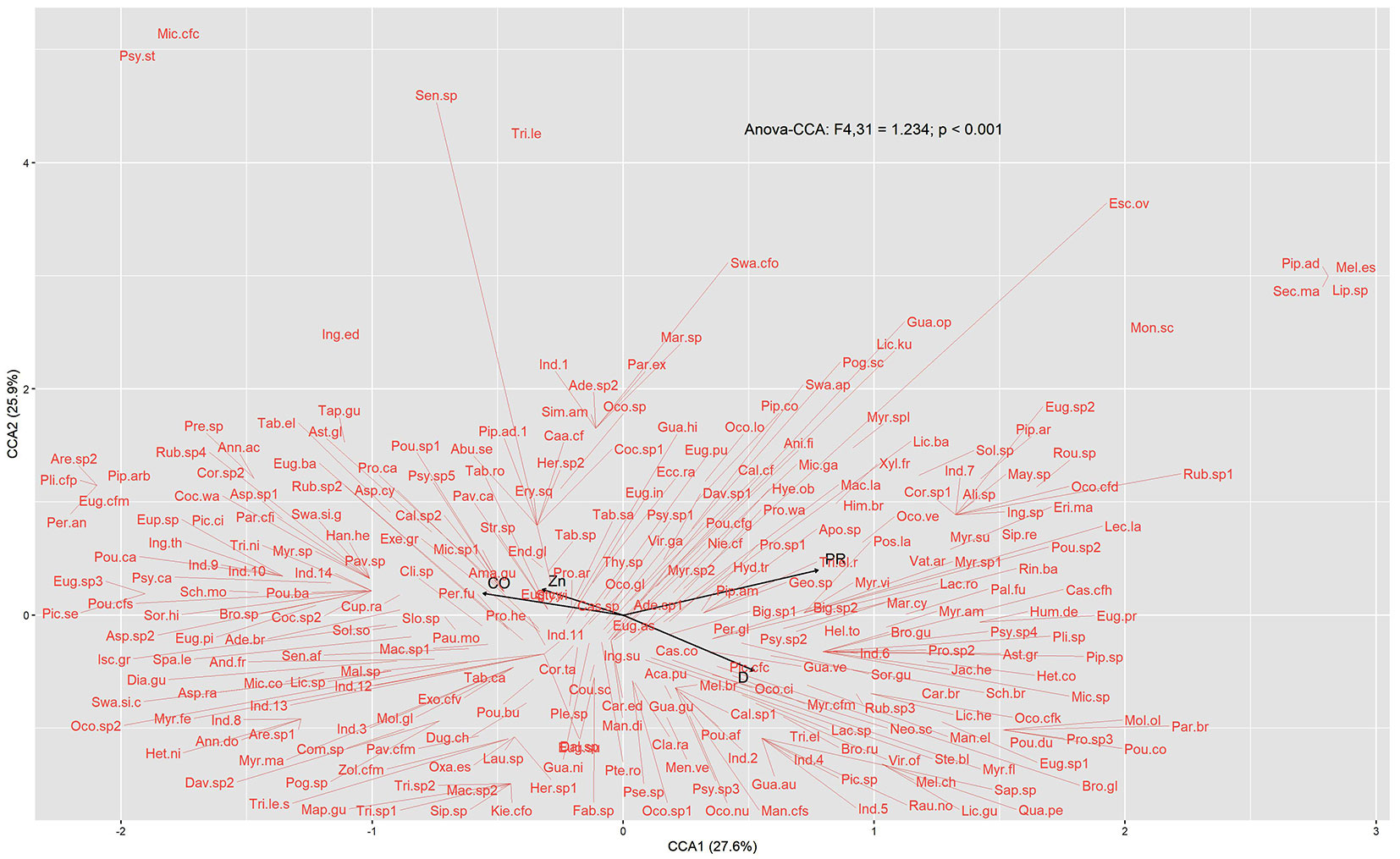
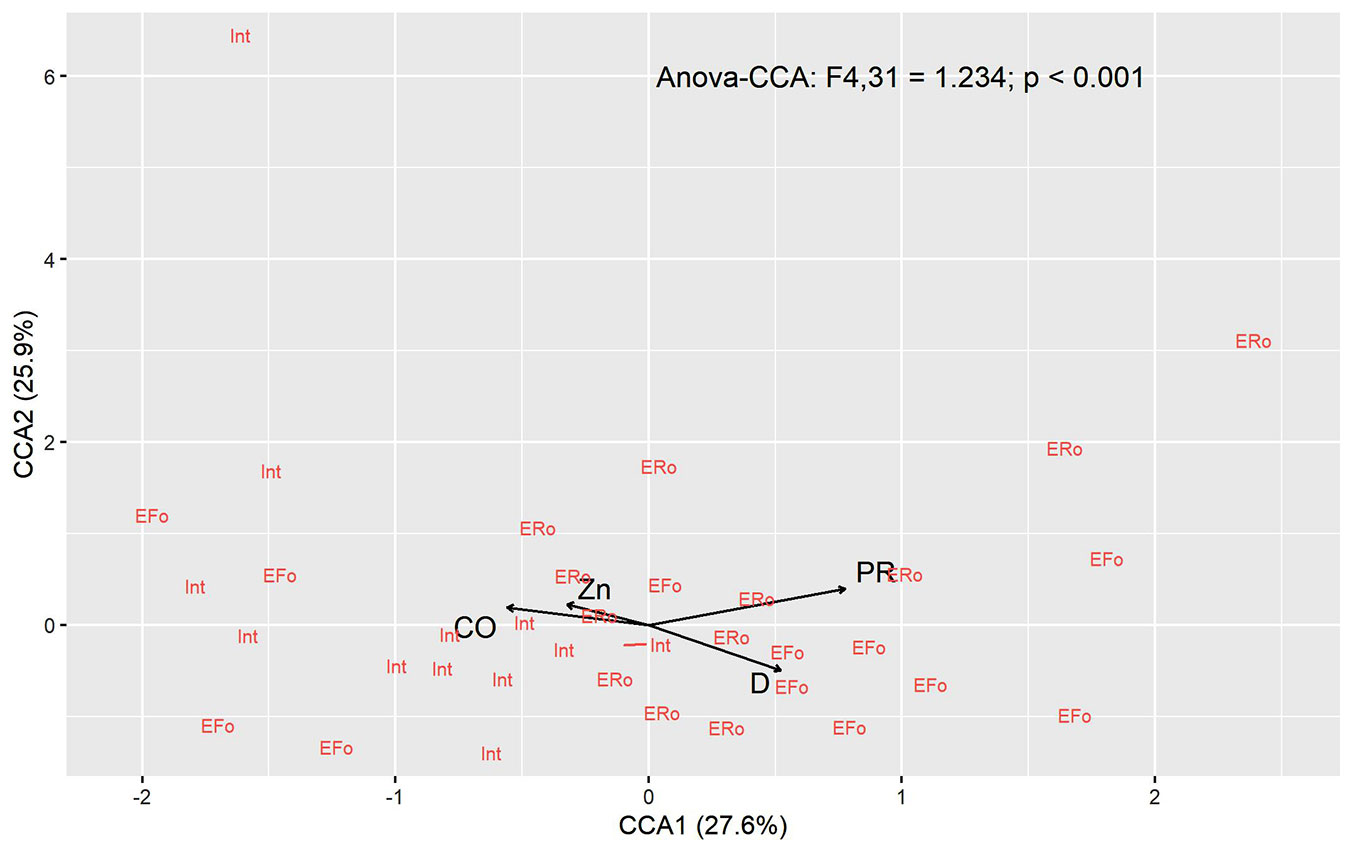
The PCA with the physical attributes of the soil was the most explanatory. The first two axes explained 72.1% of the variation in the soil’s physical data (Fig. S1 in Supplementary material). The PCA1 (45.3%) was positively correlated with clay content (R= 0.92, p< 0.01), penetration resistance (R= 0.89, p< 0.01), and the negatively correlated with sand (R= -0.89, p<0.01). The first two CCA axes explained 53.5% of all data variation (axis 1 = 27.6%; axis 2 = 25.9% - Fig. 4). The variables selected by the model were PR (anova.cca: Pseudo-F[1, 31]=1.293, p= 0.006), D (anova.cca: Pseudo-F[1, 31]=1.255, p = 0.012), CO (anova.cca: Pseudo-F[1, 31]= 1.155, p= 0.024) and Zn (anova.cca: Pseudo-F[1, 31]=1.2, p< 0.015). The species Securidaca macrocarpa, Melicoccus espiritosantensis, Piper aduncum, Lippia sp., and Monteverdia schummaniana were related to higher values of PR. While Miconia cf. cinnamomifolia, Psychotria stachyoides, and Inga edulis were associated with CO and Zn. Protium sp., Pouteria durlandii, Pouteria coelomatica, Parinari brasiliensis, and Mollinedia oligantha were correlated to D (Fig. 4). The CCA showed that the majority of the roadside edge plots were positively correlated with PR, whereas the interior was positively correlated with CO and the forest-side edge with D (Fig. 5).
Fig. 4 - Canonical correspondence analysis (CCA) of the species recorded the regeneration layer in function of soil properties sampled at the RBCG. Abiotic variables were canopy openness (CO), soil bulk density (D), soil penetration resistance (PR), and zinc (Zn).
Fig. 5 - Canonical correspondence analysis (CCA) of the plots in function of soil properties sampled at the RBCG. Abiotic variables were canopy openness (CO), soil bulk density (D), soil penetration resistance (PR), and zinc (Zn). The evaluated environments were roadside edge (ERo), forest-side edge (EFo), and fragment interior (Int).
Discussion
The results showed that the edge effect can be a factor that influences both the composition and structure of the community. The edge with forest showed a difference in richness and diversity with the interior of the fragment. Concerning the environment, PR was one of the most important factors in differentiating the environments, particularly between the roadside edge and the interior of the fragment. These results corroborate the hypothesis that different matrices have different effects on the edge of the fragment.
The species found in the regeneration layer have an important role in the succession process to compose the vertical structure of the community. The species Dialium guianense, Eschweilera ovata, Ecclinusa ramiflora, and Sorocea guilleminiana are frequent in tabuleiro forests ([42]). Among these, D. guianense, E. ovata, and S. guilleminiana are especially relevant for the composition and structure of high tabuleiro forests ([43]). The most abundant species on the roadside edge was a liana, Adenocalymma sp.1. This result is expected to be related to a positive growth association between lianas and high luminosity ([6]), especially in environments like the roadside edges.
At the forest-side edge, the highest density species was Cordia taguahyensis, perceptibly abundant in the understory at this study site. Cordia taguahyensis is a shrubby late secondary species usually related to forest transitions into advanced successional stages ([42]). In the fragment’s interior, the highest abundance species was Protium heptaphyllum. This species can be found in the mature tree layer and the regeneration layer of lowland Atlantic rainforests ([42]). Protium heptaphyllum occurs in forests with different successional stages but is frequently associated with later stages ([34]).
The presence of many species of Sapotaceae, Rubiaceae, Myrtaceae, and Fabaceae in the regeneration layer stands out because they are very representative families in the Atlantic Forest ([4]), especially in the lowland humid forests of the state of Espírito Santo ([44]). Myrtaceae features among the most species-rich plant families in Brazil ([22]), with a unique contribution to the composition of rainforests, where it is the richest family overall ([43], [22]). Generally pollinated by insects and dispersed by birds and mammals, Myrtaceae has a crucial ecological role in tropical rainforests ([26]). In this study, the most abundant and species-rich family in the fragment’s interior was Fabaceae, which is also the second-largest Atlantic Forest family in terms of native species number ([22]) and is also often recorded in tabuleiro forests ([30]).
As expected, we found higher richness and diversity at the forest-side edges than the fragment’s interior, as found in other similar studies ([32]). Edge effects alter the abiotic conditions of a forest and may, therefore, affect its ecological processes and species abundances ([32]). In general, forest edges display higher species richness than the forest interior ([2]), because seedling recruitment and growth tend to be higher near the exposed forest boundaries ([8]). The differences that we found between roadside edges and forest-side edges may be directly related to the type of surrounding matrix. A landscape characterized by multiple forest fragments, even artificial production forests such as Eucalyptus spp. monocultures, buffer the abiotic impacts stemming from edge effects on the forest fragment ([49]).
The results showed no significant differences in species composition and richness between the fragment’s interior and the roadside edge, indicating an influence from the land-use histories of the site’s matrix and interior ([36]). A potential explanation for this similarity is the forest fire that affected the interior of this fragment in the late 1990s. This fire could have resulted in a different successional pattern than expected for the interior of a mature forest ([19]), revealing similar composition and richness to the forest edges, especially considering that both evaluated edges (roadside and forest-side) have existed since the establishment of the protection area.
The CCA revealed that the main abiotic influence on species distribution in the environment’s regeneration layer was soil penetration resistance, soil bulk density, canopy openness, and soil zinc. Considering that tropical forest succession is influenced by environmental filters ([10]) and that the type of surrounding matrix may be a determining factor for the environmental variation along forest edges ([32]), the strength of edge environmental filters for forest succession is highly dependent on the associated matrix. Therefore, identifying which edge-associated factors influence the regeneration layer is vital for conservation. According to Baldeck et al. ([7]), microclimate variations are essential for species distribution in the plant community in several tropical forests.
Canopy openness was highest at the roadside edges and in the fragment’s interior. This may explain the higher floristic similarity found between these two environments, considering that canopy openness may impose a filter for species establishment. Light availability both directly and indirectly influences a large proportion of the processes related to plant growth ([11]). Therefore, light availability gradients strongly influence the local distribution of plant species in forest communities and, consequently, drive natural forest succession processes ([14]). The forest-side edge was negatively related to canopy openness. This relationship may be explained by the type of surrounding matrix (forested), which tends to display fewer or lower intensity abiotic changes than the open surroundings ([9]). Again, this result reinforces the importance of canopy openness for the regeneration layer.
Soil variables also have an important role in the survival and growth of regenerating individuals ([54]). The results suggest that soil characteristics influence species distribution in the study site: at the forest edges, the high soil penetration resistance that we found may influence the survival of regenerating individuals, especially at the roadside edge, where species richness was lower than at the forest-side edge. Abiotic factors directly influence individual recruitment and survival, ultimately driving forest succession ([12]). A study conducted in a tropical forest enrichment experiment in Borneo suggested that even 20 years after heavy-machinery logging, soil compaction negatively affected planted seedlings’ root growth ([28]). Soil compaction can influence seedling recruitment, growth, and mortality rates, especially in human-disturbed areas ([31]). Another influential factor is soil fertility during germination, which directly influences the growth of regenerating individuals ([48]). Therefore, the soil is a determining factor for the spatial distribution of species ([54]) and local species diversity ([52]).
Conclusion
Forest edge effects on species richness and diversity of a forest’s regeneration layer depend on the type of surrounding matrix. The differences that we found between edges associated with different matrices indicate that monitoring the relationship between the fragment’s edge and surrounding matrices is essential to guarantee effective forest preservation and ecosystem service provision, especially in highly fragmented regions such as the Atlantic Forest.
List of abbreviations
Roadside Edge (ERO); Forest-side Edge (EFO); Fragment Interior (INT); Diameter at Breast Height (DBH); Canopy Openness (CO); Potential of Hydrogen (ph); Calcium (Ca); Magnesium (Mg); Potassium (K); Sodium (Na); Phosphorus (P); Nitrogen (N); Potential Acidity (H+Al); Soil Organic Matter (SOM); Sum of Bases (SB); Cation Exchange Capacity (CEC); Carbon (C); Soil Penetration Resistance (PR); Soil Bulk Density (D); Principal Components Analysis (PCA); Canonical Correspondence Analysis (CCA); Non-metric Multidimensional Scaling (NMDS); Number of Individuals (NI), Relative Dominance (RDo), Relative Frequency (RF), Relative Density (RD), and Per Cent Importance Values (IV).
Author Contributions
LPG: methodology, formal analysis, investigation, data curation, writing (original draft), visualization, project administration; PBD: methodology, formal analysis, visualization, writing (review and editing); HMD: conceptualization, methodology, investigation, resources, writing (review and editing), supervision, project administration, funding acquisition; SHK: conceptualization, methodology, writing (review and editing), supervision.
Acknowledgments
This study was partly funded by the Coordination for the Improvement of Higher Education Personnel (CAPES) - Funding Code 001 and the Espírito Santo State Research Foundation (FAPES), Global Call no. 03/2017 (T.O.169 - SIAFEM: 80709605/18). We also acknowledge UFES and NUPEMASE for the logistical support and for the many friends who have assisted us in field data collection. To Mr. Geovane S. Siqueira for his assistance and support in plant identification at Vale’s herbarium (CVRD) and the Núcleo de Gestão Integrada - São Mateus / ICMBio for logistical support and permission for research in the Córrego Grande Biological Reserve.
References
CrossRef | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Programa de Pós-Graduação em Botnica, Laboratório de Ecologia e Evolução de Plantas - LEEP, Departamento de Biologia Vegetal, Universidade Federal de Viçosa, Viçosa, Minas Gerais, CEP 36570-900 (Brazil)
Programa de Pós-graduação em Ciências Florestais, Núcleo de Pesquisa Científica e Tecnológica em Meio Ambiente, Silvicultura e Ecologia - NUPEMASE, Universidade Federal do Espírito Santo (Brazil)
Sustanis Horn Kunz 0000-0001-6937-7787
Departamento de Ciências Florestais e da Madeira, Núcleo de Pesquisa Científica e Tecnológica em Meio Ambiente, Silvicultura e Ecologia - NUPEMASE, Universidade Federal do Espírito Santo (Brazil)
Corresponding author
Paper Info
Citation
Pereira Gomes L, Borges Dias P, Machado Dias H, Horn Kunz S (2022). Growing at the forest edges: how natural regeneration develops under fragmentation. iForest 15: 248-255. - doi: 10.3832/ifor3834-015
Academic Editor
Michele Carbognani
Paper history
Received: Apr 01, 2021
Accepted: May 11, 2022
First online: Jul 19, 2022
Publication Date: Aug 31, 2022
Publication Time: 2.30 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2022
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 10119
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 4882
Abstract Page Views: 3065
PDF Downloads: 1713
Citation/Reference Downloads: 4
XML Downloads: 455
Web Metrics
Days since publication: 1297
Overall contacts: 10119
Avg. contacts per week: 54.61
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2022): 3
Average cites per year: 0.75
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Towards a functional phytosociology: the functional ecology of woody diagnostic species and their vegetation classes in Northern Italy
vol. 14, pp. 522-530 (online: 22 November 2021)
Research Articles
Assessing the habitat conservation status by soil parameters and plant ecoindicators
vol. 7, pp. 170-177 (online: 14 February 2014)
Research Articles
Typology and synecology of aspen woodlands in the central-southern Apennines (Italy): new findings and synthesis
vol. 13, pp. 202-208 (online: 19 May 2020)
Research Articles
Classification and mapping of Spanish Mediterranean mixed forests
vol. 12, pp. 480-487 (online: 14 October 2019)
Research Articles
Drought effects on the floristic differentiation of Greek fir forests in the mountains of central Greece
vol. 8, pp. 786-797 (online: 08 April 2015)
Commentaries & Perspectives
The role of plant sociology in the study and management of European forest ecosystems
vol. 6, pp. 55-58 (online: 21 January 2013)
Short Communications
Biodiversity inventory of trees in a neotropical secondary forest after abandonment of shaded coffee plantation
vol. 10, pp. 303-308 (online: 23 February 2017)
Research Articles
Changes in tree layer and altitudinal distribution of herbaceous species in temperate old-growth forests over 30 years
vol. 15, pp. 206-212 (online: 11 June 2022)
Research Articles
The cork oak in the Mountains of Palermo (Italy): ecological insights from the south-eastern edge of its distribution range
vol. 13, pp. 336-344 (online: 07 August 2020)
Research Articles
Influences of mature Pinus nigra plantations on the floristic-vegetational composition along an altitudinal gradient in the central Apennines, Italy
vol. 13, pp. 279-285 (online: 03 July 2020)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword