Drought effects on the floristic differentiation of Greek fir forests in the mountains of central Greece
iForest - Biogeosciences and Forestry, Volume 8, Issue 6, Pages 786-797 (2015)
doi: https://doi.org/10.3832/ifor1214-007
Published: Apr 08, 2015 - Copyright © 2015 SISEF
Research Articles
Abstract
Greek fir (Abies cephalonica Loudon) grows in montane Mediterranean climates characterized by dry, warm summers. Drought is an important climatic feature of these montane ecosystems as it affects the floristic composition, structure and distribution of plant communities. The Oxia-North Vardousia mountain system is one of the few areas in Greece with an extensive, well-preserved Greek fir forest. This study aims at describing the Greek fir forest vegetation of such area and determining the drought-related factors affecting their floristic composition and differentiation. Vegetation relevés were classified and ordinated. The impact of drought-related variables on the vegetation composition was analyzed. A cluster analysis was used to reveal the most important factor for the discrimination of the main plant communities and to determine the drought threshold between them. Two plant communities that reflect the differentiation of the Greek fir forests in central Greece into xerophytic and mesophytic forest communities were described. Each community was divided into two sub-communities. The elevational distribution of Greek fir forests in the mountains of central Greece follows a drought gradient linked to the two main climatic components of drought, precipitation and potential evapotranspiration. The combination of these two drought-related variables into a suitable humidity index was found to adequately differentiate the xerophytic from the mesophytic forest communities and define their drought threshold.
Keywords
Abies cephalonica, Aridity, Habitat Differentiation, Humidity Index, Plant Communities, Synecology, Syntaxonomy, Sterea Ellas
Introduction
Fir (Abies spp.) forests are a distinctive, widespread landscape feature with high ecological and economic importance in the mountains of Greece, especially in the southern and central part of the country. They cover an area of 543 300 ha (16.17% of the total forested area) and comprise 38% of the coniferous forests of Greece ([2]). Three closely related fir species occur in Greece, Abies cephalonica Loudon (Greek fir), A. alba Mill. (silver fir) and their natural hybrid A. × borisii-regis Mattf. ([61], [16]). The endemic Greek fir is the dominant forest tree in the mountains of Peloponnisos (southern Greece) and Sterea Ellas (south-central Greece). In the northern mainland, Greek fir is replaced by A. × borisii-regis. The silver fir can only be found in the northernmost parts of the mainland ([16]). Greek fir is very variable in morphology and often co-occurs with A. × borisii-regis in mixed stands ([51]). Nevertheless, is very difficult to distinguish between the two species. Recent studies on the genetic variation of Greek fir populations have confirmed that the two species cannot be distinguished based on isozyme or molecular markers ([27], [77], [24]). For the purposes of our study it was considered that all fir trees found in the study area belong to the species A. cephalonica s.l.
The phytosociological research of Greek fir forests started with Knapp ([46]), who described two vegetation units on the island of Kefalonia. Since then, many researchers have studied the floristic composition and structure of Greek fir forests and several plant communities have been described from different mountains of southern and central Greece ([4], [23], [8], [44], [55], [22], [95], [47], [74]). For a detailed phytosociological review see Samaras ([73]). However, the ecological causes underlying the floristic differentiation of the Greek fir forests have not yet been studied.
Although Greek fir can be found on soils originating from different bedrock including gneiss, serpentine, flysch, schist, limestone and dolomite, the species shows no association with a specific soil type ([78]). Its natural distribution ranges from 300 to 2300 m a.s.l. ([3], [30]). It forms forests that extend from the middle and upper part of the meso-mediterranean zone (600-900 m a.s.l.) to the lower part of the oro-mediterranean zone (1900-2000 m a.s.l.), with an optimum distribution in the supra- and montane-mediterranean vegetation zones (900-1900 m a.s.l. - [64], [68], [69]). In the area of its natural distribution the mean annual precipitation varies from 500 mm (lower elevations of SE Greece) to more than 1800 mm (western part of south-central Greece - [29]). Greek fir forests receive abundant rainfall during autumn and winter, though may suffer from severe drought in summer ([3]).
Drought is a complex environmental factor having both climatic and soil components. Drought-related variables have been successfully used to explain the distribution of vegetation types on local ([103]), regional ([80]) and on continental to global scales ([79], [28]). Drought seems to play a crucial role in the floristic differentiation of Greek fir forests in southern Greece ([8]), but to which degree and how, is still not known. We hypothesize that drought could affect the floristic composition of the Greek fir forests in central Greece through their differentiation into distinct plant communities.
To test such hypothesis, we chose a representative mountain range in central Greece covered by extensive fir forests, to analyze and quantify the influence of drought on their floristic differentiation. Our objectives were:
- to describe the Greek fir plant communities found in the study area;
- to identify the components of drought that determine the floristic gradients in the Greek fir forests and;
- to quantify the influence of drought by determining a drought threshold among plant communities.
Because tree species composition of a forest is more likely to be influenced by human activities, we analyzed the ground vegetation composition. It has been demonstrated that ground vegetation can serve as an indicator of site conditions ([25]). Furthermore, ground vegetation has been shown to be particularly useful in identifying the ecotones induced by drought ([76], [34], [103]).
Material and methods
Study area
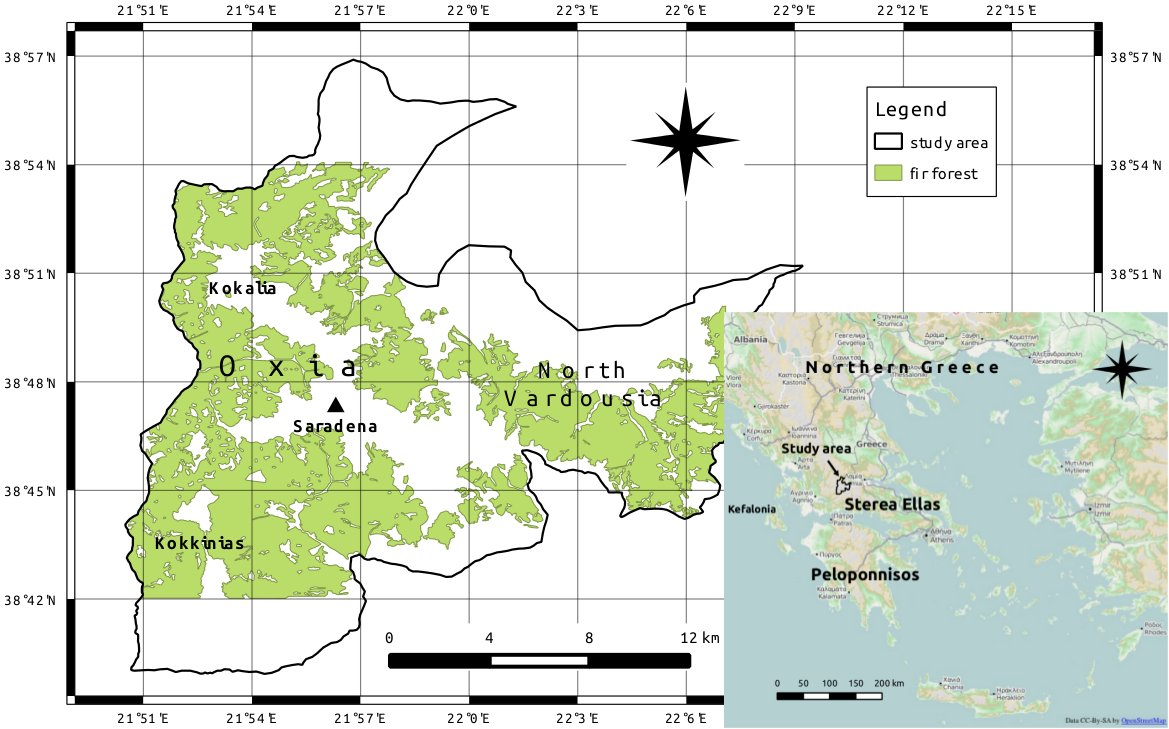
The study area is located in the Oxia-North Vardousia mountain system in south-central Greece (Sterea Ellas). The area is characterized by three mountain ranges (Kokalia, Kokkinias and North Vardousia) reaching 1923 m a.s.l. (summit of Saradena). The ranges lie between 38° 39′ 36″ and 38° 57′ 0″ N of latitude and 21° 50′ 24″ and 22° 9′ 0″ E of longitude, and cover an area of 414 km2 (Fig. 1).
The most abundant forest type in the region is the montane Greek fir coniferous forest, which covers 44.6% of the study area ([73]). Although the fir forest attains its optimum distribution in the supra-mediterranean zone (900-1400 m a.s.l.), it also occurs in the middle and upper part of the meso-mediterranean zone (600-900 m a.s.l. - [73]). In the montane-mediterranean zone (1400-1900 m a.s.l.), the fir forest is one component of a matrix that also includes pastures and forest clearings. In this zone the forest area has declined due to grazing and logging.
The dominant bedrock on the Oxia-North Vardousia mountain system is flysch, while Jurassic limestones and scree slope (or terrace) deposits occur to a small extent ([43]). The climate is typically Mediterranean with wet, cool winters and dry summers. Nevertheless, there are large local differences due to the complexity and variety of the topography. January is the coldest month with an average minimum temperature between -2.7 and 0.7 °C. The warmest month is July with an average maximum temperature between 23.1 and 28.6 °C. The annual mean temperature ranges between 8.5 and 13.3 °C. Most of the precipitation falls between October and April. Summer rains are scant and irregular, mostly in the form of thunderstorms. The annual precipitation varies from 1090 mm at the lower elevations (~600 m a.s.l.) to 1710 mm at the higher (~1600 m a.s.l. - [73]).
Quantification of drought
There are two basic concepts of drought: the first considers it as a temporary climatic aberration (meteorological anomaly), characterized by a “prolonged and abnormal moisture deficiency” ([65]), while the second describes it as a seasonal climatic feature (also called aridity) occurring in places with well-defined rainy and dry seasons ([87]). For our study we used the second concept.
Three different approaches for drought quantification were followed in this study ([96]):
- the classical approach using simple climatic variables: the main climatic factors related to drought, i.e., global (total) irradiation (Rs), air temperature (T), precipitation (P), reference potential evapotranspiration (PETref), were spatially estimated for the study area and raster maps were generated for different time periods (monthly, driest period, vegetation period, growing season, and annual period - see Tab. 1 for the definitions);
- the second approach using an index obtained through the combination of two or more climatic factors related to drought: we used a modified version of the Transeau’s humidity index (HI - [90]), which combines the two main factors related to drought; the humidity index (HI) was calculated as the ratio of P to PETref (HI = P/PETref);
- the third approach (called “site water balance approach”) considers also the soil components of drought, along with the above climatic factors: in this study both reference actual evapotranspiration (AETref) and water deficit (D) were included in the water balance; water deficit is defined as the difference between PETref and AETref ([79]); for the calculation of the above variables, a monthly version of Thornthwaite’s water balance model was applied ([99], [60], [73]).
Tab. 1 - Climatic parameters and climatic-soil components of water balance used in the ordination (as fitted environmental vectors) and the recursive partitioning analysis (as explanatory variables). The variables were calculated for different time periods: monthly, driest period (the four driest months of the year - June-September), vegetation period (beginning of April until end of October), growing season (daily mean temperature remains above 6 °C), and annual period.
| Group | Variable | Abbreviation | Units |
|---|---|---|---|
| Climatic parameters | Mean air temperature | Tav | °C |
| Minimum air temperature | Tmin | °C | |
| Maximum air temperature | Tmax | °C | |
| Precipitation | P | mm | |
| Global (total) radiation | Rs | Mj/m2 | |
| Reference potential evapotranspiration | PETref | mm | |
| Humidity index | HI | dimensionless | |
| Climatic-soil components of water balance | Reference actual evapotranspiration | AETref | mm |
| Water deficit | D | mm |
To calculate the monthly mean of daily Rs, the “r.sun” model ([38], [85]) was used in the GRASS-GIS software version 6.4.0 ([31]). For the spatial estimation of T and P, ordinary kriging, linear regression and regression-kriging models were used, taking into account the elevation and the spatial correlation of climatic data from 19 weather stations ([73]). The computational steps followed the general framework for geostatistical mapping of environmental variables ([35]). The PETref was calculated by using the empirical equation of Abtew ([1]), which requires maximum air temperature (Tmax) and Rs values, calibrated for the local conditions ([75]).
The methods used here for drought quantification took into account the spatial variation of fine-scale physiographic features such as elevation, slope, exposition and topographic shadowing effects. Any changes of these features within a distance of less than 100 m were considered as “micro-scale” changes, whereas those greater than 100 m were considered as “local-scale”.
Sampling design and data collection
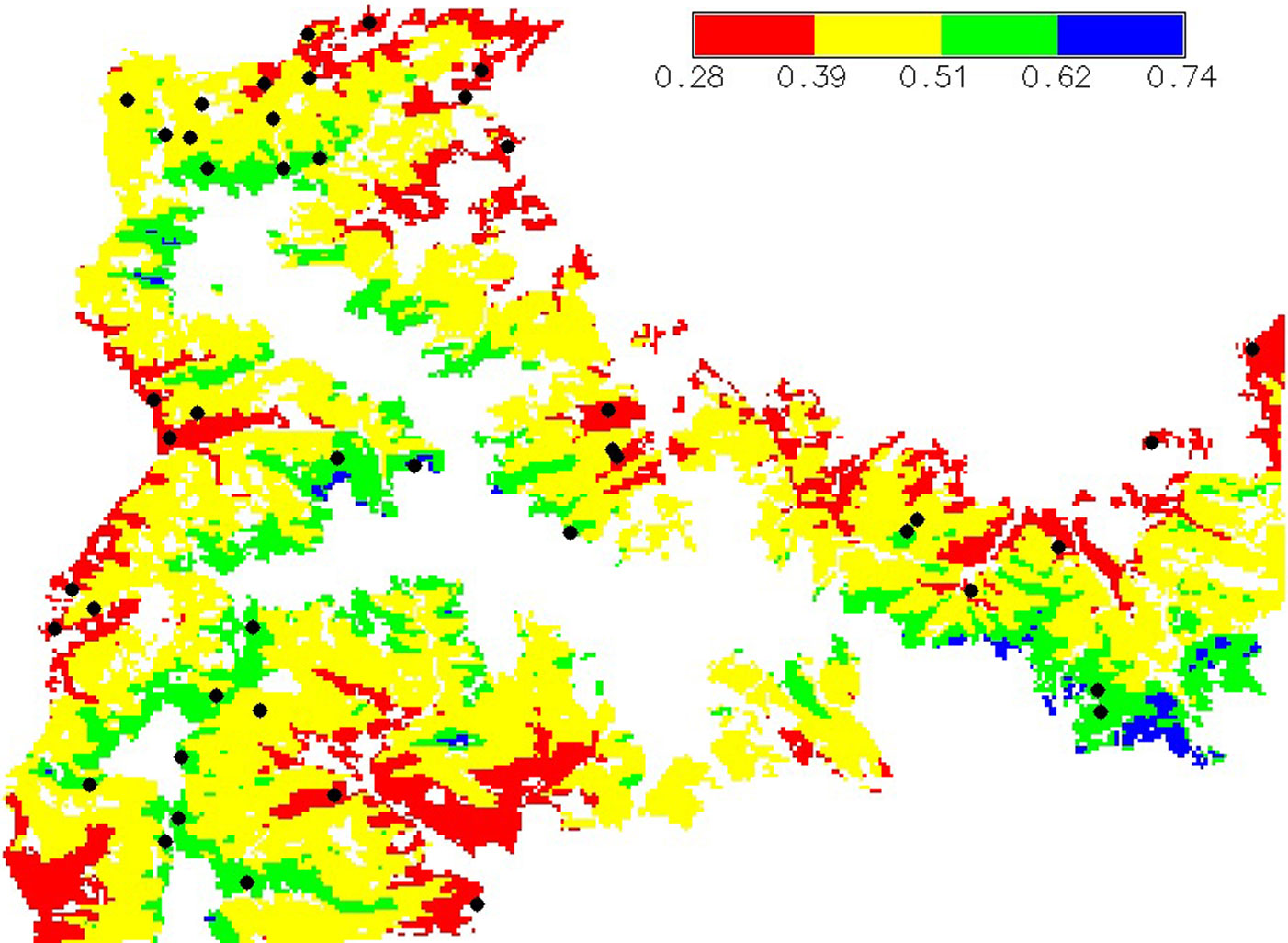
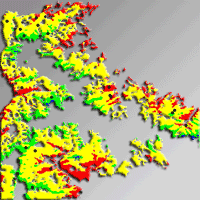
The humidity index (HI) was used to spatially quantify drought and to capture the whole range of drought intensity over the study area. The whole area was classified and mapped into four strata based on equal intervals of HI during the vegetation period (HI: 0.28-0.39, 0.39-0.51, 0.51-0.62, 0.62-0.74). The underlying assumption was that the four strata represent forest site conditions, each with a different drought intensity. Based on the map of HI intervals obtained (Fig. 2), a total of 45 locations were randomly selected. At each location a rectangular plot of 200 m2 was sampled. In order to minimize the influence of environmental factors other than climate, only fir forest stands on flysch were sampled. Since no information were available on the anthropogenic disturbances at the different locations, this aspect was neglected.
Fig. 2 - Stratification of the study area based on HI.veg values (humidity index of the vegetation period). Each stratum is indicated with a different color. The black dots indicate the locations of the 45 sampling plots.
In each plot, the species composition (including vascular plants and mosses growing on mineral soil or humus) was recorded. Structural information was obtained by assessing four layers of vegetation cover (moss, herb, shrub and tree). The moss layer included only mosses, while the herb layer included herbaceous and woody species up to 50 cm in height. The woody species between 50 cm and 5 m tall were included in the shrub layer, and those above 5 m in the tree layer. For all species and layers, the cover-abundance was estimated using the extended (9-point) Braun-Blanquet scale ([94]). Plant specimens were collected during the field work and stored in the herbarium of the Laboratory of Forest Botany-Geobotany in the Aristotle University of Thessaloniki, Greece (TAUF). The nomenclature of vascular plants follows, in order of priority, Flora Hellenica ([82], [83]), Exkursionsflora für Kreta ([42]), Mountain Flora of Greece ([84], [81]), Med-Checklist ([32]) and Flora Europaea ([91], [92]). The families Asteraceae, Poaceae and Rosaceae follow the more recent reviews of Euro+Med ([26]). The nomenclature for orchids follows Baumann et al. ([5]), and for mosses Hill et al. ([37]). We based the evaluation of vascular plant ecological traits on Böhling et al. ([15]), Pignatti et al. ([67]) and Guarino et al. ([33]). The nomenclature for the phytosociological units follows Horvat et al. ([39]) and Bergmeier & Dimopoulos ([6], [7]), and in accordance with the International Code of Phytosociological Nomenclature of Weber et al. ([97]).
In each of the 45 sampling plots a pit was dug to expose the soil profile up to 1 m in depth or until the parent material was reached. The rooting depth was measured and the soil skeleton content was estimated. Undisturbed soil samples were taken using soil sample rings of 100 and 163 cm3, and analyzed for the determination of the soil water retention curve ([18]). The soil data were used for the calculation of the available soil water storage capacity (ASWSC) of the total rooting space at each site (plot), expressed in mm. For details concernig the soil analysis and the calculation of ASWSC, see Samaras ([73]). Additionally, topographic information was recorded for each plot including elevation, inclination, exposition, slope form and landscape position, as well as environmental conditions (humus depth, cover of litter and cover of exposed rocks and stones). Exposition (measured in degrees) is considered a poor variable for quantitative analysis ([58]) and therefore was transformed prior to data analysis. Two new variables were created, “exposition to the north” and “exposition to the east”, with the use of trigonometric functions ([53]).
Data analysis
The vegetation data was classified based on the floristic composition and species cover values. A hierarchical agglomerative cluster analysis based on Bray-Curtis distance ([11]) was performed. Flexible beta ([52]) was used as a clustering algorithm, with β = -0.25. The original species cover-abundance values of the alpha-numeric extended Braun-Blanquet scale were replaced by the 1-9 Ordinal Transform Scale (OTS) proposed by Van Der Maabel ([93]). In order to reduce the noise in the dataset, all infrequent taxa (occurring in only one or two plots) were discarded from the analysis. Data were organized using the database managing system TVSBPWFH ([36]). Cluster analysis was performed using the packages “cluster” ([54]), “vegan” ([63]) and “labdsv” ([71]) of the R software, version 2.12.2 ([70]).
For the identification of the diagnostic taxa of vegetation units, fidelity values were calculated for each species using a modification of the Φ index (phi coefficient of association - [88]). Species presence/ absence values were used as they provide more robust estimates of fidelity compared with cover values ([13], [100]). The statistical significance of fidelity values was assessed by 1000 permutations of the species data ([20]). The fidelity value of each taxon was computed for each vegetation unit separately, compared with all others in the dataset, as well as for all possible combinations of units against the remaining units ([89], [21]). The R package “indicspecies” ([19]) was used for this purpose.
Non-Metric Multidimensional Scaling (NMDS - [49], [50]) was applied to a Bray-Curtis distance matrix to identify the main vegetation gradients. The NMDS is considered the method of choice for ecological community data due to its flexibility and robustness ([45], [59], [17], [72], [57]). According to McCune & Grace ([57]), it does not require the normal distribution of the data nor linear relationships among variables, relieving the “zero-truncation” problem. NMDS allows the use of any distance measure (including semi-metric like Bray-Curtis) and preserves the ordering relationships (rank order) among objects. Additionally, NMDS tends to linearize the relationship between distances measured in species space and distances measured in environmental space ([57]). To identify the underlying environmental gradients, the environmental variables (Tab. 1, Tab. 2, Appendix 1) were fitted on the ordination axis. The significance of the relationships between the NMDS axis and the environmental variables was assessed with 1000 random permutations. We conducted the analysis with the functions “metaMDS” and “envfit” of the R package “vegan” ([63]).
Tab. 2 - Topographic, structural and environmental/abiotic conditions (mean ± standard deviation) of each vegetation unit.
| Variable | Community A |
Sub-comm. A1 |
Sub-comm. A2 |
Community B |
Sub-comm. B1 |
Sub-comm. B2 |
|---|---|---|---|---|---|---|
| Elevation (m a.s.l) | 973 ± 154 | 928 ± 127 | 1005 ± 168 | 1375 ± 143 | 1401 ± 152 | 1303 ± 85 |
| Inclination (%) | 52 ± 16 | 45 ± 17 | 56 ± 14 | 46 ± 17 | 50 ± 15 | 33 ± 19 |
| Cover of tree layer (%) | 65 ± 10 | 71 ± 11 | 61 ± 8 | 67 ± 14 | 65 ± 16 | 73 ± 6 |
| Cover of shrub layer (%) | 19 ± 13 | 27 ± 14 | 13 ± 7 | 11 ± 10 | 12 ± 11 | 7 ± 3 |
| Cover of herb layer (%) | 31 ± 24 | 44 ± 30 | 22 ± 14 | 34 ± 22 | 31 ± 23 | 42 ± 18 |
| Cover of moss layer (%) | 29 ± 28 | 28 ± 31 | 31 ± 27 | 8 ± 7 | 8 ± 7 | 7 ± 9 |
| Total vegetation cover (%) | 80 ± 10 | 89 ± 8 | 74 ± 7 | 81 ± 9 | 80 ± 9 | 85 ± 5 |
| Cover of litter (%) | 28 ± 23 | 37 ± 25 | 21 ± 20 | 43 ± 29 | 33 ± 22 | 72 ± 29 |
| Cover of exposed rocks and stones (%) | 20 ± 21 | 23 ± 22 | 18 ± 22 | 22 ± 18 | 28 ± 17 | 4 ± 1 |
| Height of highest trees (m) | 18 ± 3 | 20 ± 3 | 17 ± 3 | 21 ± 4 | 21 ± 4 | 22 ± 6 |
| Diameter of highest trees (cm) | 45 ± 10 | 42 ± 8 | 47 ± 10 | 52 ± 23 | 51 ± 20 | 56 ± 32 |
| Humus depth (cm) | 3 ± 2 | 3 ± 2 | 4 ± 2 | 4 ± 3 | 4 ± 3 | 2 ± 1 |
| Soil depth (cm) | 66 ± 31 | 79 ± 33 | 57 ± 28 | 75 ± 27 | 78 ± 26 | 67 ± 32 |
| ASWSC (mm) | 92 ± 65 | 119 ± 76 | 73 ± 52 | 128 ± 66 | 122 ± 56 | 146 ± 93 |
To determine the influence of each drought related variable on vegetation patterns, recursive partitioning based on species composition was used to derive classification trees for the categorical response variable ([12]). A non-parametric conditional inference tree approach was used ([40]) to explain the variation in the response variables (plant communities) as a function of the explanatory (environmental) variables, implemented in the R package “party” ([41]). At each step of the analysis, one explanatory variable was selected from all the available variables (Tab. 1, Tab. 2, Appendix 1), based on the best separation of two homogeneous groups using a permutation test. The split point was determined by a numerical value (threshold) of the explanatory variable. The relationships between the response and explanatory variables were presented in a dichotomous tree diagram, where nodes represent split points, while branches connecting nodes and leaves or terminal nodes represent the final groups.
Results
Floristic differentiation of the Greek fir forest
The classification of the vegetation plots analyzed revealed two distinct forest communities (indicated as A and B in Tab. 3), clearly distinguishable by their floristic and ecological characteristics (Appendix 2).
Tab. 3 - Synoptic table of the fir forest vegetation units (communities and sub-communities) in the study area based on cluster analysis (flexible beta with β = -0.25, Bray-Curtis distance). Frequency values of taxa are displayed. Only the diagnostic taxa are presented with their fidelity values (Φ) and their statistical significances. (*): p < 0.05, (**): p < 0.01, (***): p < 0.001. The frequency values of taxa considered as diagnostic for the vegetation units are reported in italic. Woody species occurring in different layers are referred to with a letter (t = tree; s = shrub; h = herb). Mosses are referred to with the letter m. Communities and sub-communities: (A): Crepis fraasii-Abies cephalonica comm.; (A1): Sub-comm. with Castanea sativa; (A2): Sub-comm. with Trifolium grandiflorum; (B): Sanicula europaea-Abies cephalonica comm.; (B1): Sub-comm. with Silene multicaulis ssp.’€‰multicaulis; (B2): Sub-comm. with Rubus hirtus; (C): Common diagnostic taxa of two or more vegetation units.
| Comm. (subcomm.) |
Species | Form | A (n=22) | B (n=23) | Φ | ||
|---|---|---|---|---|---|---|---|
| A1 (n=9) | A2 (n=13) | B1 (n=17) | B2 (n=6) | ||||
| (A) | Crepis fraasii | - | 67 | 92 | 6 | 0 | 0.778*** |
| Quercus frainetto | t | 44 | 23 | 0 | 0 | 0.451* | |
| Quercus frainetto | s | 56 | 54 | 6 | 0 | 0.571** | |
| Quercus frainetto | h | 100 | 100 | 24 | 33 | 0.746*** | |
| Juniperus oxycedrus ssp. oxycedrus | s | 78 | 92 | 24 | 0 | 0.733*** | |
| Hypnum cupressiforme var. cupressiforme | m | 100 | 92 | 65 | 33 | 0.528** | |
| Thymus longicaulis ssp. chaubardii | - | 67 | 85 | 29 | 17 | 0.526** | |
| Homalothecium aureum | m | 67 | 92 | 41 | 17 | 0.507** | |
| Trifolium patulum | - | 44 | 46 | 6 | 0 | 0.495* | |
| Galium mollugo agg. | - | 56 | 62 | 18 | 17 | 0.427* | |
| (A1) | Rosa arvensis | h | 67 | 0 | 0 | 0 | 0.775*** |
| Scutellaria columnae ssp. columnae | - | 56 | 0 | 0 | 0 | 0.696** | |
| Rubus canescens | s | 44 | 0 | 0 | 0 | 0.612** | |
| Hedera helix ssp. helix | s | 33 | 0 | 0 | 0 | 0.522** | |
| Hedera helix ssp. helix | h | 56 | 15 | 0 | 0 | 0.572** | |
| Viola alba ssp. alba | - | 56 | 0 | 0 | 17 | 0.563** | |
| Cyclamen hederifolium | - | 33 | 0 | 0 | 0 | 0.522* | |
| Asplenium onopteris | - | 44 | 15 | 0 | 0 | 0.477* | |
| Platanthera montana | - | 33 | 0 | 6 | 0 | 0.457* | |
| Ruscus aculeatus | s | 22 | 0 | 0 | 0 | 0.42* | |
| Ruscus aculeatus | h | 33 | 8 | 0 | 0 | 0.439* | |
| Castanea sativa | s | 56 | 15 | 6 | 17 | 0.439* | |
| Carex flacca ssp. serrulata | - | 22 | 0 | 0 | 0 | 0.42* | |
| Scabiosa sp. | - | 22 | 0 | 0 | 0 | 0.42* | |
| Trifolium heldreichianum | - | 22 | 0 | 0 | 0 | 0.42* | |
| (A2) | Trifolium grandiflorum | - | 22 | 100 | 0 | 0 | 0.87*** |
| Origanum vulgare ssp. hirtum | - | 11 | 85 | 0 | 0 | 0.821*** | |
| Myosotis ramosissima ssp. ramosissima | - | 0 | 85 | 12 | 0 | 0.817*** | |
| Anisantha sterilis | - | 0 | 69 | 0 | 0 | 0.792*** | |
| Trifolium arvense | - | 0 | 69 | 6 | 0 | 0.746*** | |
| Cistus creticus ssp. creticus | h | 11 | 69 | 0 | 0 | 0.708*** | |
| Trifolium physodes | - | 44 | 100 | 29 | 0 | 0.658*** | |
| Cerastium brachypetalum ssp. roeseri | - | 33 | 100 | 47 | 0 | 0.637*** | |
| Festuca circummediterranea | - | 0 | 62 | 18 | 0 | 0.605** | |
| Cynosurus echinatus | - | 0 | 54 | 12 | 0 | 0.584** | |
| Vicia lathyroides | - | 22 | 77 | 24 | 0 | 0.579** | |
| Trifolium scabrum | - | 0 | 38 | 0 | 0 | 0.565** | |
| Trifolium ochroleucon | - | 0 | 46 | 12 | 0 | 0.52* | |
| Anthyllis vulneraria ssp. bulgarica | - | 0 | 31 | 0 | 0 | 0.5* | |
| Chamaecytisus austriacus | - | 0 | 31 | 0 | 0 | 0.5* | |
| Lathyrus digitatus | - | 0 | 31 | 0 | 0 | 0.5* | |
| Leontodon tuberosus | - | 0 | 31 | 0 | 0 | 0.5* | |
| Torilis arvensis | - | 0 | 31 | 0 | 0 | 0.5* | |
| Syntrichia ruralis | m | 0 | 31 | 0 | 0 | 0.5* | |
| Asplenium adiantum-nigrum | - | 33 | 69 | 18 | 0 | 0.493* | |
| Cota tinctoria ssp. parnassica | - | 22 | 62 | 18 | 0 | 0.48* | |
| Hypericum spruneri | - | 33 | 69 | 24 | 0 | 0.469* | |
| Neotinea maculata | - | 11 | 46 | 12 | 0 | 0.442* | |
| (B) | Epilobium lanceolatum | - | 0 | 23 | 88 | 67 | 0.663*** |
| Poa nemoralis ssp. nemoralis | - | 11 | 0 | 88 | 50 | 0.657*** | |
| Sanicula europaea | - | 33 | 8 | 88 | 83 | 0.654*** | |
| Viola reichenbachiana × riviniana | - | 11 | 0 | 47 | 83 | 0.624*** | |
| Fragaria vesca | - | 0 | 0 | 53 | 50 | 0.589** | |
| Lapsana communis | - | 11 | 15 | 71 | 67 | 0.563** | |
| Lactuca muralis | - | 56 | 46 | 94 | 100 | 0.526** | |
| Pteridium aquilinum ssp. aquilinum | - | 44 | 38 | 82 | 100 | 0.526** | |
| Geum urbanum | - | 0 | 0 | 35 | 50 | 0.521* | |
| Geocaryum capillifolium | - | 22 | 15 | 76 | 50 | 0.452* | |
| (B1) | Silene multicaulis ssp. multicaulis | - | 0 | 8 | 82 | 0 | 0.827*** |
| Rumex acetosella ssp. acetoselloides | - | 0 | 15 | 59 | 0 | 0.598** | |
| Arabis alpina ssp. caucasica | - | 0 | 0 | 35 | 0 | 0.539** | |
| Myosotis sylvatica ssp. cyanea | - | 56 | 31 | 100 | 50 | 0.48* | |
| Arrhenatherum elatius | - | 11 | 0 | 47 | 17 | 0.42* | |
| (B2) | Geranium robertianum ssp. purpureum | - | 22 | 8 | 12 | 100 | 0.78*** |
| Rubus hirtus | h | 11 | 0 | 12 | 83 | 0.742*** | |
| Moehringia trinervia | - | 0 | 0 | 6 | 50 | 0.6** | |
| Bromopsis riparia | - | 0 | 0 | 29 | 67 | 0.576*** | |
| Ilex aquifolium | s | 0 | 0 | 0 | 33 | 0.522* | |
| Polystichum lonchitis | - | 0 | 0 | 0 | 33 | 0.522* | |
| Tephroseris integrifolia ssp. integrifolia | - | 0 | 0 | 0 | 33 | 0.522* | |
| Epipactis greuteri ssp. preinensis | - | 0 | 0 | 18 | 50 | 0.51** | |
| Calamintha grandiflora | - | 22 | 0 | 24 | 67 | 0.495* | |
| (C) | Dactylis glomerata | - | 100 | 77 | 53 | 0 | 0.671*** |
| Campanula spatulata ssp. spruneriana | - | 78 | 100 | 82 | 17 | 0.657*** | |
| Doronicum orientale | - | 44 | 77 | 82 | 0 | 0.588** | |
| Cynosurus effusus | - | 56 | 85 | 59 | 0 | 0.574** | |
| Juniperus oxycedrus ssp. oxycedrus | h | 56 | 85 | 41 | 0 | 0.526** | |
| Pilosella piloselloides ssp. bauhinii | - | 56 | 77 | 41 | 0 | 0.506* | |
| Cardamine hirsuta | - | 33 | 100 | 82 | 0 | 0.747*** | |
| Carlina biebersteinii et corymbosa | - | 22 | 77 | 71 | 0 | 0.634*** | |
| Pilosella hoppeana ssp. testimonialis | - | 0 | 46 | 29 | 0 | 0.483* | |
| Sedum amplexicaule ssp. tenuifolium | - | 0 | 46 | 29 | 0 | 0.483* | |
| Sanguisorba minor ssp. muricata | - | 0 | 31 | 35 | 0 | 0.445* | |
| Sedum hispanicum | - | 0 | 38 | 24 | 0 | 0.428* | |
Crepis fraasii - Abies cephalonica community
General appearance: the community is comprised of pure stands of Abies cephalonica and mixed stands of A. cephalonica (dominant tree species) with Quercus frainetto and occasionally Castanea sativa. The shrub layer consists mainly of A. cephalonica, Juniperus oxycedrus ssp. oxycedrus and Q. frainetto. Many other woody species appear with low frequency in the shrub layer. Most of them are diagnostic elements of the Ostryo-Carpinion (Fraxinus ornus, Carpinus orientalis ssp. orientalis, Hippocrepis emerus ssp. emeroides etc. - [7]).
Distribution: this community occurs on Mt. Oxia and Mt. Vardousia in the meso-mediterranean and the lower part of the supra-mediterranean zones between 690 and 1360 m a.s.l. It covers the upper, middle and lower sections of gentle to steep slopes (25-90%) in all expositions (Fig. 3b, Fig. 3c).
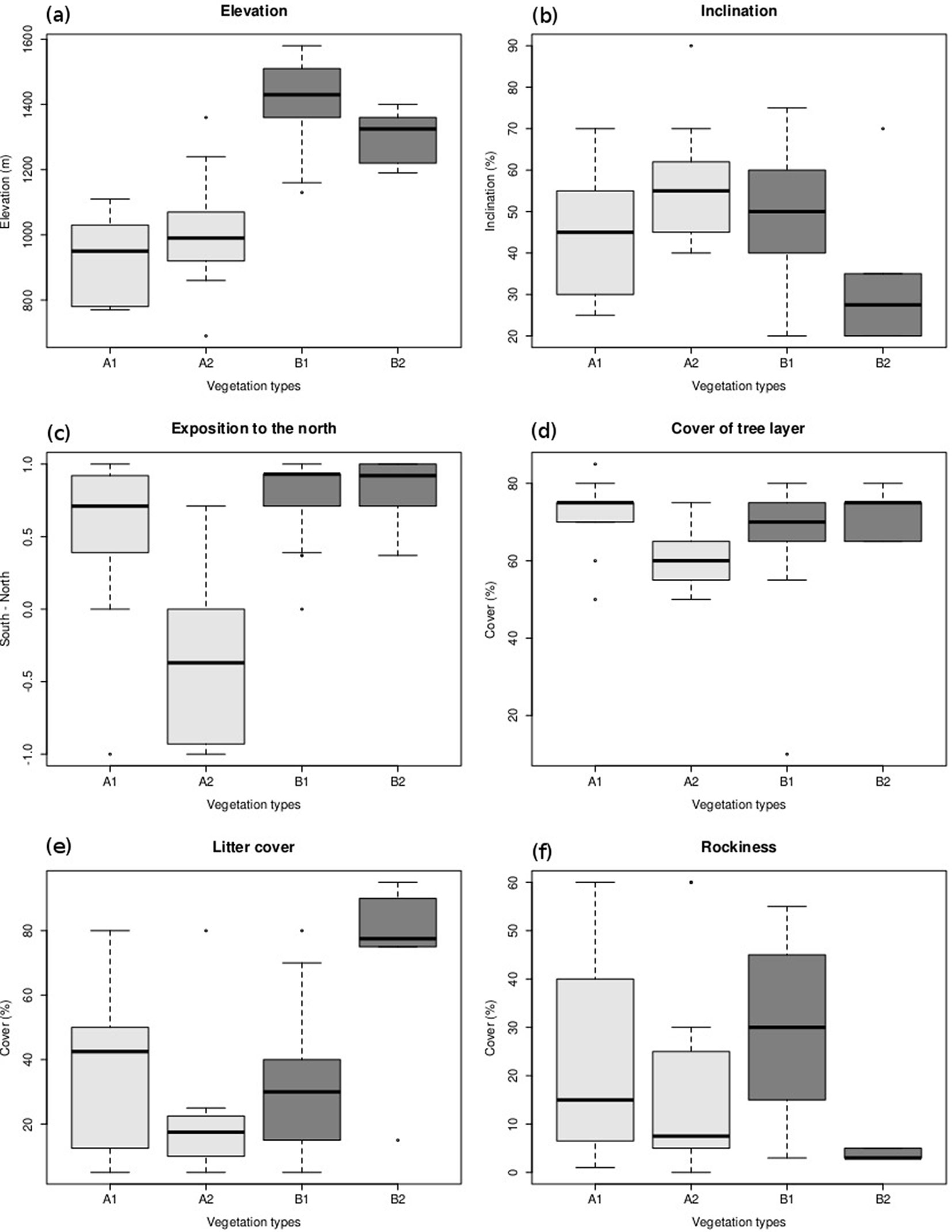
Fig. 3 - Boxplots of the environmental variables in the different vegetation units. Median, mid-spread and range of variables are displayed. For the mid-spread (interquartile range) the 25% and 75% quartiles were used. Whiskers extend up to 1.5 times the interquartile range. Outliers are indicated with small circles. Rockiness (f) is the cover of exposed rocks and stones. The exposition to the north (c) is dimensionless, ranging from -1 (south) to 1 (north).
Sub-communities: two sub-communities could be distinguished (A1 and A2 - Tab. 3). The first was characterized by the presence of Castanea sativa and a group of typically semi-shade to semi-light forest plants ([15], [67]). Most of these species (such as Rosa arvensis, Scutellaria columnae ssp. columnae, Rubus canescens, Viola alba ssp. alba, Platanthera montana, Ruscus aculeatus are diagnostic of the sub-mediterranean thermophilous oak woodlands (Quercet(-ea)alia pubescentis - [62], [7]). Abies cephalonica dominates and together with Castanea sativa, Juniperus oxycedrus ssp. oxycedrus and Quercus frainetto forms a moderately dense shrub layer covering up to 45% of the surface (average cover 27% - Tab. 2). This sub-community is restricted to the mountain range of Oxia. The second sub-community (A2) with Trifolium grandiflorum was characterized by a large group of plants that indicate low nutrient availability (Tab. 3). Most of these taxa are semi-light to light-demanding thermophilous plants indicative of grasslands and other open plant communities (Myosotis ramosissima ssp. ramosissima, Trifolium arvense, Cistus creticus ssp. creticus, Festuca circummediterranea, Cynosurus echinatus, Vicia lathyroides, T. scabrum etc. - [62], [15], [67]). Such taxa reflect not only the stand structure but also some site characteristics which differ from the previous sub-community (A1). The stands are more open (average cover almost 60% - Tab. 2) and established preferentially on south facing and steep slopes (Fig. 3b, Fig. 3c), though their elevation range does not differ from the previous sub-community (Fig. 3a). The shrub layer is less dense and species-rich as compared with the previous sub-community, and mainly composed by Abies cephalonica and Juniperus oxycedrus ssp. oxycedrus. The shrub layer covers up to 25% of the surface with an average of 13% (Tab. 2). This sub-community is distributed throughout the study area.
Sanicula europaea - Abies cephalonica community
General appearance: this community is made up of pure Abies cephalonica forest stands. In the shrub layer A. cephalonica predominates and other woody species (Juniperus oxycedrus ssp. oxycedrus, Castanea sativa, Fagus sylvatica ssp. sylvatica, Ilex aquifolium, etc.) are only rarely found. It was differentiated from the previous community by a group of species indicating moderately moist to fresh and warm (not hot) conditions.
Distribution: this community can be found on Mt. Oxia and Mt. Vardousia at higher altitude (1130-1580 m a.s.l.) as compared with community A (Fig. 3a). It ranges up to the lower part of the montane-mediterranean zone and reaches the timberline. It is largely restricted to the upper and middle parts of north facing slopes.
Sub-communities: two sub-communities may be distinguished (B1 and B2 - Tab. 3). The first sub-community with Silene multicaulis ssp. multicaulis (B1) was characterized by a small group of diagnostic taxa, while the second sub-community with Rubus hirtus (B2), was differentiated by a larger group of taxa. The distribution of sub-community B2 is restricted to the northwest part of the study area, mainly on gentle slopes with very few rocks and a north to northeast orientation (Fig. 3b, Fig. 3c, Fig. 3f).
Plant communities along the drought gradient
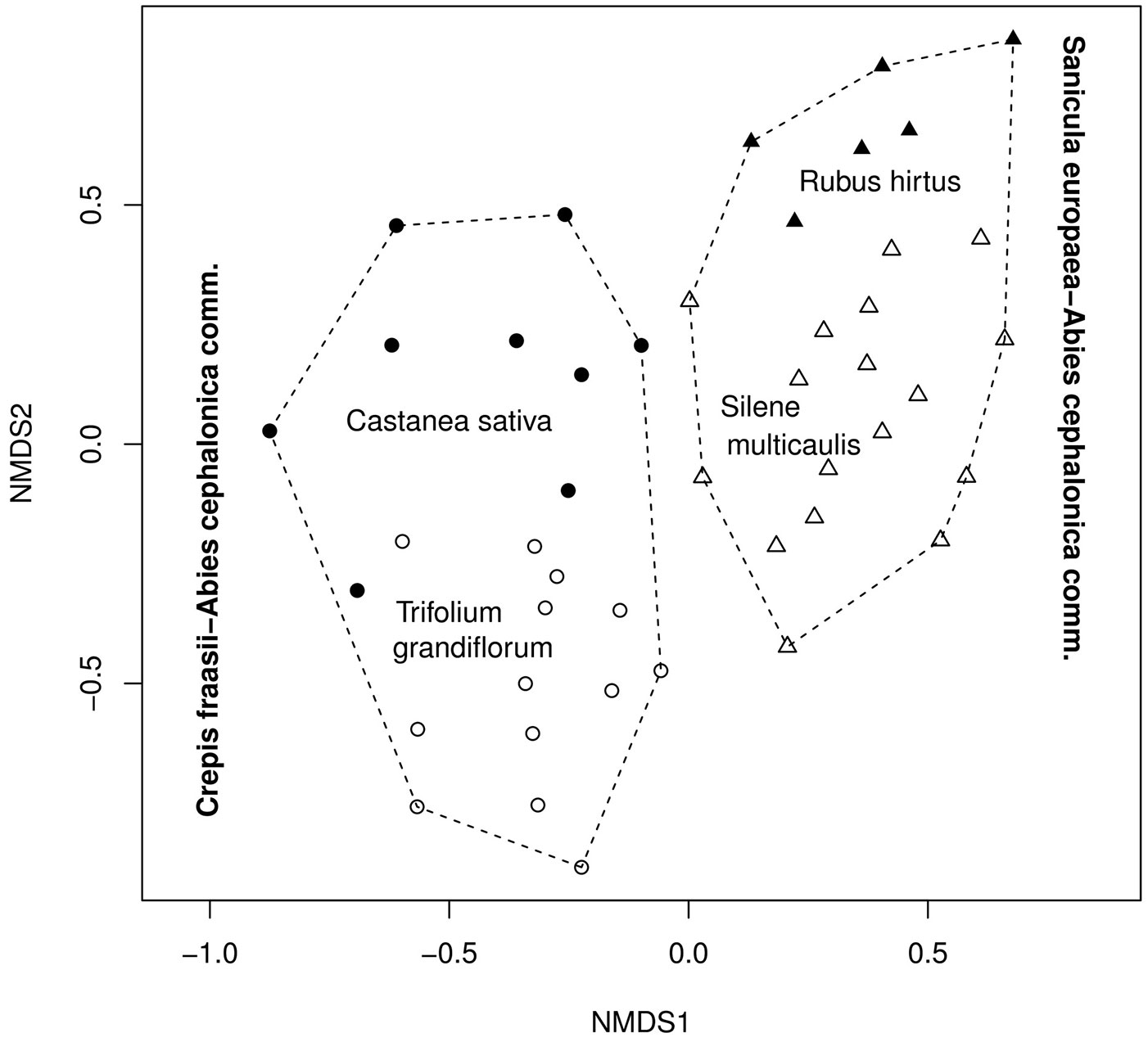
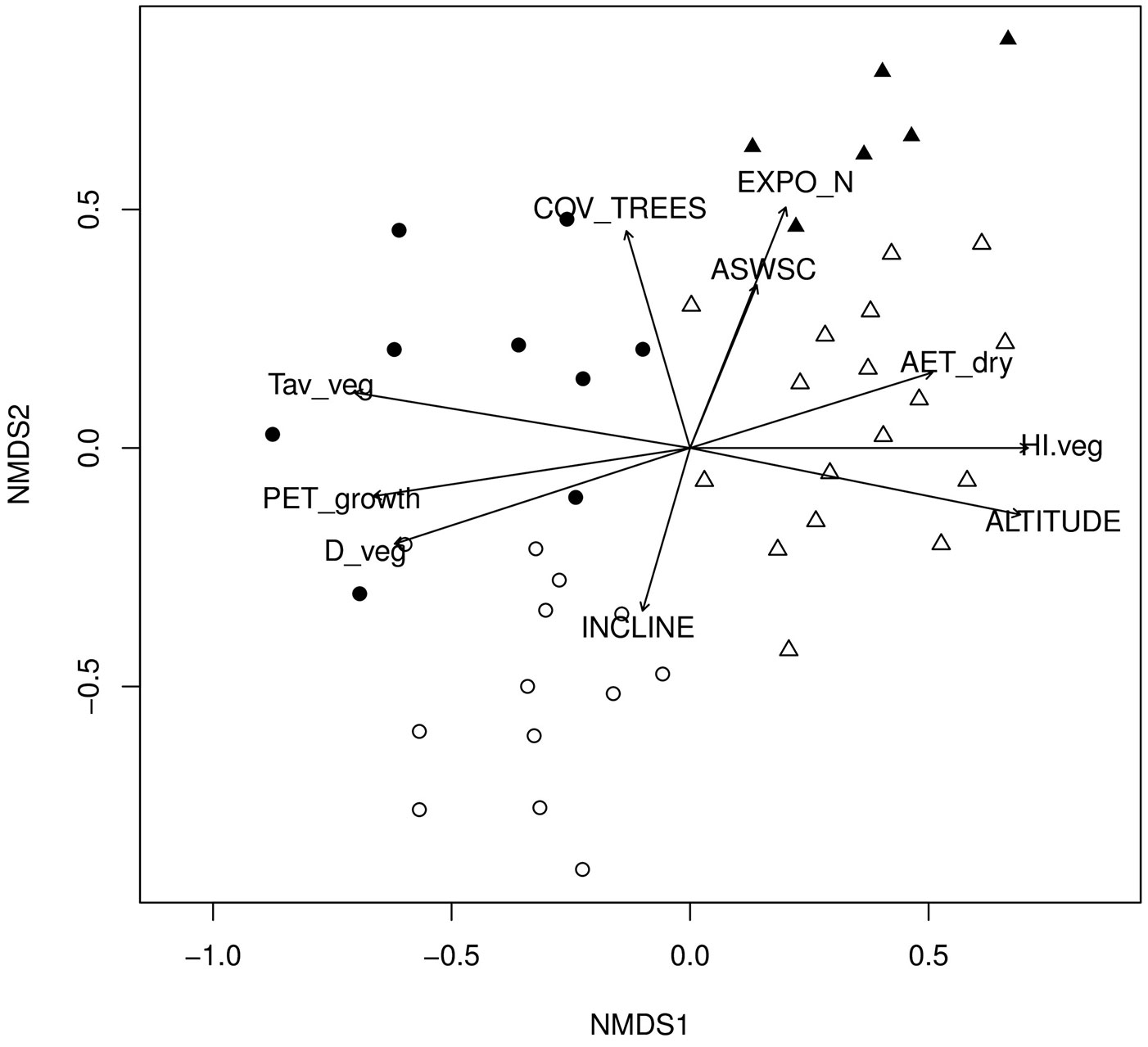
Multivariate analysis on floristic data allowed to identify two main vegetation gradients in the study area. The first floristic gradient was depicted by the horizontal axis (NMDS1) of the ordination diagram displayed in Fig. 4. The two plant communities described above (A: Crepis fraasii-Abies cephalonica and B: Sanicula europaea-Abies cephalonica) appeared well differentiated along the NMDS1 (horizontal) axis. The second floristic gradient, depicted by the vertical axis (NMDS2) of the ordination diagram displayed in Fig. 4, differentiated the sub-communities within the A and B.
Fig. 4 - Distribution of the vegetation units along the two axes (NMDS1, NMDS2) of the ordination (NMDS). The different symbols indicate site groups (vegetation units) formed by the cluster analysis. The envelopes comprise the two main plant communities A and B. The stress for the solution with two axes is equal to 13.6.
The underlying ecological gradients were detected by fitting the different environmental variables onto the ordination scores (Fig. 5). The direction of the environmental vectors (Fig. 5) and their coefficients of determination (Tab. 4, Appendix 1) indicate the direction and the strength of the ecological gradients respectively. The first floristic gradient (NMDS1) reflects a climatic gradient related to drought. Almost all of the climatic (T, P, PETref, HI) and the edapho-climatic (D, AETref) components of drought were strongly correlated with the horizontal axis (Fig. 5, Tab. 4). Temperature and precipitation showed the highest correlation with the horizontal axis for all the periods analyzed (driest, vegetation and annual), except for the growing season (Tab. 4, Appendix 1). The left part of the ordination diagram depicted in Fig. 5, occupied by the community A (Crepis fraasii-Abies cephalonica), represents areas with low elevation and low humidity, while the right part, occupied by the community B (Sanicula europaea-Abies cephalonica), represents the more humid areas of the high-altitude fir forest (Fig. 4, Fig. 5).
Fig. 5 - Projection of the environmental variables (as vectors) on the ordination axes (NMDS). The direction and strength of the gradients is represented by the direction and length of the vectors respectively. For the abbreviations of the environmental variables and their coefficients of determination see Tab. 4.
Tab. 4 - Relationships between the species ordination’s scores (NMDS) and the fitted environmental variables. Coefficients of determination (r2) and their significances assessed using 1000 random permutations. Only variables with significant coefficients are shown. (*): p < 0.05; (**): p < 0.01; (***): p < 0.001.
| Variables | Abbreviations | r2 |
|---|---|---|
| Elevation (m a.s.l.) | ALTITUDE | 0.843 *** |
| Inclination (%) | INCLINE | 0.205 * |
| Exposition to the north | EXPO_N | 0.487 *** |
| Cover of tree layer (%) | COV_TREES | 0.394 *** |
| Available soil water storage capacity (mm) | ASWSC | 0.215 ** |
| Solar radiation during growing period (Mj/m2) | Rs_growth | 0.522 *** |
| Maximum air temperature during driest period (°C) | Tmax_dry | 0.857 *** |
| Mean air temperature during vegetation period (°C) | Tav_veg | 0.856 *** |
| Precipitation during vegetation period (mm) | P_veg | 0.852 *** |
| Reference actual evapotranspiration during driest period (mm) | AET_dry | 0.462 *** |
| Reference potential evapotranspiration during growing period (mm) | PET_growth | 0.753 *** |
| Humidity index during driest period | HI_dry | 0.824 *** |
| Humidity index during vegetation period | HI_veg | 0.839 *** |
| Water deficit during driest period (mm) | D_dry | 0.690 *** |
| Water deficit during vegetation period (mm) | D_veg | 0.696 *** |
Differences in topography (exposition and inclination - Fig. 3b, Fig. 3c), soil water availability (ASWSC - Tab. 2) and stand structure (cover of tree layer - Fig. 3d) were found between the sub-communities A1 and A2. These structural, topographic and edaphic changes along the vertical axis result in specific micro-climatic conditions that differentiate the two sub-communities at a micro-scale (a scale of tens of meters). In the case of the community B (Sanicula europaea-Abies cephalonica), the structural differentiation between its two sub-communities B1 and B2 was less apparent (Fig. 3d). The main topographic difference between B1 and B2 was related to slope steepness (Fig. 3b). Despite their dissimilarity in rockiness (Fig. 3f), their difference in ASWSC was very small (Tab. 2). However, higher accumulation of litter on the surface was detected for B2 (Rubus hirtus sub-community - Fig. 3e).
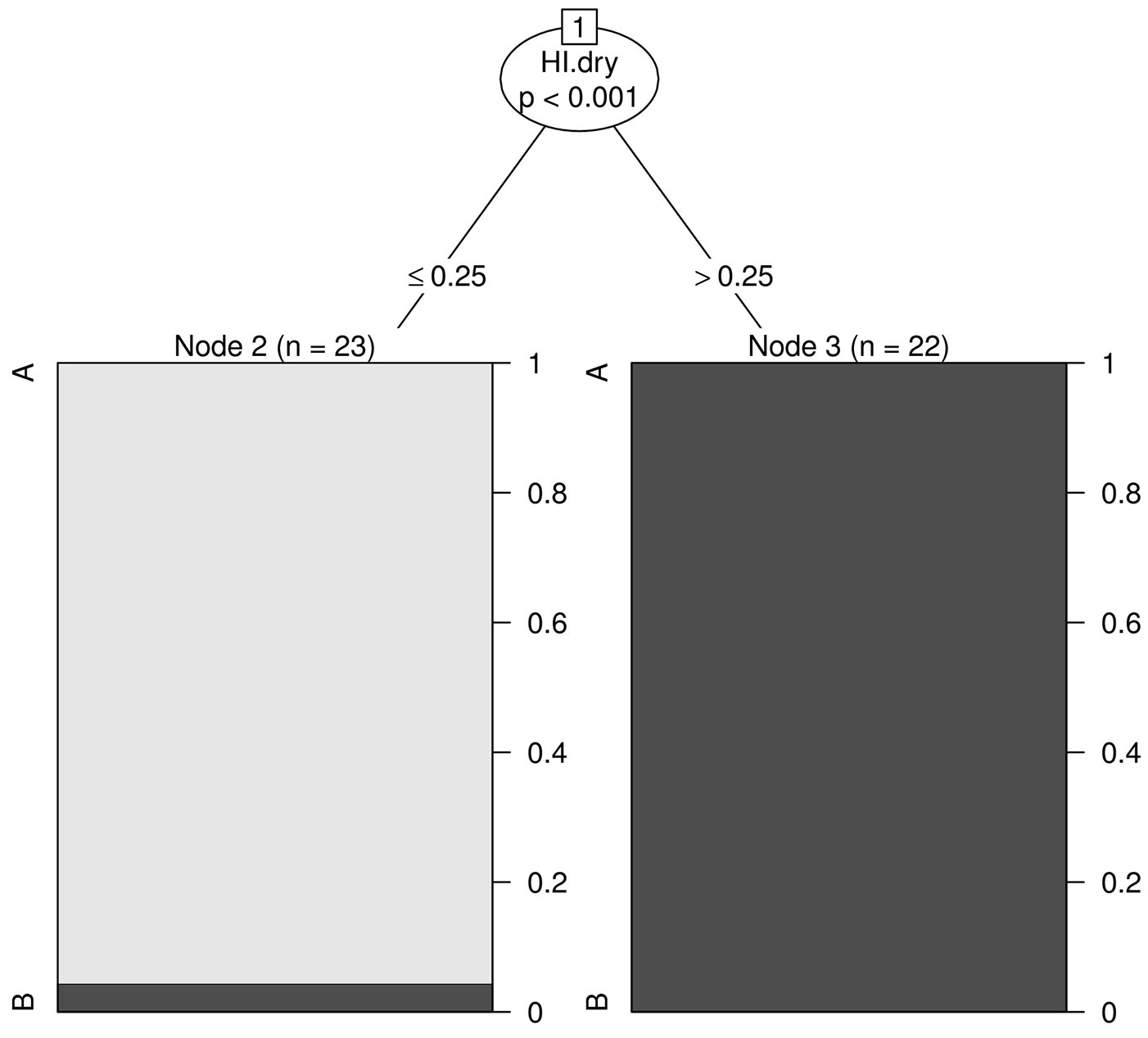
Threshold values of drought
Recursive partitioning based on the HI during the driest period (HI.dry) revealed the existence of two groups of plots (Fig. 6). HI.dry was the best discriminating variable among all the factors analyzed (p-value < 0.001). The first group (Fig. 6, left panel) included all plots where HI of the driest period was less or equal to 0.25 (all plots from community A plus one plot from community B). The second group (Fig. 6, right panel) included all plots where HI of the driest period was higher than 0.25 (the majority of the plots belonging to the B community, except for one that was misclassified). No further plot subgrouping was detected using any of the remaining environmental variables analyzed in this study.
Fig. 6 - Classification tree for the two fir forest communities (A: Crepis fraasii-Abies cephalonica comm.; B: Sanicula europaea - Abies cephalonica comm.) based on the environmental variables. The drought threshold is indicated by HI.dry (humidity index of driest period). The vertical axis show the proportions of each community in the relative group. Level of significance α = 0.05.
Discussion
Syntaxonomy of the Greek fir forests
The Crepis fraasii-Abies cephalonica community has many floristic elements in common with the association Trifolio grandiflori-Abietetum borisii-regis [4]. This association was described by Barbéro & Quézel ([4]) from the mountains of Oxia, Timfristos and South Vardousia and confirmed later by Dimitrellos ([22]) and Vlachos ([95]) for Timfristos and South Vardousia, respectively. The actual distribution of the association is still unknown, though it seems restricted to the northern and western part of Sterea Ellas. The small number of relevés used by Barbéro & Quézel ([4]), Dimitrellos ([22]) and Vlachos ([95]) to describe this association (14-15 in each study) did not allow a full delineation of the Trifolio grandiflori-Abietetum borisii-regis. From the character species of the association (Abies borisii-regis, Trifolium grandiflorum, Trifolium aurantiacum, Luzula forsteri, Helleborus odorus ssp. cyclophyllus and Cicer montbretii), given by Barbéro & Quézel ([4]), only Trifolium grandiflorum has a diagnostic value for the sub-community A2, but it is almost absent from the more dense north facing stands represented by the sub-community A1 with Castanea sativa. Therefore, despite their floristic similarity and geographic closeness, we decided to describe this fir forest type of the lower elevation zone as a rankless Crepis fraasii-Abies cephalonica community (and not an association).
The Sanicula europaea-Abies cephalonica community shares many floristic elements with the association Lilio chalcedonicae-Abietetum cephalonicae Barbéro & Quézel ([4]). This association was first described by Barbéro & Quézel ([4]) from different mountains of Peloponnisos and later confirmed by several authors not only in southern Greece ([23], [8]), but also in central Greece ([44], [22], [95], [47]). Several taxa have been described by the above authors as “character or diagnostic species” of the Lilio chalcedonicae-Abietetum cephalonicae (Lilium chalcedonicum, Calamintha grandiflora, Ribes uva-crispa, Polygonatum multiflorum, Scilla bifolia, Stellaria cupaniana, Galium taygeteum, Digitalis ferruginea etc.). Most of these species were recorded in only one or few locations and should therefore be considered as local diagnostic taxa. Lilium chalcedonicum is the only species that is present on almost all of the sites where the above association is described. In this study, Lilium chalcedonicum was not detected in any of the plots, though it occurs in the study area. The species grows mainly in forest gaps or edges, and not in the interior of forest stands. Common floristic elements can be also found between the Sanicula europaea-Abies cephalonica community and the Abies cephalonica community from the neighboring Mt. Iti ([44]). Taxa with common diagnostic values between the two communities are: Lapsana communis, Pteridium aquilinum ssp. aquilinum and Arrhenatherum elatius. The Abies cephalonica community, though very similar to the association Lilio chalcedonicae-Abietetum cephalonicae, is also distinguished by the absence of Lilium chalcedonicum. Based on the above considerations, we decided to describe this fir forest type of the higher elevation zone as a rankless Sanicula europaea-Abies cephalonica community (and not an association).
Horvat et al. ([39]) assigned all fir forests of southern Greece to the Abietion cephalonicae alliance without providing any character species group. Barbéro & Quézel ([4]) assigned the fir and black pine forests of southern Greece to the Abieti-Pinion alliance. The names Abietion cephalonicae and Abieti-Pinion have been alternatively used since then by several authors and are now considered as synonyms. Following Bergmeier ([8]), the name Abietion cephalonicae [39] was adopted and used in this study to describe the fir forests of the area. The presence of many diagnostic taxa of the order Quercetalia pubescenti-petraeae Klika 1933 in the two communities analyzed (Appendix 2) indicates their close floristic relation to the thermophilous deciduous forests. At the class level, there is an ongoing debate whether to include thermophilous deciduous forests of south-eastern Europe together with beech forests into Querco-Fagetea, or to separate them into distinct classes ([9]). Following the recent syntaxonomical revisions for Greece by Bergmeier & Dimopoulos ([6], [7]), we decided to include the fir forests of central Greece in Quercetea pubescentis Doing-Kraft ex Scamoni et Passarge 1959.
In contrast to the aforementioned authors ([39], [4], [8]), Brullo et al. ([14]) included the orophilous communities (above 1500 m a.s.l.) of Greek fir of central Greece in the Berberido creticae-Juniperion foetidissimae alliance of the Pino-Juniperetea class. This class describes the open coniferous woodlands and shrublands of the supra- to oro-Mediterranean zones characterized by the lack of nemoral species and does not apply to the more or less dense Greek fir forests in the study area. A broad syntaxonomic review of all fir forests in Greece is needed to relate all fir forest vegetation units to a wider phytogeographical background, giving a better understanding of their floristic differentiation.
A syntaxonomic synopsis for the communities analyzed in this study is given below:
Class: Quercetea pubescentis Doing-Kraft ex Scamoni et Passarge 1959
Order: Quercetalia pubescenti-petraeae Klika 1933
Alliance: Abietion cephalonicae [39]
1. Community: Crepis fraasii-Abies cephalonica comm.
- Sub-community: with Castanea sativa
- Sub-community: with Trifolium grandiflorum
2. Community: Sanicula europaea-Abies cephalonica comm.
- Sub-community: with Silene multicaulis ssp.multicaulis
- Sub-community: with Rubus hirtus
Synecology of the Greek fir forests
The spatial scale of the analysis should be taken into consideration for any ecological interpretation. Indeed, it is important to distinguish the environmental factors with relevance only at particular scales of analysis from other variables showing an explanatory power across all scales of analysis ([98]). In this study, two spatial scales should be considered for the investigated area: the local and the micro-scale.
At the local scale, the floristic differentiation of Greek fir forest communities depends largely on climate. Elevation was the main factor affecting the regional climatic water balance, thus determining the patterns of floristic composition within the Greek fir forest. Elevation does not have any direct ecological or physiological impact on species ([66]), but it is highly correlated with precipitation and air temperature. The increase in precipitation and the decrease in temperature with increasing elevation lead to a strong drought gradient. This gradient can be better expressed by the combination of the most important climatic parameters (P, T and PETref) in the Humidity Index (HI).
The HI and other related moisture indexes have been repeatedly used to express the climatic water balance and explain the vegetation distribution ranging from regional ([56], [34]) to global scales ([10], [101]). Our results confirm the suitability of HI for describing the climatic water balance of the Greek fir forests at a local scale, as well as the vegetation gradients and thresholds between Greek fir forest communities.
A principal differentiation of the Greek fir forest vegetation into mesophytic and xerophytic plant communities could be found in our study area. Bergmeier ([8]) found the same pattern and suggested that water supply was the crucial factor driving the floristic variation of coniferous forests and woodlands of Abies cephalonica, Pinus nigra and Juniperus drupacea on Mt. Parnon (southern Greece). Our results confirm the hypothesis that drought affects the floristic composition of the Greek fir forests in central Greece, determining the occurrence of mesophytic and xerophytic plant communities. Patterns of vegetation differentiation along a gradient of increasing drought are likely common in most Greek fir forests of Greece, as they cover a wide elevational range ([23], [8], [44], [22], [95]).
At the micro-scale, the floristic differentiation depends on micro-environmental interactions (micro-climate and soil conditions), since the local water balance is further affected by site-specific variations in topography (exposition, inclination) and available soil water storage capacity (ASWSC). The division of the two main Abies forest types in the study area into sub-communities reflects the occurrence of such micro-climatic conditions. Other abiotic parameters (geology, nutrients) as well as biotic interactions (logging, grazing) could also have a significant impact on vegetation. For example, the detected difference in litter accumulation between the two sub-communities of the Sanicula europaea-A. cephalonica community may suggest a nutrient gradient. A similar gradient, from nutrient-rich to nutrient-poor sites, was found on Mt. Parnon ([8]). For a better understanding of these complex relationships, more micro-scale studies in the Greek fir forests are needed, including a more detailed assessment of soil characteristics and, whenever possible, historic land use records and management plans.
Although historic records can be very difficult to find and interpret, such information would help to clarify the interactions of drought with grazing, logging and fire and their subsequent impact on vegetation patterns. The differences in the tree layer cover between the two sub-communities A1 and A2 of the Crepis fraasii-Abies cephalonica community might be the result of such anthropogenic disturbances. Indeed, the presence of Juniperus oxycedrus (prickly juniper) in the shrub layer of such community indicates site degradation due to anthropogenic activities. Wildfires are common in the thermo- and meso-mediterranean zones, sometimes reaching the lower part of the fir forest zone ([48], [102]). Grazing by sheep and goats is a common practice in the study area. It has been reported that forest fires, overgrazing and illegal logging can lead to degradation and the replacement of forests by prickly juniper shrublands. Where such disturbances have stopped, the degraded land can recover to support a forest with a juniper understory ([86]).
Conclusions
The floristic variation within the Greek fir forest vegetation of the study area reflects a principal differentiation between mesophytic and xerophytic forest communities. This pattern, mainly driven by climatic factors related to elevation, appears on a local scale. On the Oxia-North Vardousia mountain system the wide altitudinal distribution of Greek fir follows a drought gradient influenced by an increase in precipitation and a decrease in potential evapotranspiration. Greek fir follows a similar elevation gradient in all mountains of southern and central Greece. This suggests that the pattern from mesophytic to xerophytic plant communities is likely typical of all Greek fir forests.
The modified Transeau’s humidity index calculated over the driest season appears to be the most suitable variable for the quantification of drought and the climatic water balance on a local scale in central Greece. It is also suitable to delineate the distribution threshold between the mesophytic and xerophytic Greek fir forest communities and to predict their occurrence.
Acknowledgments
The field work was partly funded by the Deutscher Akademischer Austausch Dienst (DAAD) through the IKYDA-programm. We are grateful to Bernd Künemund, Rodrigo Vargas, Cristabel Durán, Osvaldo Vidal and Carl Höcke for their help in the field. Special thanks go to Nikos Alexandris for helping with the climatic analysis, to Günter Gottschlich for verifying or identifying the specimens of the genus Hieracium, to Michael Lüth for the identification of the mosses, to Christina Petschke for her guidance and help during the soil analysis and to Bernhard Thiel for improving the English of the manuscript. We would also like to thank two anonymous reviewers for their useful comments and recommendations.
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Stefanie Gaertner
Albert Reif
Chair of Site Classification and Vegetation Science, Faculty of Environment and Natural Resources, University of Freiburg, D-79085 Freiburg i.Br. (Germany)
Konstantinos Theodoropoulos
Laboratory of Forest Botany-Geobotany, Faculty of Forestry and Natural Environment, Aristotle University of Thessaloniki, GR-54124 Thessaloniki (Greece)
Corresponding author
Paper Info
Citation
Samaras DA, Gaertner S, Reif A, Theodoropoulos K (2015). Drought effects on the floristic differentiation of Greek fir forests in the mountains of central Greece. iForest 8: 786-797. - doi: 10.3832/ifor1214-007
Academic Editor
Paola Mairota
Paper history
Received: Dec 23, 2013
Accepted: Dec 19, 2014
First online: Apr 08, 2015
Publication Date: Dec 01, 2015
Publication Time: 3.67 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2015
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 54749
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 45438
Abstract Page Views: 3022
PDF Downloads: 4737
Citation/Reference Downloads: 25
XML Downloads: 1527
Web Metrics
Days since publication: 3904
Overall contacts: 54749
Avg. contacts per week: 98.17
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2015): 4
Average cites per year: 0.36
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Typology and synecology of aspen woodlands in the central-southern Apennines (Italy): new findings and synthesis
vol. 13, pp. 202-208 (online: 19 May 2020)
Research Articles
Towards a functional phytosociology: the functional ecology of woody diagnostic species and their vegetation classes in Northern Italy
vol. 14, pp. 522-530 (online: 22 November 2021)
Research Articles
Assessing the habitat conservation status by soil parameters and plant ecoindicators
vol. 7, pp. 170-177 (online: 14 February 2014)
Research Articles
Post-fire recovery of Abies cephalonica forest communities: the case of Mt Parnitha National Park, Attica, Greece
vol. 11, pp. 757-764 (online: 15 November 2018)
Research Articles
First results on early post-fire succession in an Abies cephalonica forest (Parnitha National Park, Greece)
vol. 5, pp. 6-12 (online: 06 February 2012)
Commentaries & Perspectives
The role of plant sociology in the study and management of European forest ecosystems
vol. 6, pp. 55-58 (online: 21 January 2013)
Research Articles
The cork oak in the Mountains of Palermo (Italy): ecological insights from the south-eastern edge of its distribution range
vol. 13, pp. 336-344 (online: 07 August 2020)
Research Articles
Influences of mature Pinus nigra plantations on the floristic-vegetational composition along an altitudinal gradient in the central Apennines, Italy
vol. 13, pp. 279-285 (online: 03 July 2020)
Research Articles
Growing at the forest edges: how natural regeneration develops under fragmentation
vol. 15, pp. 248-255 (online: 19 July 2022)
Research Articles
Changes in tree layer and altitudinal distribution of herbaceous species in temperate old-growth forests over 30 years
vol. 15, pp. 206-212 (online: 11 June 2022)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword