Age and thinning effects on elemental composition of Pinus pinaster and Pinus radiata needles
iForest - Biogeosciences and Forestry, Volume 14, Issue 3, Pages 299-306 (2021)
doi: https://doi.org/10.3832/ifor3575-014
Published: Jun 08, 2021 - Copyright © 2021 SISEF
Research Articles
Abstract
The information about the impacts of thinning on the availability of micro-nutrients, as well as macro-nutrients other than N, P and K, is still scarce. We assessed the changes in the concentrations of 12 elements (Al, B, Ca, Cu, Fe, K, Mg, Mn, N, Na, P and Zn) with needle age (current year or 1-year-old) and three early thinning treatments in two of the most widely distributed pines in SW Europe: P. pinaster and P. radiata. Four treatments were setup in triplicate: control (C), light thinning (LT), heavy thinning (HT) and selection thinning of dominant trees (ST), with 0%, 10%, 20% and 20% of total basal area removed, respectively. Needle δ15N varied little with needle age and most thinning treatments in both species, but ST triggered an increase of N in P. pinaster needles. Needle Ca and Na increased with age, but were unaffected by treatment. Foliar K, Zn and Cu decreased with age in both species and increased with ST only in P. pinaster. Jointly considering all treatments, there was no needle age effect on Mn concentration, neither in P. radiata nor in P. pinaster, but in the latter species Mn levels increased with age in the selection thinning plots. There were significant thinning effects on Mn levels in both P. pinaster (ST>C) and P. radiata (HT > LT, ST). Foliar Fe and Al concentration increased with age in both pines; the former increased with ST only in P. pinaster while the latter was affected by thinning only in current year needles and without a clear tendency. Neither age nor treatment effects on needle Mg and B were found, while for P needle age had a significant effect only in P. pinaster.
Keywords
Introduction
As leaves account for a high proportion of nutrients in the aboveground biomass of trees, nutrient concentrations in leaves have been frequently used for assessing the nutritional status of forest stands ([27], [13], [1], [2], [23], [24]).
In forest management, thinning is a common practice aiming to improve or redistribute tree growth, increase timber quality, regulate tree species, ameliorate wildlife habitat and reduce risks due to wildfire, snow breakage or windthrow ([19], [22], [39], [20], [43]). By reducing stem density, thinning alters a variety of abiotic and biotic factors (light, water and nutrient availability; soil temperature; moisture and microbial activities; inter- and intra-specific competition), leading to improve stand growth and productivity ([22], [5], [28], [8], [4]). Besides, thinning has been considered an important management practice for conserving soil fertility ([22]).
Regarding the effects of thinning treatments on forest stands, Chase et al. ([9]) highlighted that the response of trees to nutrient availability (less frequently studied) was higher than that to light availability, at least in Douglas-fir forests. However, most research focused on N, P, and K as major limiting nutrients for forest growth ([29], [21], [38]), as well as on foliar Ca and Mg ([27]). To assess the effects of thinning on N cycling, both the leaf N concentration ([22]) and the foliar δ15N have been employed ([4], [43]). Nonetheless, studies about the impacts of pre-commercial thinning or thinning on foliar micronutrient status are more recent and still scarce ([9], [31], [14]).
At present, a significant part of European forests are “semi-natural” plantations of native and non-native species, i.e., even-aged stands under intensive management, with low species richness and simplified structure ([15]). Two pine species with contrasting origins and characteristics are currently among the most widely planted in SW Europe: the native Pinus pinaster Ait. and the exotic Pinus radiata D. Don. While the former is able to grow even under harsh environmental conditions (unfertile soils and limited water availability), the latter requires a better supply of water and nutrients ([36]).
Taking into account all this information, our objective was to evaluate the effects of three early thinning treatments, differing in the intensity of canopy reduction, on the availability of nutrients and trace elements in trees. With this aim, the concentrations of 12 elements (Al, B, Ca, Cu, Fe, K, Mg, Mn, N, Na, P and Zn) were assessed in current year and 1-year-old needles of P. pinaster and P. radiata, using unthinned plots as control.
Material and methods
Study areas
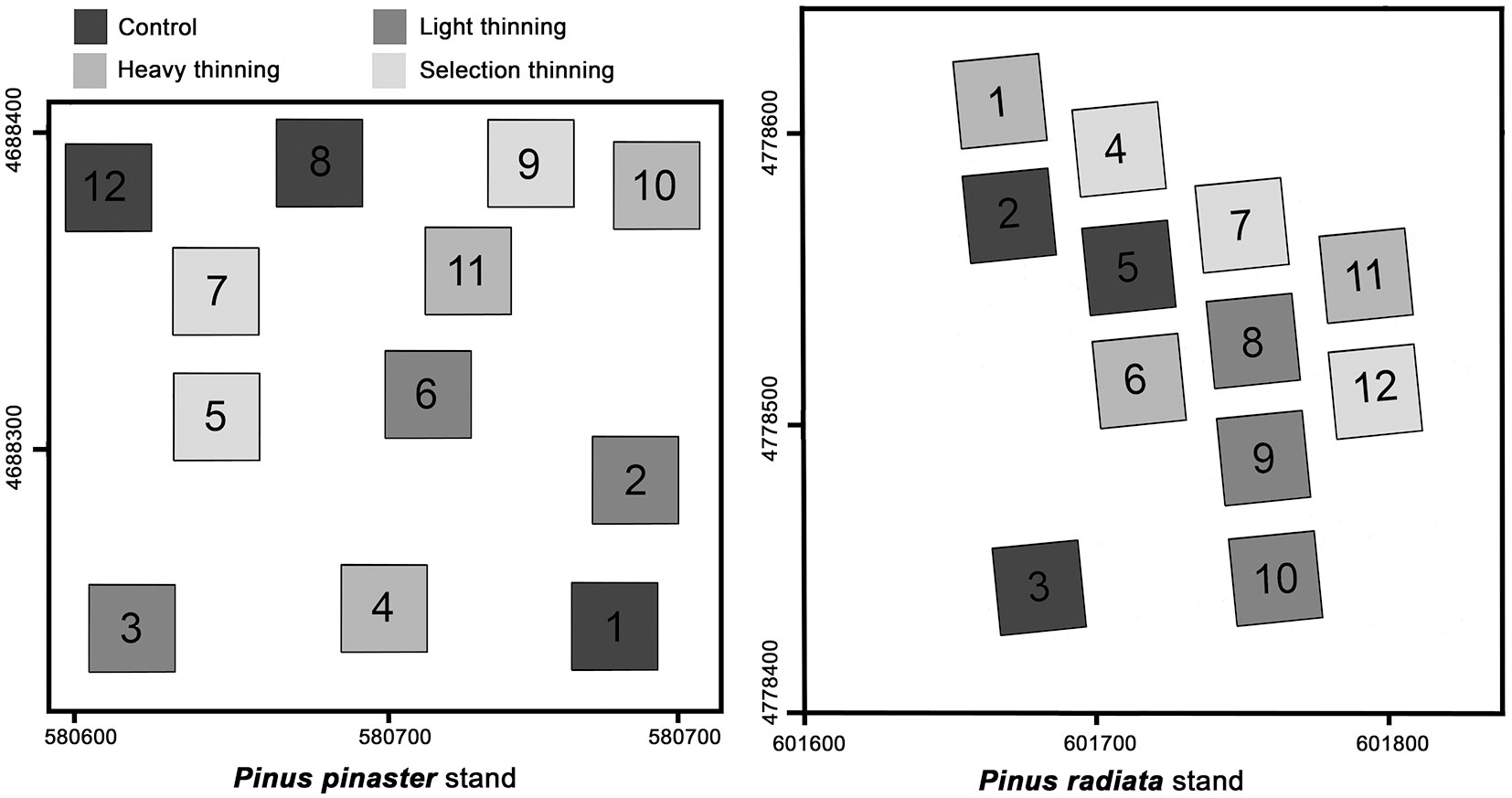
The study was conducted in Galicia (NW of Spain) at Barbantes site, a Pinus pinaster Aiton stand naturally regenerated after a wildfire that completely destroyed the original plantation, and at the Baamonde site, a plantation of Pinus radiata D. Don. Tab. 1shows the main site and stand characteristics of both sites before thinning. When the stands where 12-year-old, four treatments were randomly setup at both sites in triplicate: C, control unthinned; LT, light thinning from below with 10% of total basal area at breast height (m2 ha-1) and 19% of trees removed; HT, heavy thinning from below with 20% of total basal area and 37% of trees removed; ST, selection thinning of trees with worst shape or growth, with 20% of the total basal area and 24% of trees removed (Fig. 1).
Tab. 1 - Main characteristics of the studied sites before thinning.
| Characteristic | Sites | |
|---|---|---|
| Barbantes (Pinus pinaster) |
Baamonde (Pinus radiata) |
|
| Geographic coordinates | 42°20′ 29″ N 08°01′ 15″ W |
43°08′ 59″ N 07°45′ 04″ W |
| Altitude (m a.s.l.) | 370 | 475 |
| Slope (%) | 9-10 | 3 |
| Mean annual temperature (°C) | 14.0 | 12.2 |
| Mean annual precipitation (mm) | 900-1100 | 830-1430 |
| Stand size (ha) | 2.7 | 2.6 |
| Plot size (m) | 20 × 25 | 30 × 30 |
| Stand age (years) | 13 | 12 |
| Density (trees/ha) | 3750 | 1670 |
| Site Index (m) | 14.8 | 26 |
| Mean diameter at breast height (cm) | 9.6 | 13.0 |
| Total basal area at breast height (m2 ha-1) | 26 | 22 |
| Soil type | Leptic umbrisol | Haplic umbrisol |
| Parent material | Adamellitic granite | Graphitic schists |
| pHH2O | 4.2 | 5.1 |
| Sand (%) | 75.7 | 38 |
| Silt (%) | 11.1 | 38.8 |
| Clay (%) | 13.2 | 23.2 |
| Total C (g kg-1 dry soil) | 70.9 | 57.5 |
| Total N (g kg-1 dry soil) | 3.1 | 3.6 |
Needle sampling, processing and analysis
Two years after thinning, six pines per stand were randomly selected to collect current year (“young”) and 1-year-old (“old”) needles from the upper third of live crown in mid-January, according to the recommendations reported by Stefan et al. ([35]). Overall, we sampled 24 trees for each pine species, of which 12 for each age class, and 6 for each thinning treatment.
Needle samples were oven-dried at 60 °C to a constant mass and finely ground (< 100 µm) in a planetary ball mill (PM 100®, Retsch GmbH, Haan, Germany) for nutrient analyses. Between consecutive samples, vessel and balls (both made of zirconium oxide) were thoroughly cleaned with water, DI-water and ethanol. The dry matter content of needles was assessed by oven-drying subsamples at 110 °C to constant weight. Needle total N and δ15N were measured with an elemental analyser (CNS 1508®, Carlo Erba, Milan, Italy) coupled on-line to an isotopic ratio mass spectrometer (delta c™, Finnigan Mat, Bremen, Germany). For determining the total concentration of other elements, a subsample (500 mg) was digested for 55 min with 8 mL of 65% HNO3 and 25 mL of 30% H2O2 in teflon containers in a high performance microwave digestion unit (Milestone 1200 Mega, Sorisole, Italy). Once cooled, the solutions were filtered through quantitative filter paper (Filter-laboratory 1242, 90-mm diameter), transferred to 25 mL volumetric flasks and made to volume with water. Analytical-grade chemicals were obtained from Merck Chemical Co. (Darmstadt, Germany), and all aqueous solutions were prepared with type I water ([3]). The total concentration of Al, B, Ca, Cu, Fe, K, Mg, Mn, Na, P and Zn was measured by simultaneous ICP-OES (Varian Vista Pro, Mulgrave, Australia).
Calculation and statistical analysis
Data were examined by two-way ANOVA with age and thinning treatment as factors. The fulfillment of the assumptions of normal distribution of the data was verified by the Shapiro-Wilk’s W test, and the equality of variances among groups by the Levene’s test. In the case of departure from normality or heteroscedasticity, the original data were subjected to Tukey’s ladder of powers to yield normal distribution and equality of variances. The Bonferroni’s test for multiple comparisons was used to detect significant differences between the group means; throughout the text the values given are the mean ± standard deviation. The proportion of the variation accounted for each factor or interaction in the ANOVA was determined by the partial eta-squared (ηp2) statistic. Statistical procedures were performed with SPSS® v. 25.0 for Windows (IBM Corp., Armonk, NY, USA).
Results
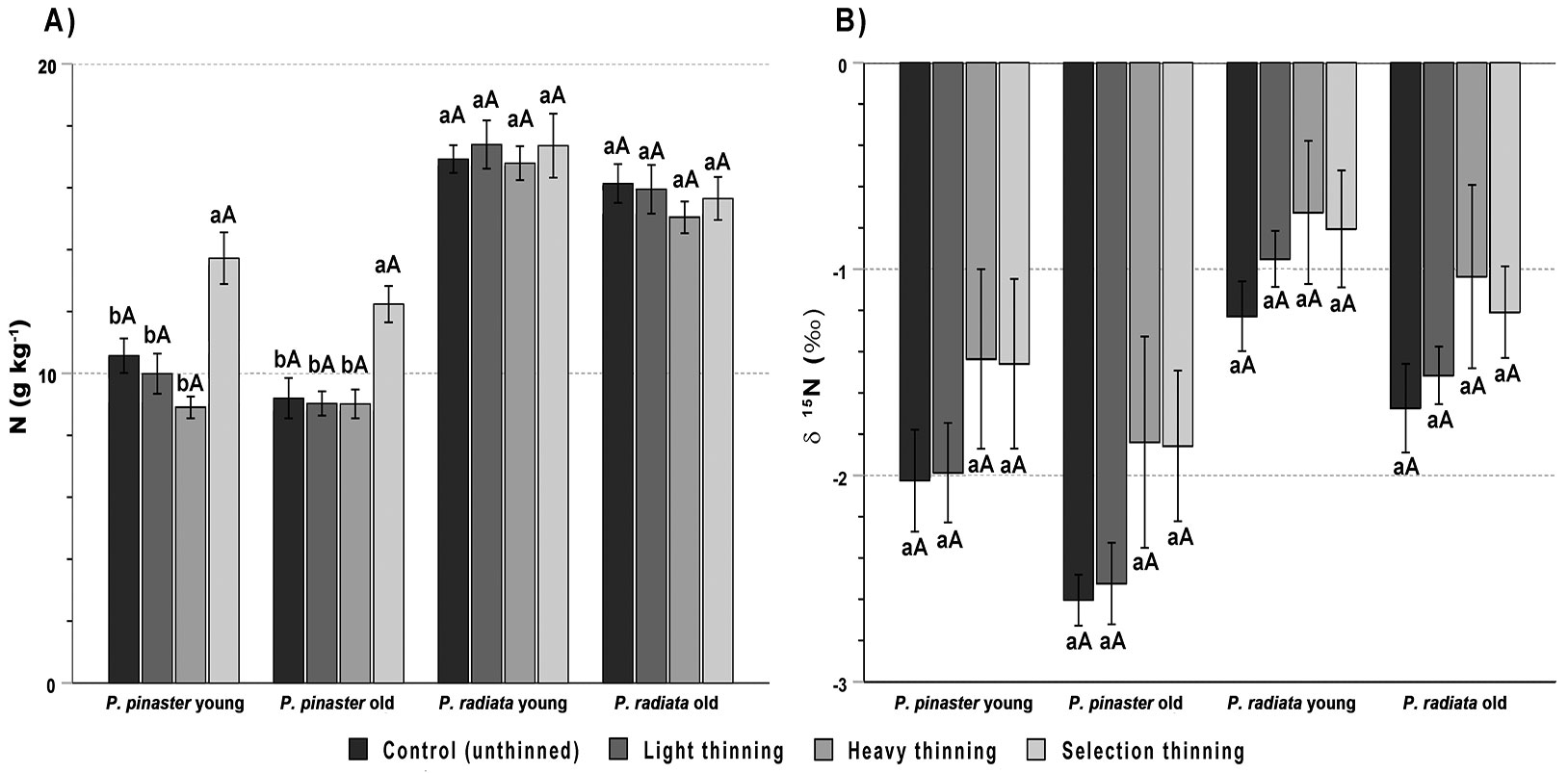
The needle N concentration in P. pinaster (11.08 ± 2.32 g kg-1 and 10.04 ± 1.87 g kg-1 in young and old needles, respectively) was not significantly affected by needle age, but strongly influenced by the treatment which accounted for 60% of the total variance, with higher values in ST than in the other treatments (Tab. 2, Fig. 2A). Conversely, N concentration in P. radiata needles (17.13 ± 1.66 and 15.77 ± 1.42 g kg-1 in young and old needles, respectively) was unaffected by the studied factors (Tab. 2, see also Fig. 2A for the mean values and significant differences among groups). The needle 15N isotopic signature in P. pinaster (-1.76 ± 0.75 ‰ and -2.24 ± 0.71 ‰ in young and old needles, respectively) and P. radiata (-0.93 ± 0.61 ‰ and -1.37 ± 0.60 ‰ in young and old needles, respectively) did not differ significantly between needle age classes (Tab. 2, Fig. 2B).
Tab. 2 - Tests of between-subjects effects for the macro- and micro-nutrient concentrations of pine needles with two-way ANOVAs. (df): degrees of freedom; (CM): Corrected model; (I): intercept; (NA): needle age; (T): thinning; (X): needle age × thinning (NA×T).
| Element | Source | df | Pinus pinaster | Pinus radiata | Element | Source | df | Pinus pinaster | Pinus radiata | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | Prob | ηp2 | F | Prob | ηp2 | F | Prob | ηp2 | F | Prob | ηp2 | ||||||
| N | CM | 7 | 8.095 | <0.001 | 0.632 | 0.691 | 0.679 | 0.131 | Mn | CM | 7 | 3.254 | 0.009 | 0.401 | 2.852 | 0.019 | 0.37 |
| I | 1 | 1940.444 | <0.001 | 0.983 | 1633.086 | <0.001 | 0.981 | I | 1 | 546.967 | <0.001 | 0.941 | 187.836 | <0.001 | 0.847 | ||
| NA | 1 | 3.889 | 0.057 | 0.105 | 0.807 | 0.376 | 0.025 | NA | 1 | 2.268 | 0.141 | 0.063 | 2.774 | 0.105 | 0.075 | ||
| T | 3 | 16.688 | <0.001 | 0.603 | 0.5 | 0.685 | 0.045 | T | 3 | 4.268 | 0.012 | 0.274 | 5.714 | 0.003 | 0.335 | ||
| X | 3 | 0.465 | 0.708 | 0.041 | 0.853 | 0.475 | 0.074 | X | 3 | 2.501 | 0.076 | 0.181 | 0.204 | 0.893 | 0.018 | ||
| δ15N | CM | 7 | 1.799 | 0.122 | 0.282 | 1.591 | 0.172 | 0.247 | Zn | CM | 7 | 9.499 | <0.001 | 0.675 | 2.03 | 0.08 | 0.295 |
| I | 1 | 281.491 | <0.001 | 0.898 | 139.998 | <0.001 | 0.805 | I | 1 | 2134.946 | <0.001 | 0.985 | 6299.779 | <0.001 | 0.995 | ||
| NA | 1 | 4.179 | 0.049 | 0.116 | 4.964 | 0.033 | 0.127 | NA | 1 | 10.696 | 0.003 | 0.251 | 7.958 | 0.008 | 0.19 | ||
| T | 3 | 2.618 | 0.068 | 0.197 | 1.874 | 0.153 | 0.142 | T | 3 | 18.546 | <0.001 | 0.635 | 1.695 | 0.186 | 0.13 | ||
| X | 3 | 0.042 | 0.988 | 0.004 | 0.063 | 0.979 | 0.006 | X | 3 | 0.345 | 0.793 | 0.031 | 0.812 | 0.496 | 0.067 | ||
| P | CM | 7 | 4.837 | 0.003 | 0.482 | 0.299 | 0.948 | 0.077 | Cu | CM | 7 | 23.972 | <0.001 | 0.832 | 6.737 | <0.001 | 0.596 |
| I | 1 | 470.763 | <0.001 | 0.948 | 69.904 | <0.001 | 0.737 | I | 1 | 6108.075 | <0.001 | 0.994 | 6356.334 | <0.001 | 0.995 | ||
| NA | 1 | 6.601 | 0.016 | 0.202 | 0.223 | 0.641 | 0.009 | NA | 1 | 44.927 | <0.001 | 0.569 | 41.084 | <0.001 | 0.562 | ||
| T | 3 | 7.966 | 0.002 | 0.38 | 0.049 | 0.985 | 0.006 | T | 3 | 33.461 | <0.001 | 0.747 | 0.504 | 0.683 | 0.045 | ||
| X | 3 | 0.8 | 0.46 | 0.058 | 0.474 | 0.703 | 0.054 | X | 3 | 3.811 | 0.019 | 0.252 | 1.477 | 0.239 | 0.122 | ||
| Ca | CM | 7 | 2.459 | 0.037 | 0.336 | 4.084 | 0.002 | 0.457 | B | CM | 7 | 0.782 | 0.607 | 0.139 | 0.439 | 0.871 | 0.083 |
| I | 1 | 414.071 | <0.001 | 0.924 | 220.705 | <0.001 | 0.867 | I | 1 | 110.8 | <0.001 | 0.765 | 121.606 | <0.001 | 0.781 | ||
| NA | 1 | 7.672 | 0.009 | 0.184 | 23.156 | <0.001 | 0.405 | NA | 1 | 2.2 | 0.147 | 0.061 | 0.011 | 0.917 | 0.001 | ||
| T | 3 | 2.608 | 0.068 | 0.187 | 0.934 | 0.435 | 0.076 | T | 3 | 0.584 | 0.63 | 0.049 | 0.336 | 0.799 | 0.029 | ||
| X | 3 | 0.322 | 0.81 | 0.028 | 0.328 | 0.805 | 0.028 | X | 3 | 0.283 | 0.837 | 0.024 | 0.592 | 0.625 | 0.05 | ||
| Mg | CM | 7 | 1.506 | 0.198 | 0.237 | 0.767 | 0.618 | 0.136 | Na | CM | 7 | 1.158 | 0.352 | 0.193 | 5.72 | <0.001 | 0.556 |
| I | 1 | 537.074 | <0.001 | 0.94 | 1386.803 | <0.001 | 0.976 | I | 1 | 2782.872 | <0.001 | 0.988 | 4693.933 | <0.001 | 0.993 | ||
| NA | 1 | 1.179 | 0.285 | 0.034 | 1.651 | 0.208 | 0.046 | NA | 1 | 5.274 | 0.028 | 0.134 | 28.289 | <0.001 | 0.469 | ||
| T | 3 | 2.541 | 0.073 | 0.183 | 0.794 | 0.506 | 0.065 | T | 3 | 0.432 | 0.731 | 0.037 | 1.409 | 0.258 | 0.117 | ||
| X | 3 | 0.52 | 0.672 | 0.044 | 0.559 | 0.646 | 0.047 | X | 3 | 0.459 | 0.712 | 0.039 | 1.425 | 0.254 | 0.118 | ||
| K | CM | 7 | 2.582 | 0.03 | 0.347 | 1.549 | 0.184 | 0.242 | Al | CM | 7 | 4.733 | 0.001 | 0.501 | 3.493 | 0.007 | 0.426 |
| I | 1 | 883.349 | <0.001 | 0.963 | 684.165 | <0.001 | 0.953 | I | 1 | 549.783 | <0.001 | 0.943 | 515.934 | <0.001 | 0.94 | ||
| NA | 1 | 3.623 | 0.065 | 0.096 | 5.973 | 0.02 | 0.149 | NA | 1 | 21.661 | <0.001 | 0.396 | 20.303 | <0.001 | 0.381 | ||
| T | 3 | 3.727 | 0.02 | 0.247 | 1.47 | 0.24 | 0.115 | T | 3 | 3.37 | 0.03 | 0.235 | 0.958 | 0.424 | 0.08 | ||
| X | 3 | 0.754 | 0.528 | 0.062 | 0.3 | 0.825 | 0.026 | X | 3 | 1.4 | 0.26 | 0.113 | 0.14 | 0.935 | 0.013 | ||
| Fe | CM | 7 | 21.911 | <0.001 | 0.823 | 7.047 | <0.001 | 0.607 | - | - | - | - | - | - | - | - | - |
| I | 1 | 952.987 | <0.001 | 0.967 | 617.617 | <0.001 | 0.951 | - | - | - | - | - | - | - | - | ||
| NA | 1 | 77.624 | <0.001 | 0.702 | 44.219 | <0.001 | 0.58 | - | - | - | - | - | - | - | - | ||
| T | 3 | 21.326 | <0.001 | 0.66 | 1.169 | 0.337 | 0.099 | - | - | - | - | - | - | - | - | ||
| X | 3 | 5.799 | 0.003 | 0.345 | 0.126 | 0.944 | 0.012 | - | - | - | - | - | - | - | - | ||
Fig. 2 - Mean nitrogen concentration (A) and δ15N (B) in young (current year) and old (1-year-old) needles of Pinus pinaster and Pinus radiata from control (unthinned) and light, heavy and selection thinning plots. For each species, different lowercase letters indicate significant differences among treatments for the same needle age, while different uppercase letters indicate significant differences between young and old needles for the same treatment (n = 6, p < 0.05).
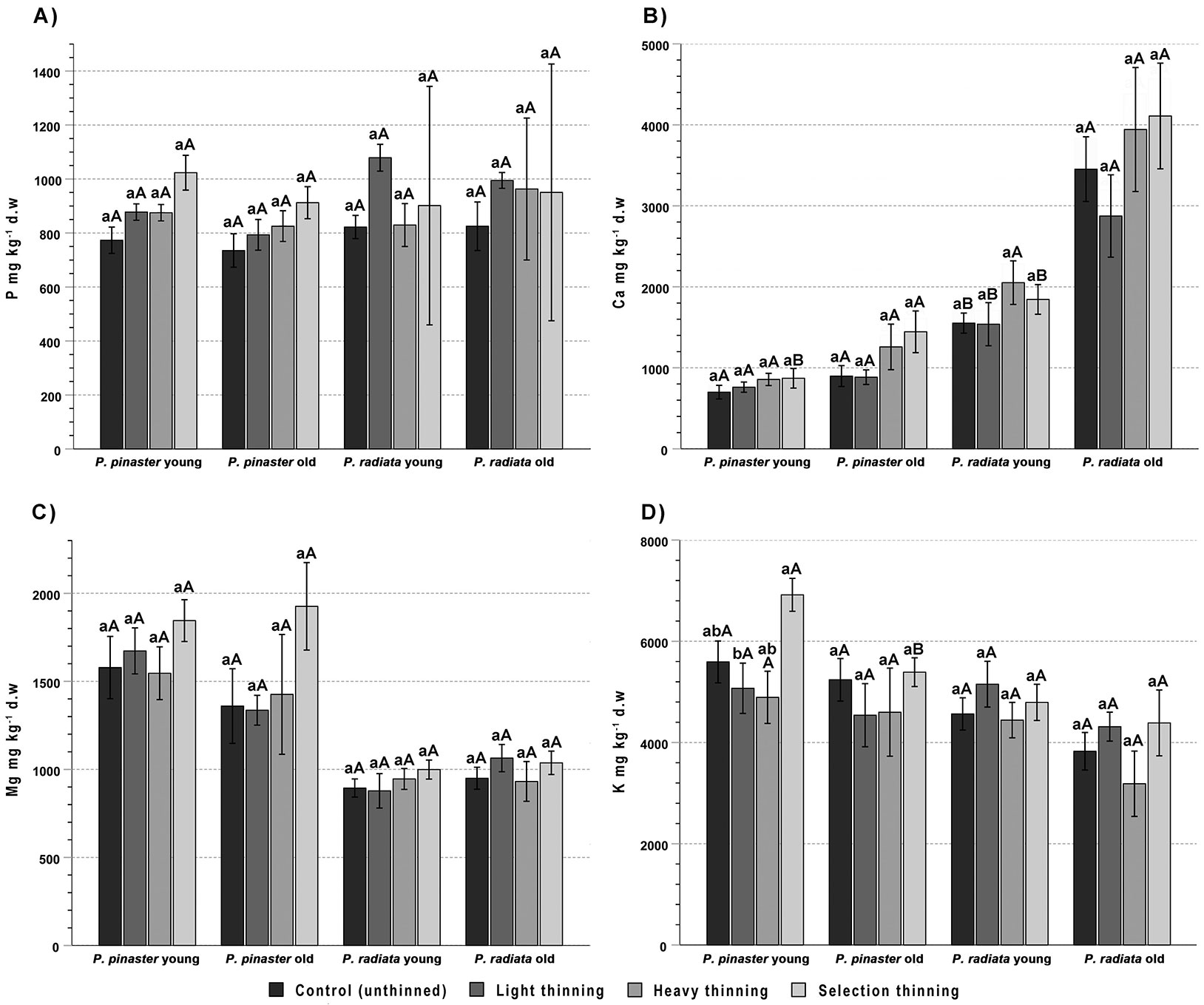
The foliar P concentration was 735 ± 240 mg kg-1 in P. pinaster and 639 ± 378 mg kg-1 in P. radiata (Fig. 3A). However, while in P. radiata no effect of age or treatment was found, in P. pinaster there were significant effects of both needle age and treatment (20% and 38% of the variance explained, respectively; ST > C, LT - Tab. 2).
Fig. 3 - Mean concentration of phosphorous (A), calcium (B), magnesium (C) and potassium (D) in young (current year) and old (1-year-old) needles of Pinus pinaster and Pinus radiata from control (unthinned) and light, heavy and selection thinning plots. For each species, different lowercase letters indicate significant differences among treatments for the same needle age, while different uppercase letters indicate significant differences between young and old needles for the same treatment (n = 6, p < 0.05).
The values of Ca concentration in P. pinaster (788 ± 213 and 1102 ± 475 mg kg-1 in young and old needles, respectively) and P. radiata (1756 ± 528 and 3672 ± 1286 mg kg-1 in young and old needles, respectively) did not show significant differences due to treatment and increased significantly with needle age in both species (18% and 41% of the variance explained, respectively - Tab. 2, Fig. 3B). Compared to young needles, the mean concentration of Ca in old needles increased moderately in P. pinaster (40%) and much more importantly in P. radiata (109% - Tab. 2, Fig. 3B).
No significant effects of either thinning or age, nor an interaction effect of these factors, was observed for needle Mg (1601 ± 524 mg kg-1 in P. pinaster; 962 ± 165 mg kg-1 in P. radiata - Tab. 2; see Fig. 3C for the mean values and significant differences among groups).
Potassium concentration tended to decrease with age in both P. pinaster (5728 ± 1237 and 4991 ± 1160 mg kg-1 in young and old needles, respectively) and P. radiata (4720 ± 858 and 3946 ± 1208 mg kg-1 in young and old needles, respectively), but the effect was only significant in the latter, accounting for 15% of the variance (Tab. 2). There was only a treatment effect in P. pinaster, explaining 25% of the variance with ST > LT, and the other two treatments in intermediate positions (Tab. 2, Fig. 3D).
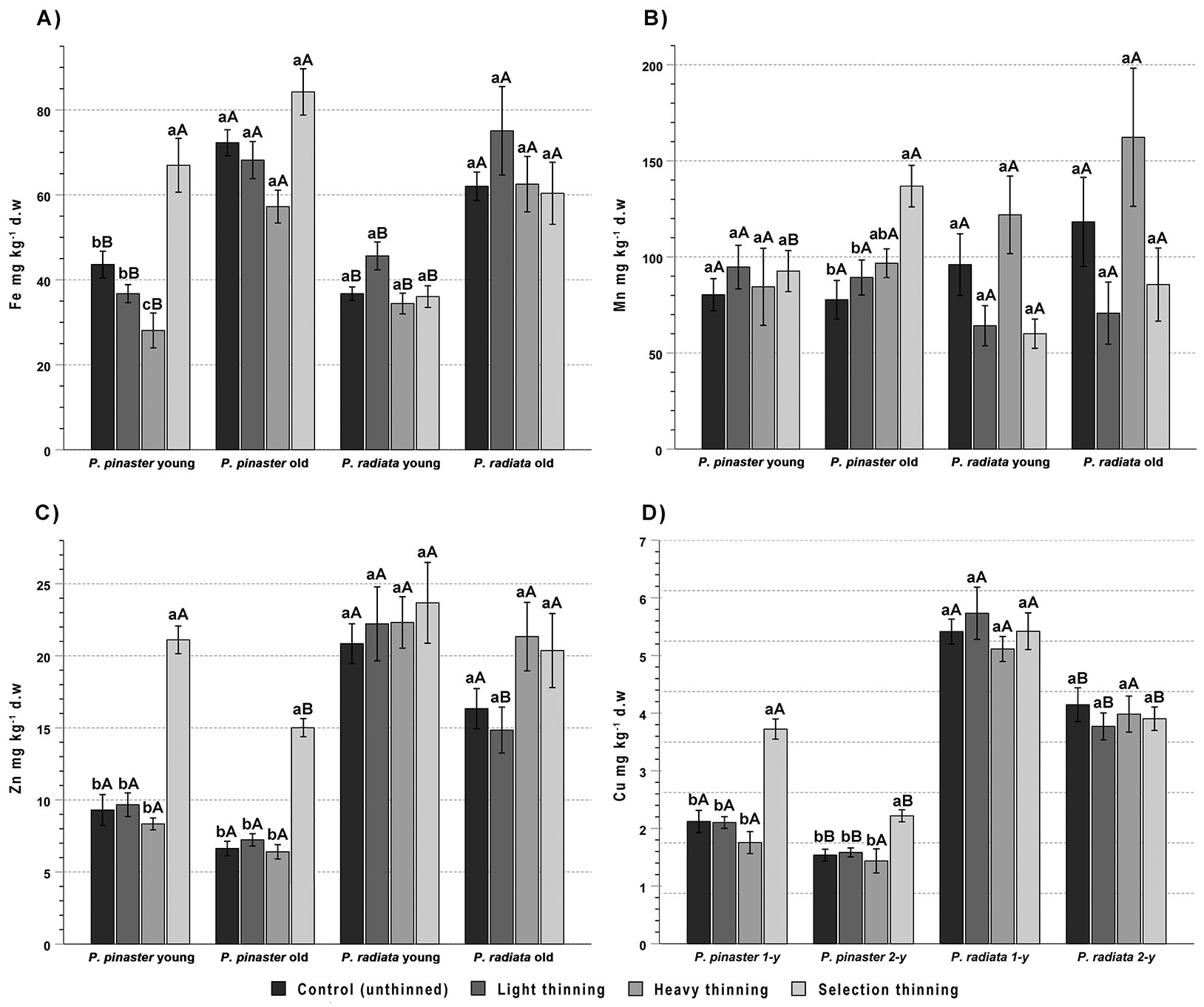
Needle age was an important factor of Fe variability in pine needles (70% and 58% of the variance explained in P. pinaster and P. radiata, respectively - Tab. 2), Fe levels increasing significantly with needle age from 44 ± 15 to 71 ± 12 mg kg-1 in P. pinaster and from 38 ± 7 to 64 ± 13 mg kg-1 in P. radiata (Fig. 4A). The two-way ANOVA also showed a significant effect of the treatment and the needle age × treatment interaction in P. pinaster, the concentration of Fe decreasing in the order ST > C, LT > HT, but differences were significant only in the young needles (Tab. 2).
Fig. 4 - Mean concentration of iron (A), manganese (B), zinc (C) and copper (D) in young (current year) and old (1-year-old) needles of Pinus pinaster and Pinus radiata from control (unthinned) and light, heavy and selection thinning plots. For each species, different lowercase letters indicate significant differences among treatments for the same needle age, while different uppercase letters indicate significant differences between young and old needles for the same treatment (n = 6, p < 0.05).
Unlike most of the other studied element, needle Mn in P. pinaster (89 ± 25 and 101 ± 33 mg kg-1 in young and old needles, respectively) and P. radiata needles (86 ± 42 and 110 ± 59 mg kg-1, in young and old needles, respectively) was not affected by needle age (except in P. pinaster after ST), but a significant effect of thinning treatment was recorded in both species (27-34% of the variance explained - Tab. 2), with ST > C in the former and HT > LT, ST in the latter (Fig. 4b).
Zinc concentration in P. pinaster (11.7 ± 1.6 and 6.8 ± 1.2 mg kg-1, in young and old needles, respectively) and P. radiata needles (21.8 ± 1.2 and 17.8 ± 1.3 mg kg-1, in young and old needles, respectively) was moderately affected by needle age (19-25% of the variance explained - Tab. 2), decreasing by about 29% in P. pinaster and 17% in P. radiata (Fig. 4C). In P. pinaster there was also a strong treatment effect (64% of the variance explained - Tab. 2), with higher values in selection thinning (ST) than in the other treatments.
The concentration of Cu in P. pinaster (2.9 ± 1.0 and 2.0 ± 0.5 mg kg-1, in young and old needles, respectively) and P. radiata needles (6.2 ± 0.8 and 4.5 ± 0.6 mg kg-1, in young and old needles, respectively) was strongly affected by age, which explained 56-57% of Cu variance (p < 0.001 - Tab. 2); in both species, Cu concentration decreased from current year to 1-year-old needles, by 31% in P. pinaster and 22% in P. radiata (Fig. 4D). In P. pinaster there was also a strong treatment effect and a needle age × treatment interaction (75% and 25% of the variance explained, respectively - Tab. 2), with higher values in selective thinning (ST) than in the other treatments.
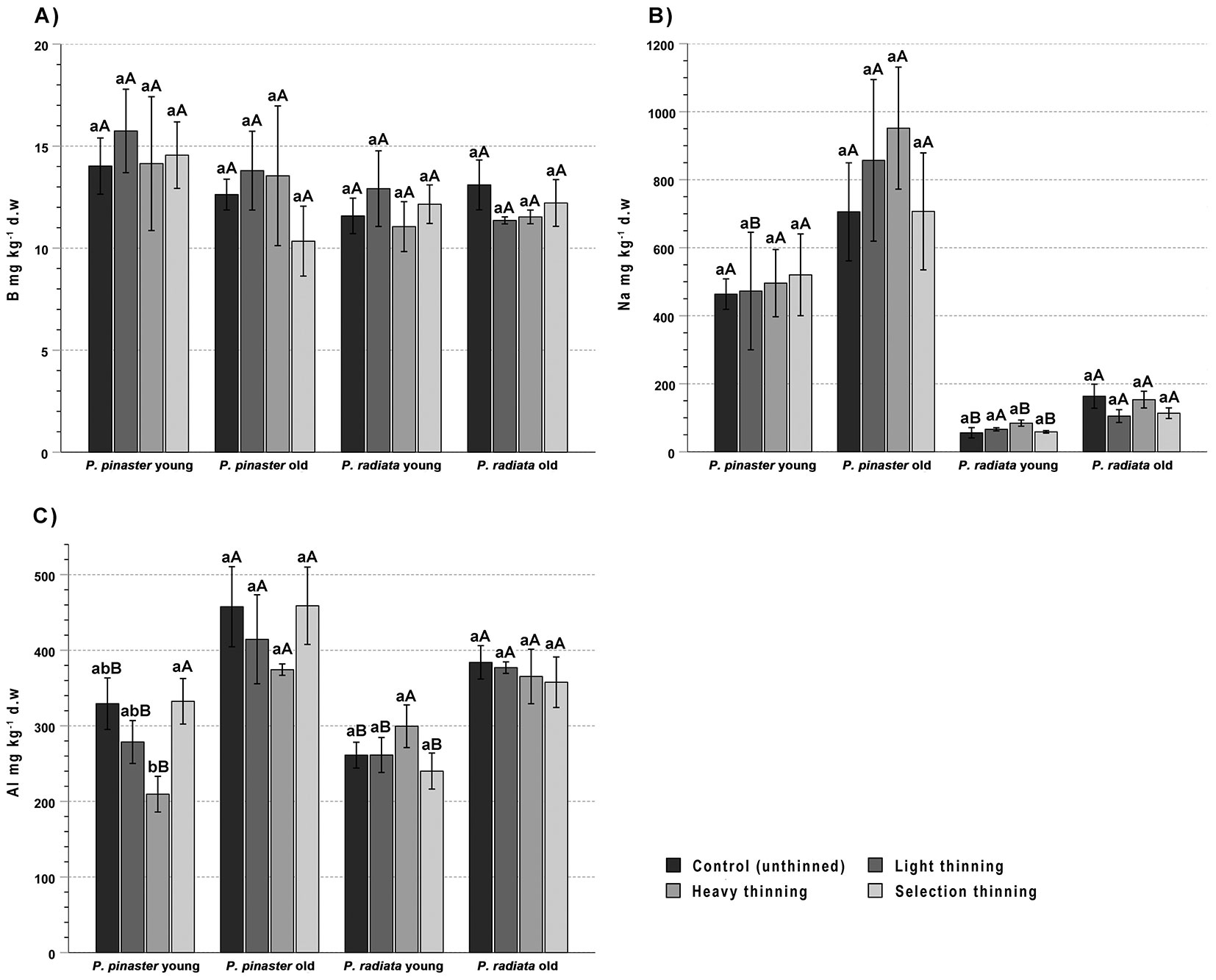
Like for Mg, needle B (3.2 ± 1.8 and 2.1 ± 1.2 mg kg-1 in P. pinaster and P. radiata, respectively) did not show significant effects of either thinning or needle age, nor an interaction effect of these factors (Fig. 5A).
Fig. 5 - Mean concentration of boron (A), sodium (B) and aluminium (C) in young (current year) and old (1-year-old) needles of Pinus pinaster and Pinus radiata from control (unthinned) and light, heavy and selection thinning plots. For each species, different lowercase letters indicate significant differences among treatments for the same needle age, while different uppercase letters indicate significant differences between young and old needles for the same treatment (n = 6, p < 0.05).
The two-way ANOVA showed a significant increase of Na concentration with needle age: from 487 ± 270 to 784 ± 423 mg kg-1 in P. pinaster, and from 67 ± 25 to 138 ± 61 mg kg-1 in P. radiata, explaining 13% and 47% of the variance, respectively, but no differences due to treatment nor a needle age × treatment interaction (Tab. 2, Fig. 5B).
The Al concentration in P. pinaster was 297 ± 79 mg kg-1 in young needles and 434 ± 120 mg kg-1 in old needles, while the corresponding values for P. radiata were 268 ± 55 mg kg-1 and 373 ± 50 mg kg-1, respectively (Fig. 5C). The two-way ANOVA showed a moderate effect of thinning on Al concentration only in P. pinaster needles (24% of the variance explained - Tab. 2), differences being significant only between the highest (ST) and the lowest values (HT). Contrastingly, needle age accounted for about 40% of Al variability in both P. pinaster and P. radiata (38-40% of the variance explained - Tab. 2), with mean values around 39% (P. pinaster) and 53% (P. radiata) higher in young than in old needles and significant differences due to needle age in most treatments.
Discussion
Nitrogen concentration in the foliar organs is directly related with photosynthetic activity and water use efficiency ([6]) and, consequently, is a key factor for tree growth. In the investigated P. pinaster stands, needle N concentration was always within the range of values of 7 to 16 g kg-1 reported for pine needles by several authors ([45], [37], [10], [42], [11]), but in some cases it was below the N deficiency threshold of 9.8 g kg-1 proposed for the species in NW Spain ([16]). In P. radiata our values were ever in the upper end of the mentioned range or even above it. The insignificant decrease of N concentration with needle age in our stands contrasts with the reduction of foliar N with age reported for other Pinus species by Zhang & Allen ([45]) and Choi et al. ([10]). Regarding the effects of thinning on needle N concentration, we found contrasting results, as it was significantly modified only by selection thinning in P. pinaster (with the highest value for this species); therefore, except for this single combination (ST in P. pinaster) the thinning treatments did not affect the foliar N concentration, thus not improving the photosynthetic activity and water use efficiency of trees. Similarly, contrasting results have been reported about the effects of thinning on leaf N concentration, ranging from increased values ([38], [27], [22]), to no changes ([31]), and even reductions ([9]). The decrease of δ15N with needle age that we observed in both Pinus species agrees with the trend most frequently reported ([18], [17], [11], [12]), though inconclusive results have also been found ([10]). Although a foliar δ15N increase and more open N cycling in thinned areas relative to unthinned plots have been reported ([4], [43]), we did not find significant changes in needle δ15N due to thinning.
Phosphorous concentrations were in the lower half of the usual range for P. pinaster (0.6-1.5 g kg-1 - [32], [37], [16]), but below the normal levels in P. radiata (1-1.6 g kg-1 - [40], [44], [37]). In our stands, neither needle age nor thinning treatment impacted on the P concentration of P. radiata needles, agreeing with the results of Piatek et al. ([31]), but both factors affected it in P. pinaster. In the latter species, the increase of foliar P concentration in thinned plots shows the beneficial effects of thinning in P nutrition which has been already reported by many authors ([29], [21], [38], [27], [9], [14]).
Most values of needle Ca in our P. pinaster stands were well below the expected range of 1.6-2.7 g kg-1 reported in the references compiled by Tausz et al. ([37]), but above the Ca deficiency level (0.6 g kg-1) proposed for the species in NW Spain ([16]). In P. radiata needles, all values were within the expected range for Ca nutrition (1-4 g kg-1 - [40], [44], [37]). The slight increase of Ca concentration we observed from young to old needles in P. pinaster contrasted with the substantial increment (from 1.7 ± 0.1 g kg-1 to 2.9 ± 0.6 g kg-1) observed by Saur ([32]) in this species and with the two-fold increase in our P. radiata stands. The lack of treatment effects on needle Ca clearly showed that thinning was unable to improve the foliar levels of Ca in P. pinaster and P. radiata growing in the studied acidic soils. This result agrees with those of Piatek et al. ([31]), as well as with the immobility of Ca inside the plant that explains the little effects of silvicultural treatments on needle Ca in P. halepensis ([27]).
Magnesium concentration was ever within the normal ranges of 0.5-2 g kg-1 in P. pinaster ([32], [37], [16]) and 0.4-2.4 g kg-1 in P. radiata ([40], [44], [37]), as well as within the optimum range for P. sylvestris (0.5-2 g kg-1 - [30]). Neither needle age nor thinning did affect the concentration of Mg in the studied P. pinaster and P. radiata needles. As for Ca, the lack of thinning effects on needle Mg was likely explained by its immobility inside the plant ([27]) and shows that thinning was unable to improve foliar Mg nutrition in the studied stands.
All K values we found in P. pinaster needles were in the upper half or above the range (3-5 g kg-1) compiled for the species ([32], [37]) and above the threshold of K deficiency (4.5 g kg-1) proposed for NW Spain ([16]). Similarly, K concentration in P. radiata needles were ever within the range of 3-8 g kg-1 usually reported ([40], [44], [37]). Most of the values in both pine species were also within the optimum range (4.5-6 g kg-1) suggested for P. sylvestris ([30]). Needle K concentrations decreased not significantly with needle age in P. pinaster but significantly in P. radiata, as also reported for P. taeda ([45]). Although increased foliar K levels are more frequent in thinned plots ([21], [38], [27], [14]), we only found a significant increase in current year P. pinaster needles from selection thinning plots, the increment being not significant in 1-year-old needles, thus agreeing with the findings by Lopez-Serrano et al. ([27]). Moreover, the lack of significant changes in needle K in our P. radiata stands and two of the three thinning treatments in P. pinaster agreed with the results of Chase et al. ([9]) and Piatek et al. ([31]) and showed that thinning has little effect on foliar K nutrition, at least in stands without K deficiency as in our case.
In P. pinaster Fe concentration in young and old needles were below and within the respective ranges (52 ± 8 mg kg-1 and 66 ± 12 mg kg-1) observed by Saur ([32]), and the same holds for P. radiata needles when compared with the range (71 ± 28 mg kg-1) reported for this species by Zas & Serrada ([44]). In young needles of P. pinaster (only LT and HT treatments) and P. radiata, Fe concentrations were also below the optimum range of 40-100 mg kg-1 previously reported for P. sylvestris ([30]). Our results suggested that even in pinewoods with sub-optimum foliar Fe levels, thinning had not significant effects on tree Fe nutrition, except the selection thinning which improved Fe nutrition in P. pinaster, the effect being significant in young needles.
Manganese concentration was quite similar in P. pinaster and P. radiata needles and well within the ranges usually reported for these and other Pinus species: 40-300 mg kg-1 in P. pinaster ([32], [34], [33]), 50-500 mg kg-1 in P. radiata ([40], [44], [37]) and 70-400 mg kg-1 in P. sylvestris ([30]). Manganese was the only studied element not affected by needle age, and showing significant differences among thinning treatments, but without a common trend in both species. Significant differences were found for treatment ST > C in P. pinaster and HT > LT, ST in P. radiata. These results suggest that the strong thinning treatments (ST and HT) can improve foliar Mn nutrition, contrasting with the lack of effects reported by Piatek et al. ([31]).
In our stands, needle Zn in P. pinaster was below the range of 15-65 mg kg-1 reported for this species and other conifers ([32], [7], [34], [33], [44], [37]), except for ST plots; whereas all P. radiata values fall within that range and were above the critical level of 11-12 mg kg-1 indicated by Turner & Lambert ([40]). Regarding needle age effects on Zn concentration, our results for both Pinus species contrasted with the decrease in foliar Zn concentration after thinning in Douglas-fir reported by Chase et al. ([9]). No treatment effects were found for P. radiata while a positive influence of ST on Zn nutrition was observed in P. pinaster; these difference could be related with the respective Zn nutritional status in our stands, which was well above the critical level in the former and below it in the latter, especially in the old needles.
Foliar Cu concentrations in the studied P. pinaster stands were in the lower part of the usual range of 2-9 (31) mg kg-1 ([32], [34], [33], [37]) and in most cases below the optimum range (3-6 mg kg-1) for P. sylvestris ([30]). Unlike P. pinaster, needle Cu levels in our P. radiata sites were in the middle of the range of 2-10 mg kg-1 usually reported ([26], [41], [44], [37]) and above the optimum range for P. sylvestris ([30]). Copper concentration decreased significantly with needle age in both P. pinaster and P. radiata, as also found by Saur ([32]) for the former species. Agreeing with the lack of thinning effects on foliar Cu found by Chase et al. ([9]), only one of the assayed thinning treatment (ST) improved Cu nutrition and only in one of the studied species (P. pinaster).
Boron levels in P. pinaster were within the ranges previously found by Saur ([32]) for current year and 1-year-old needles (15.9 ± 2.9 and 13.6 ± 3.2 mg kg-1, respectively), and were also close to those of 12 and 16 mg kg-1 compiled by Saur et al. ([34]) and Tausz et al. ([37]), respectively. In P. radiata the concentration of B was also within the normal range of 10-15 mg kg-1 reported for the species and above the critical deficiency threshold of 8 mg kg-1 ([25], [40]), which is identical to that for P. sylvestris ([30]). Although Chase et al. ([9]) reported an increase of foliar B in thinned plots of Douglas-fir, no thinning effects on needle B were found in our plots, a result which can be related with the good B levels in the unthinned plots.
In both Pinus species, foliar Na levels increased with needle age and did not varied due to the thinning treatment.
Compared to the concentration of Al reported by Saur ([32]) for current year (215 ± 62 mg kg-1) and 1-year-old (240 ± 78 mg kg-1) needles in P. pinaster, Al values in our stands were similar in the former case, but noticeably higher in the latter. In P. radiata, needle Al concentration was always below the most frequent threshold of 500 mg kg-1 ([40]). In both pine species, needle Al increased with needle age and was affected by thinning only in young needles, but no clear tendency can be discerned, likely due to the fact that this element is not essential for plant growth, but it is toxic for trees at high concentration.
Conclusions
Needle age affected the foliar concentrations of 8 out 12 of the elements considered in the same way for both pine species studied: needle δ15N, K, Zn and Cu decreased with needle age, whereas the levels of P (only in P. pinaster), Ca, Fe, Na and Al increased. Conversely, except for Mn, the effects of thinning on foliar elemental concentrations were restricted to P. pinaster and more specifically to the selection thinning treatment which enhanced the levels of N, K, Fe, Mn, Zn and Cu.
Acknowledgements
This research was done on the experimental stands setup by Dr. A. Rojo Alboreca, Dr. J.G. Álvarez González and Dr. R. Rodríguez Soalleiro, who are acknowledged for their invaluable collaboration. We also thank Teresa Pérez and Jorge Benítez for their technical assistance in the laboratory.
This work was supported by the Spanish Ministry of Science and Technology through the project AGL2004-07976-C02-02-FOR. The participation of ACV and MXGR was supported, respectively, by a pre-doctoral CSIC-I3P contract and a post-doctoral JAE-DOC contract co-financed by the European Social Fund. The isotopic ratio mass spectrometer was partly financed by the European Regional Development Fund (EU).
References
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Alejandra Couto-Vázquez
Serafín J González-Prieto 0000-0003-2157-3505
Instituto de Investigaciones Agrobiológicas de Galicia, CSIC, Apartado 122, E-15780 Santiago de Compostela (Spain)
Corresponding author
Paper Info
Citation
Gómez-Rey MX, Couto-Vázquez A, González-Prieto SJ (2021). Age and thinning effects on elemental composition of Pinus pinaster and Pinus radiata needles. iForest 14: 299-306. - doi: 10.3832/ifor3575-014
Academic Editor
Emanuele Lingua
Paper history
Received: Jul 07, 2020
Accepted: Apr 10, 2021
First online: Jun 08, 2021
Publication Date: Jun 30, 2021
Publication Time: 1.97 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2021
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 34268
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 29041
Abstract Page Views: 2514
PDF Downloads: 2170
Citation/Reference Downloads: 2
XML Downloads: 541
Web Metrics
Days since publication: 1707
Overall contacts: 34268
Avg. contacts per week: 140.52
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2021): 2
Average cites per year: 0.40
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Soil nutrient status, nutrient return and retranslocation in poplar species and clones in northern Iran
vol. 6, pp. 336-341 (online: 29 August 2013)
Research Articles
Nursery fertilization affected field performance and nutrient resorption of Populus tomentosa Carr. ploidy levels
vol. 15, pp. 16-23 (online: 24 January 2022)
Research Articles
Nutrient uptake, allocation and biochemical changes in two Chinese fir cuttings under heterogeneous phosphorus supply
vol. 11, pp. 411-417 (online: 05 June 2018)
Research Articles
Combined effects of short-day treatment and fall fertilization on growth, nutrient status, and spring bud break of Pinus tabulaeformis seedlings
vol. 10, pp. 242-249 (online: 11 February 2017)
Research Articles
Links between phenology and ecophysiology in a European beech forest
vol. 8, pp. 438-447 (online: 15 December 2014)
Research Articles
Carbon and nutrient contents in the miscellaneous fraction of litterfall under different thinning intensities in a semiarid Pinus halepensis afforestation
vol. 12, pp. 375-382 (online: 12 July 2019)
Research Articles
Salinity strongly drives the survival, growth, leaf demography, and nutrient partitioning in seedlings of Xylocarpus granatum J. König
vol. 10, pp. 851-856 (online: 26 October 2017)
Research Articles
Nutrient accumulation and export in teak (Tectona grandis L.f.) plantations of Central America
vol. 8, pp. 33-44 (online: 04 June 2014)
Review Papers
Recent insights in soil nutrient cycling: perspectives from Pinus and Eucalyptus forest studies around the world
vol. 17, pp. 394-404 (online: 20 December 2024)
Research Articles
Modifying harvesting time as a tool to reduce nutrient export by timber extraction: a case study in planted teak (Tectona grandis L.f.) forests in Costa Rica
vol. 9, pp. 729-735 (online: 03 June 2016)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword