Drought-induced oak decline in the western Mediterranean region: an overview on current evidences, mechanisms and management options to improve forest resilience
iForest - Biogeosciences and Forestry, Volume 10, Issue 5, Pages 796-806 (2017)
doi: https://doi.org/10.3832/ifor2317-010
Published: Sep 25, 2017 - Copyright © 2017 SISEF
Review Papers
Abstract
Increased forest vulnerability is being reflected as more widespread and severe drought-induced decline episodes. In particular, the Mediterranean area is revealing a high susceptibility to phenomena of loss in tree vitality across species. Within tree species, oaks (Quercus spp.) are experiencing extensive decline in many countries. However, in the wake of the so-called “oak decline phenomenon”, the attention on these species has generally been limited. In this paper, we review the current available literature on oak-decline cases reported within the Mediterranean Basin, with particular remark for those occurred in Italy and Spain. More specifically our main aims were to: (i) provide an update on the patterns and mechanisms of decline by focusing on tree-ring and wood-anatomical variables; (ii) provide some hints for improving the resistance and resilience of oak stands experiencing decline. Our review reveals that drought is reported as the main driver triggering oak decline within the Mediterranean Basin, although other causes (i.e., increasing temperature, pathogens attack or excessive stand density) could exacerbate decline. In most reported cases, drought induced a substantial reduction of growth and changes in some wood anatomical properties. Indeed, growth decline prior death is also indicated as an early-warning signal of impending death. In ring-porous oak species, declining trees were often characterized by a very low production of latewood and a decrease in lumen area of the widest earlywood vessels, suggesting a potential reduction of hydraulic conductivity. Moreover, hydraulic dysfunction is reported as the main cause of decline. Finally, regarding management actions that should be considered for improving the resilience of declining stands and preserve the species-specific stand composition, it could be useful to shorten the rotation period of coppice stands or promoting their gradual conversion towards high forests, and favoring more drought-resistant species should also be considered. In addition, regeneration prior to regeneration cuts should be improved by anticipating seed dispersal or by planting oak seedlings obtained from local germoplasm.
Keywords
Growth, Adaptive Forest Management, Quercus, Resilience, Forest Dieback
Introduction
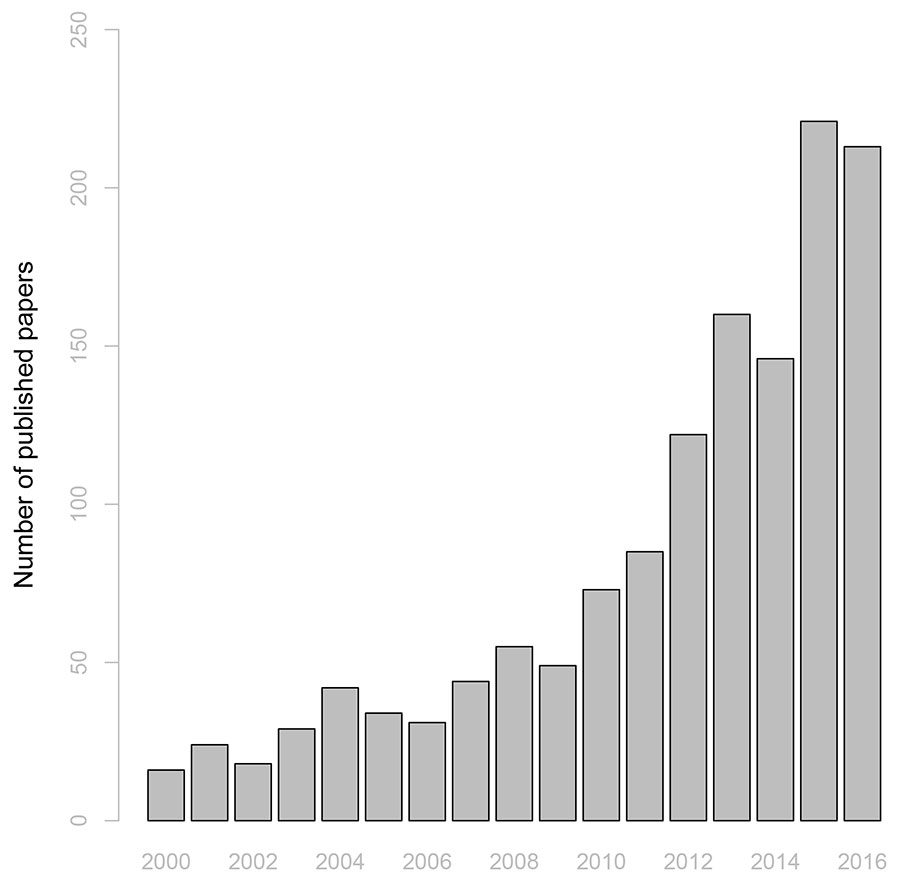
Recent studies have highlighted an increased susceptibility of forest ecosystems to climate change and a reduced adaptive capacity of forests to respond to such negative impacts ([61]). Understanding the causes of such decline and predicting its consequences has emerged as one of the greatest challenges for the international scientific community. The 88 cases of forest drought- and heat-induced tree mortality documented by Allen et al. ([3]) had almost doubled in 2014, involving more areas and biomes ([47]), and testifying an increasing scientific attention appreciable in terms of number of published studies. In fact, searching the terms “forest, drought and mortality” in the ISI Web of Knowledge® database returns an increasing number of papers, which almost tripled in 2016 (213 papers) compared to 2010 (73 papers - Fig. 1).
Fig. 1 - Temporal trend of the number of published studies containing the terms “drought, forest, mortality”. These studies were published from 2000 to 2016, and were retrieved from the ISI Web of Knowledge® database.
Most of these studies reported widespread reductions of growth and increased mortality worldwide, which affect several tree species and biomes, including tropical forests ([36]), temperate mountain conifer and hardwood forests ([60], [4]), drought-prone mountain conifer forests ([1]), Mediterranean conifer forests ([59]) and Mediterranean oak forests ([62], [15], [16], [17]).
The Mediterranean region has been identified as one of the climate-change hotspots with major risks in the near future, including warmer and drier conditions ([52]). These trends would imply that more forest areas will become particularly vulnerable under drier conditions. These more drought-susceptible areas across the Mediterranean Basin include oak (Quercus spp.) forests that represent one of the most widespread genus across the Northern hemisphere ([73]), and it is considered of great importance in ecological, cultural and economical terms across the Mediterranean Basin ([7]). However, despite the relevance of Mediterranean oak forests, the biogeographical patterns and the management implications of decline episodes in this type of forest have not been deeply investigated.
In this paper, we discuss the available knowledge on oak decline in the western Mediterranean area by analyzing concrete evidences from two study cases in Italy and Spain, where oak forests are experiencing decline episodes. Moreover, we propose useful actions of adaptive forest management for improving the resilience and resistance of declining oak stands within the Mediterranean area.
The oak decline phenomenon
Oak decline was first described in 1739 in Germany, and subsequently in Hungary in 1877 ([41]); since then, those phenomena were not as frequently reported as in the late 20th century ([29], [84]). Greater attention on this topic started around the 1990s after the first international congress held in Poland ([83]).
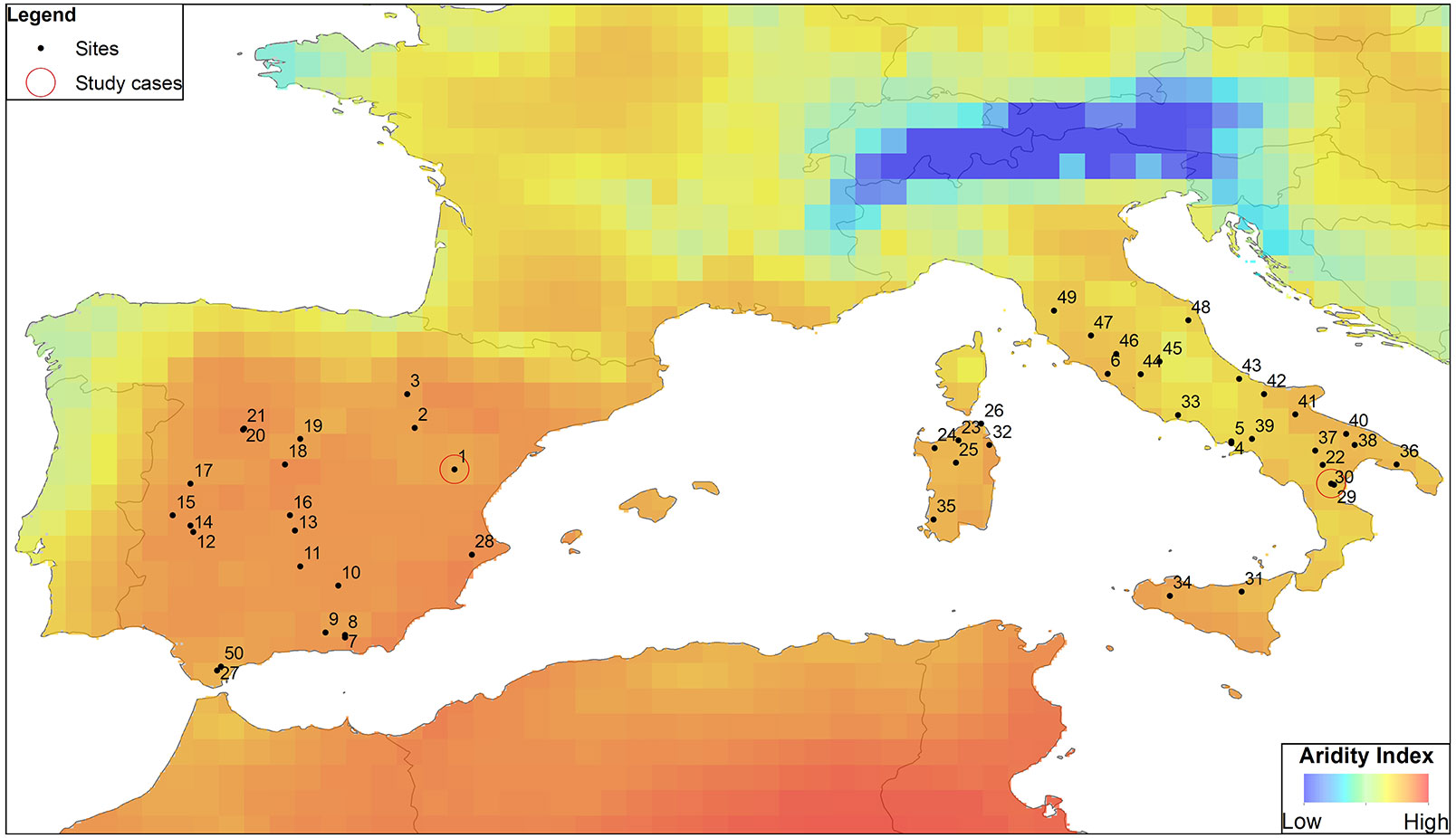
Currently, it is widely recognized that oak decline is an ongoing phenomenon in Europe since at least the early 1980s. Its incidence seems to spread over larger areas in southern Europe, at least in the two countries compared here, namely Spain and Italy (Fig. 2). Furthermore, oak decline seems to be a global phenomenon, as it has been also described in Japan ([72]) and northern America ([33]).
Fig. 2 - Location of sites in the western Mediterranean Basin, with particular focus on Italy and Spain, reporting oak decline, as retrieved from literature studies. Background colors in the map indicate the aridity index, evaluated as the ratio between precipitation and potential evapotranspiration for the 2007-2012 period. Climate data were obtained from the 0.5°-gridded Climatic Research Unit (CRU TS 3.23) climate dataset. Red circles indicate the study cases reported in the text.
In the Mediterranean Basin, the Spanish authorities officially recognized oak decline (the so-called “la seca”) since the 1990s, to designate a group of symptoms where many different parasitic agents can be involved, giving rise to a process of quick loss in tree vigor ([41]). La seca occurred more frequently at savanna-type dehesas (i.e., a high forest with scattered and pruned adult trees which is commonly exploited as an agro-silvopastoral system), where the affected oak trees (usually Q. ilex and Q. suber), showed a rapid death often connected to a disease caused by the Phytophtora cinnamomi pathogen ([12], [13], [41]). However, decline episodes have been also detected in Spanish temperate oak forests ([78], [79]) and mainly in low drought-prone oak forests, often formerly coppiced ([24], [25], [62], [23]).
In Italy, isolated oak decline cases have been observed since the end of the last century. Such decline has been always considered as a sporadic phenomenon of site-specific importance (i.e., local or regional), due to the fragmentation of local reports, thus it has not been object of adequate attention at the national level so far. Initially identified in northeastern Italy forests, where Q. robur was the most affected species, it has spread thereafter in central and southern Italy, mainly on Q. cerris, Q. frainetto and Q. pubescens in both pure and mixed forests ([8], [31], [80]). Nevertheless, relatively few studies on oak decline have been carried out to date ([8], [88], [55], [31], [80], [27]).
Causes and mechanisms leading to oak decline
The oak decline is widely recognized as a complex phenomenon due to multiple causes that occur slowly over several years, even if mortality can suddenly follow decline. Manion ([63]) conceptualized the tree decline by categorizing predisposing, inciting and contributing factors. For example, predisposing factors might be associated with high competition or the presence of shallow, rocky soils, ridge top and upper slope site locations; inciting factors are interconnected to extreme climatic events as dry spells, and contributing factors are often related to secondary factors (e.g., pathogens), affecting trees already weakened. However, recent findings suggest that long-term or acute drought stress often act as a major driver of oak decline, usually linked to site-specific soil conditions, such as the characteristics of soil parent material that inhibits root development or access to groundwater ([26]), the low organic horizon that in some cases can lead to a nutrient deficiency ([30]) or to a low water retention capacity ([3]). Therefore, the conceptual model of Manion ([63]) is not always easily transferable to study cases where the same factor (e.g., drought stress) acts at different time scales and can predispose, incite or contribute to oak decline.
In general, hydraulic failure induced by drought is considered the primary mechanism responsible of widespread decline of several forest species around the globe including oaks, which are mainly anisohydric species ([20]). Drought-induced xylem embolism reduces the capacity of plants to move water from roots to leaves, altering the tree’s hydraulic system and leading trees to shoot and canopy dieback, leaf desiccation and branch mortality ([1]). Although the hydraulic failure is recognized as the main mechanism of decline, other mechanisms are often reported, such as the phloem dysfunction and carbon starvation, in addition to biotic stressors as fungi, bacteria and insects ([67]).
For example, recent studies evidenced how some functional traits as wood density ([43]), stand conditions as inter- and intra-specific competition, the level of crown transparency or the size and age of plants, co-act with drought in leading to decline. In fact, in accordance with Martínez-Vilalta et al. ([65]), intra- and interspecific competition exacerbates the negative effects of drought predisposing plants to decline. Furthermore, according to recent studies ([17], [21]), small plant size and low growth rates in the period before decline significantly predisposed some oak species to drought-induced decline (see also [66]).
Although drought is identified as the first cause of oak decline in most Mediterranean sites across Italy or Spain, in a temperate Q. robur forest located in northwestern Spain the decline was attributed to excessively elevated soil moisture ([78]). Indeed, the excess of soil water may lead to root death, increasing the predisposition of trees to other stressors ([64]).
Updating evidences of widespread oak decline in the western Mediterranean (Italy and Spain) from literature studies
We conducted a literature search through the ISI Web of Knowledge® database, and we initially selected published papers addressing the oak decline topic. Wide ranges of studies reporting evidences of oak decline from Europe were collected; however, we selected only studies conducted in the western Mediterranean Basin, with particular focus on Italy and Spain (Tab. 1).
Tab. 1 - Summary of literature studies reporting decline of Mediterranean oaks. The main traits observed in studies are reported and the symbols (+/-) indicate the observed effects (positive or negative) on considered variables. Mortality (Mor) indicates the presence of drought-killed trees. Replicated symbols emphasize their intensity. (Sym): presence of decline symptoms; (Pat): pathogens were identified; (TRW): ring width; (LW): latewood; (EW): earlywood; (HC): hydraulic conductivity; (Site number): indicate the correspondences between the published papers and the site location in Fig. 2. The main causes of observed decline are reported according to the published papers. (OR): official report available online; (ns): oak decline attributed to non-specific causes; (‡): study sites.
| Reference | Oak species |
TRW | LW | EW | HC | Sym | Mor | Pat | Causes | Site number |
|---|---|---|---|---|---|---|---|---|---|---|
| [17] | Mixed (Q. ilex-Q. faginea) |
-- | - | - | - | ++ | + | - | drought | 1 (‡) |
| [24], [25] | Mixed (Q. ilex-Q. faginea) |
-- | --- | -- | -- | ++ | + | - | drought | 2 |
| [23] | Q. pyrenaica | -- | --- | -- | -- | ++ | - | - | overaging | 3 |
| [27] | Q. ilex | - | - | - | - | - | - | - | ns | 4, 5 |
| [31] | Q. cerris | -- | - | - | - | - | - | - | drought | 6 |
| [38] | Q. pyrenaica | - | - | - | - | - | - | - | high temperature | 7-14, 16, 19, 20 |
| [38] | Q. ilex | - | - | - | - | - | - | - | high temperature | 15, 17, 18, 21 |
| [39] | Q. cerris, pubescens | -- | - | - | - | +++ | - | - | drought | 22 |
| [55] | Q. suber | - | - | - | - | ++ | - | ++ | pathogens | 23 |
| [56] | Q. suber | - | - | - | - | ++ | - | ++ | pathogens | 24 |
| [57] | Q. suber | - | - | - | - | ++ | - | ++ | pathogens | 25 |
| [58] | Q. ilex | - | - | - | - | ++ | - | ++ | pathogens | 26 |
| [71] | Q. suber | - | - | - | - | ++ | - | - | tree nutritional status | 27 |
| [75] | Q. suber | - | - | - | - | +++ | - | ++ | low temperatures | 28 |
| [21], [22] | Q. frainetto | -- | - | - | - | ++ | + | - | drought | 29 (‡), 30 (‡) |
| [80] | Q. gussonei | - | - | - | - | +++ | - | - | ns | 31 |
| [81] | Q. suber | - | - | - | - | ++ | - | ++ | pathogens | 32, 33 |
| [88] | Q. cerris | - | - | - | - | ++ | - | ++ | ns | 34, 37, 39, 42-29 |
| [88] | Q. ilex | - | - | - | - | ++ | - | ++ | ns | 35, 36 |
| [88] | Mixed | - | - | - | - | ++ | - | ++ | ns | 38, 40, 41 |
| [5] | Q. suber | - | - | - | - | ++ | +++ | ++ | pathogens | 50 |
| [13] | Mixed | - | - | - | - | - | - | - | temperature | - |
| [6] (OR) | Mixed | -- | - | - | - | ++ | - | + | pathogens | - |
| [26] | Q. suber | - | - | - | - | ++ | + | - | site-specific | - |
| [28] | Q. suber | - | - | - | - | ++ | + | ++ | interaction of factors | - |
| [35] | Mixed | - | - | - | - | - | ++ | - | ns | - |
Overall, 50 sites showing decline symptoms (crown dieback, leaf shedding, abundant dead branches or shoots, formation of epicormic shoots, increased tree mortality) were retrieved from literature studies; the stand-specific information (i.e., composition of species and management) were carefully collected from selected study sites.
The composition of sites can be summarized as follows: mixed stands (6 sites), Q. pyrenaica (12 sites), Q. ilex (9 sites), Q. cerris (12 sites), Q. suber (7 sites), Q. frainetto (2 sites), Q. gussonei (1 site), and Q. faginea (1 site). According to the distribution ranges of considered species, the elevation of sites spans from 0 up to 1600 meters above sea level, corresponding to Q. suber, Q. cerris, Q. ilex, and mixed stands situated at the lowest (100-500 m a.s.l.), and Q. pyrenaica, Q. frainetto, Q. gussonei, Q. faginea stands located at the highest (800-1200 m) elevation, respectively.
Therefore, 25 papers carried out on oak decline stands with trees growing in drought-prone areas from Mediterranean region were considered, including one national published report available online (Tab. 1).
Most of the considered studies did not directly discuss the decline of oak stands, as they often focused on pathogen attacks and soil-water dynamics. During the last decades, several defoliating insects species have been reported among the main mortality agents of oak forests, particularly in Northern America (see [45] for a review). The pathogens associated with the oak decline in Mediterranean areas are generally recognizable as secondary biotic factors (i.e., due to opportunistic organisms that develop as a consequence of the loss in tree vigor), such as root fungi of the Armillaria and Phytophthora genera ([28], [88], [75]), and stem cankers caused by Hypoxylon spp. Within these studies a large variability of symptoms is reported (i.e., foliage rarefaction and wilting, drying of branches, crown dieback, delay of spring bud burst, production of epicormic shoots, longitudinal cracks of the bark which is often detached, reduction of biomass, necrotic lesions and necrosis of absorbing roots - Fig. 3 and Fig. 4). Moreover, a reduction of primary and secondary growth and leaf area index often translates into increased risk of tree mortality.
Fig. 3 - Evidences of decline observed in oak forest stands from Southern Italy: (a) presence of dead trees; (b) diffuse brown crowns and increased crown transparency (defoliation); (c) detachment of bark; and trunk damages caused by pathogen agents such as fungi (d: Fomes fomentarius; e: Hypoxilon mediterraneum).
Fig. 4 - Two panoramic views of a Quercus ilex stand affected by the severe 2012 drought and showing canopy dieback and leaf shedding. The site is located in the Teruel province, eastern Spain (Formiche Bajo - 40° 18′ 02″ N, 0° 52′ 14″ W, 1120-1150 m a.s.l.). The triangles identified the most affected area characterized by abundant leaf death and shedding. See more details in Camarero et al. ([17]).
In the following section we underline the main characteristics of declining oak trees as depicted from tree-ring and wood-anatomical features based on published studies. Moreover, we compare site-specific evidences from two recent studies carried out in declining oak stands from the Mediterranean region to assess differences between declining and non-declining trees. Two sites subjected to Mediterranean conditions and characterized by frequent and severe droughts were chosen, the first located in eastern Spain ([17]) and the second in southern Italy ([21], [22]). These countries provide ideal settings to compare temperature effects on drought severity, since Italy is subjected to more temperate conditions, whereas most of the Peninsular Spain is subjected to more continental conditions. Moreover, the forecasted warmer conditions would exacerbate drought stress in the continental Iberian Peninsula compared to the more temperate Italian Peninsula, with severe consequences for forest ecosystems. Site-specific study cases were chosen in order to compare two broadleaf deciduous species (Q. faginea vs. Q. frainetto) forming mixed and pure forest populations managed as coppice (Q. faginea) and high forest (Q. frainetto) stands, respectively.
Drawing a declining oak from tree-rings
Studies on quantitative wood anatomy and dendrochronology have demonstrated their potential to supply useful information on the causes of tree decline, although this approach is basically observational and retrospective. Detecting growth responses to climate constitutes a powerful tool for understanding forest dynamics under climate-change scenarios especially in cases of forest decline ([60]). Moreover, the long-term reconstruction of wood anatomical features, strictly linked to the evolution of xylem anatomy plasticity through time, allows better investigating tree hydraulic adjustments in response to changing environmental conditions such as drought stress ([23]).
By comparing wood variables of declining vs. non declining trees, the following differences have been often detected: (i) a reduction of ring width; (ii) reduced diameter of earlywood vessels; (iii) a sharp decrease, or even an absence, of latewood production in ring-porous species; and (iv) abundant lumen vessels filled with tyloses, indicating a potential decrease of xylem hydraulic conductivity.
Most tree species showed a particularly low rate of growth in the years immediately preceding tree death, a feature recently recognized as related to drought-induced mortality ([9]). Levanic et al. ([54]) stated that periods of low growth rates preceding mortality are consistent with the carbon starvation mechanism of decline, but this does not exclude reduced water transport capacity in the final years before death, due to a low xylem production. Moreover, the reduced growth rate has been observed not only within the period of dieback symptoms occurrence, but also during the whole life of declining trees ([86]). However, in other studies on oak decline higher growth rates have been described in trees prone to drought-induced damage, particularly in temperate areas where water shortage rarely occurs ([54]).
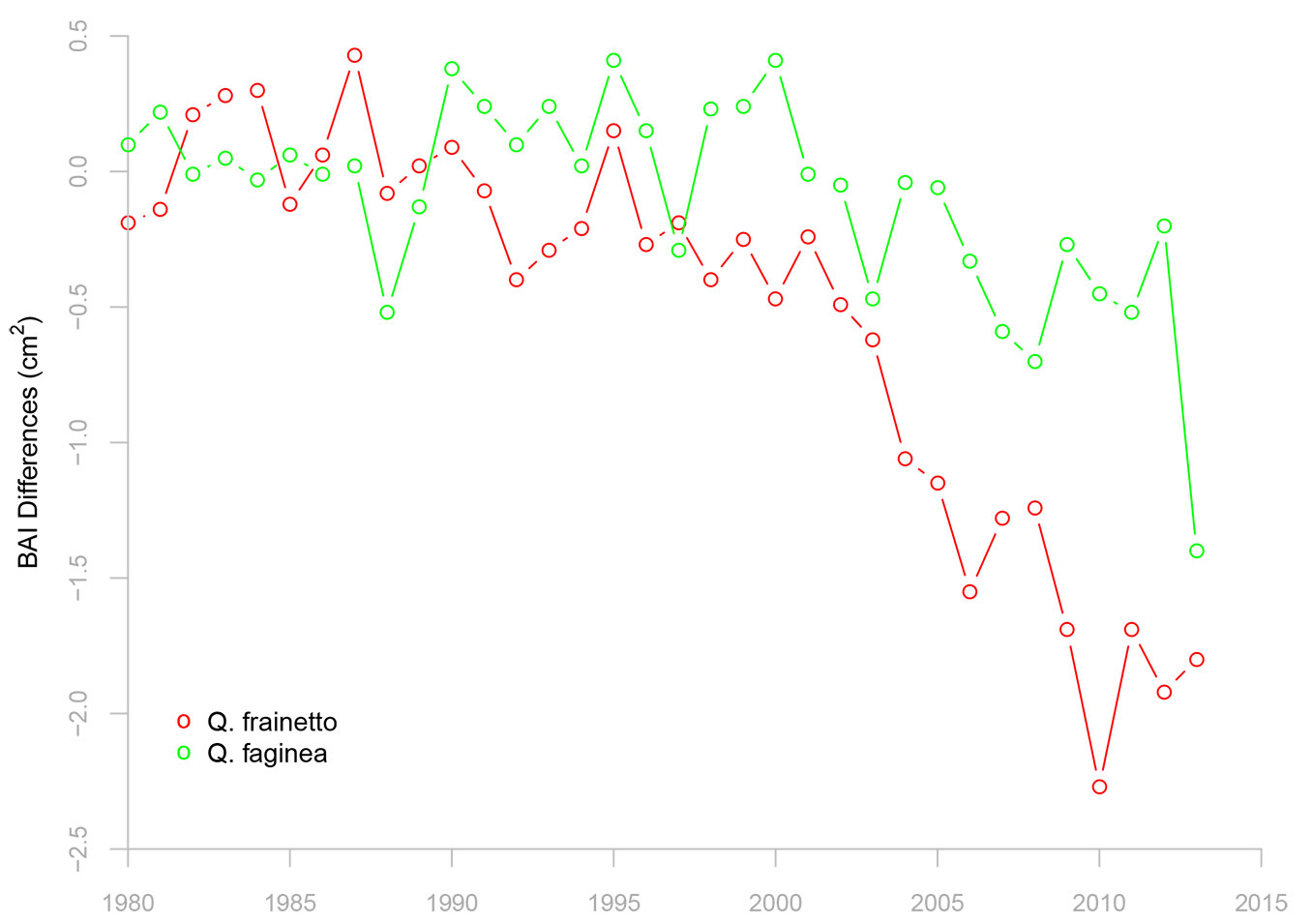
In our recent studies carried out on declining oak stands situated in southern Italy (40° 00′ 10″ N, 16° 23′ 30″ E; 770 m a.s.l.) and eastern Spain (40° 18′ 02″ N, 0° 52′ 14″ W; 1120-1150 m a.s.l.) a general and significant decreasing trend in ring width following several drought episodes was found overall after 2000s in declining trees. This reduction is reflected in an accentuated divergence in terms of basal area increment between declining and non-declining trees (Fig. 5). This irreversible growth decline evidences a long-term predisposition of trees to death (i.e., 10 to 20 years before). The usefulness of such sharp or gradual drops in growth after drought as early-warning signal of tree death has been proven based on diverse statistical tools ([22]).
Fig. 5 - Differences in basal area increment (BAI) between declining and non-declining oak trees from two Mediterranean study sites located in eastern Spain (Q. faginea, Formiche Bajo, 40° 18′ 02″ N, 0° 52′ 14″ W; 1120-1150 m a.s.l.) and southern Italy (Q. frainetto, San Paolo, 40° 00′ 10″ N, 16° 23′ 30″ E; 770 m a.s.l.). The divergence in growth between the two vigor classes abruptly increased after the 2000s droughts.
Often the growth reduction of declining trees translates in fewer and smaller earlywood vessels, thus leading to a reduced hydraulic conductivity ([37]). However, drought- and frost-induced xylem cavitation can make earlywood vessels lose their functionality in sites under Mediterranean continental conditions ([23]) and increase forest susceptibility to further water deficit with subsequent reduced production of latewood. Thus, a drop in latewood production with consequent changes in its wood anatomical features is often observed in declining trees.
Wood anatomy
Wood anatomical traits are mainly linked to hydraulic conductivity affecting xylem embolism, growth and carbon use ([49]). These traits constitute valuable indicators for determining the vulnerability of trees to water shortage.
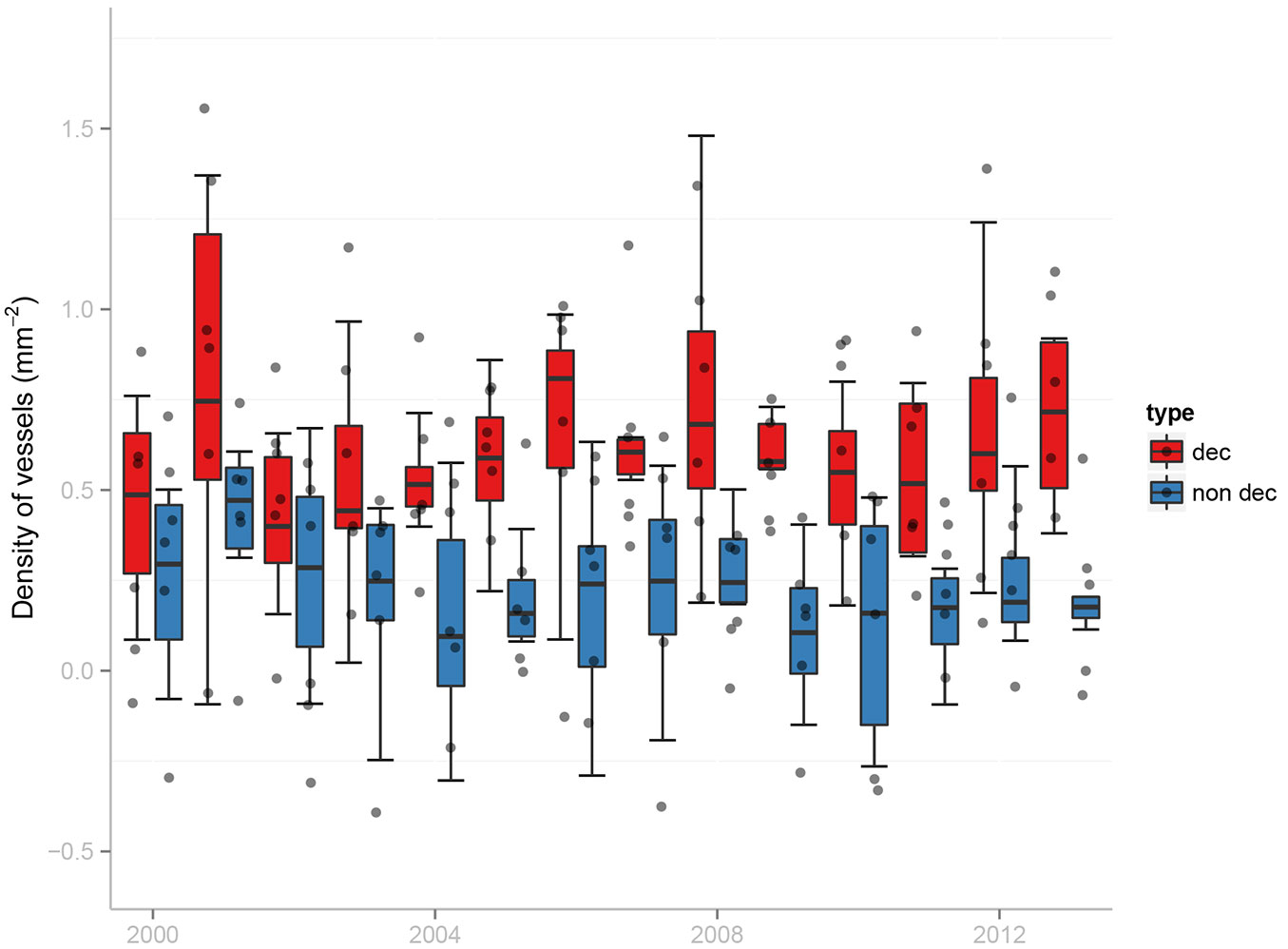
Recently, Colangelo et al. ([22]) underlined the presence of very few differences in wood anatomy features by analyzing oak trees belonging to different vigor classes, thus suggesting a low plasticity of xylem traits, in response to drought. In that study a higher latewood vessel density was observed in declining trees as compared to non-declining trees (Fig. 6). This divergence was interpreted as a compensation mechanism to provide hydraulic conductivity if earlywood vessels experience embolism.
Fig. 6 - Recent trends in latewood vessel density of declining (dec) and non-declining (non dec) Q. frainetto trees in a forest from southern Italy (modified from [21]). The graph shows an increase in latewood vessel density in declining trees. Note that vessel density data are log-transformed. Each box represents the 75th to 25th percentiles, the horizontal line shows the median, the upper and lower marks identify the ranges of variation, and the points located outside the lower-upper mark range are outliers.
Rainfall exclusions performed on Q. suber trees under semi-arid conditions underlined a high resilience of this species to inter-annual precipitation variability, with a remarkable recovery capacity after severe drought events ([10]). Moreover, recent observations from NE Spain ([6]) indicated that Q. ilex and Q.suber were vulnerable to drought-induced damage, but they were also able to recover rapidly. In a Q. ilex forest located in NE Spain, the 150 monitored trees showed a mean defoliation of 44% and 22% of trees with defoliation > 75%, after the severe 2012 drought, although only 2 (i.e., 1.3%) of highly defoliated trees died ([15]).
Noteworthy, wood anatomy may differ among oak species showing good resilience ability, as demonstrated for species with high wood density (see [40] for a review on xylem anatomical features in a global comparison of woody species). However, there is a research gap concerning the variability of wood features within oak species considering individuals of different vigor or showing different vulnerability to drought-induced mortality. For instance, Greenwood et al. ([43]) concluded that tree mortality increased with drought severity, but functional traits as wood density explained less than 10% of the variation in drought responses, although tree species with denser wood showed lower mortality responses. Remarkably, only 17% of their 58 study cases considered oak species. O’Brien et al. ([74]) also considered wood density as a potential functional proxy of susceptibility to drought-induced mortality, but they failed to find direct relationships between tree growth, functional traits and drought-induced decline and mortality.
Recent studies have introduced a new interpretation of hydraulic functioning in trees by relating phloem and xylem function ([68]). In this sense, the hydraulic capacitance of trees assumes a key role in xylem vulnerability, more than the water availability itself. This is an open area of research since we lack information on how much water trees are able to store in their sapwood and how it is transported across organs.
Climate-growth relationships
In general, most studies carried out on declining oak forests showed stand-specific growth responses to climate variables ([24], [25]). Furthermore, the few investigations comparing climate-growth relationship among trees of different decline degree, reported slight differences between non-declining and declining trees ([31], [17], [22]).
In the circum-Mediterranean region, oak radial growth is improved by wet and cool conditions during spring and summer ([85]). In particular, ring-porous and often deciduous oaks typically have high water requirements during the spring ([53], [2], [31]), due to their earlier phenology as compared to diffuse-porous oaks. Indeed, latter oaks are often evergreen species whose growth depends more on summer water availability ([24], [25], [70]). Within diffuse-porous oak species, Q. ilex and Q. suber show a particular sensitivity to spring precipitation ([24], [44], [10]).
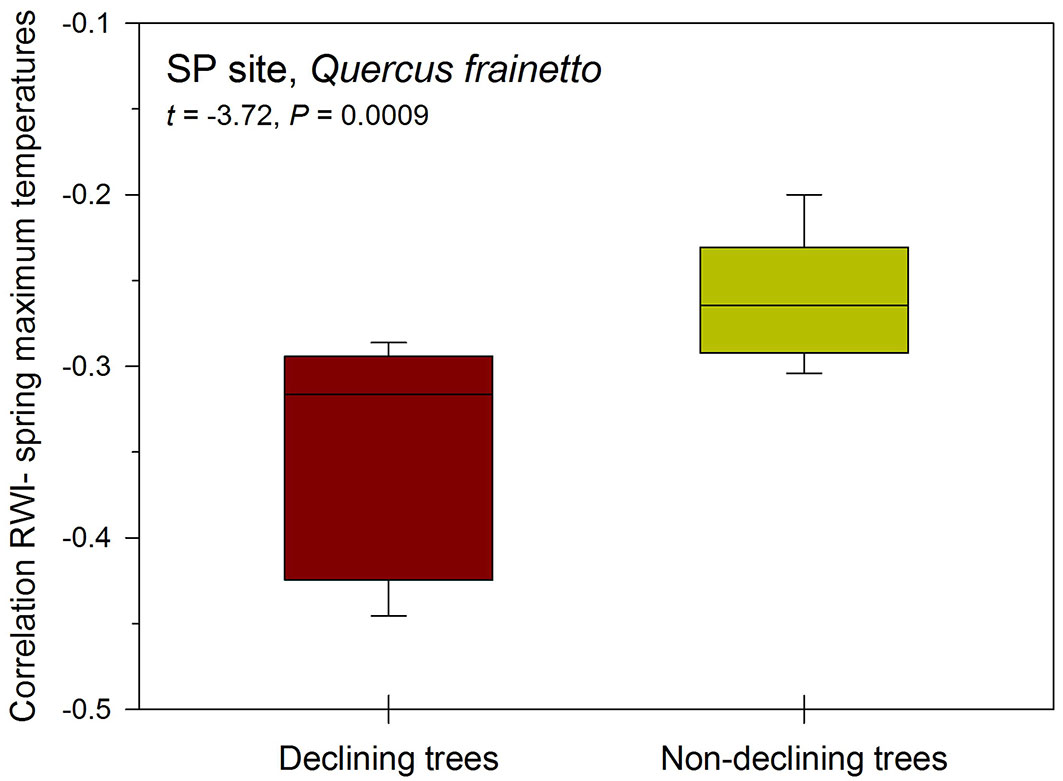
In our study cases in southern Italy we found that declining Q. frainetto trees (Fig. 7) responded more negatively to warmer spring conditions (higher mean maximum temperatures) than non-declining trees, whereas correlations with precipitation or with water balance did not differ between the two vigor classes. Interestingly, similar findings were obtained by Camarero et al. ([17]) in another Mediterranean ring-porous deciduous oak (Q. faginea). This suggests that heat stress could be a major trigger of dieback in similar Mediterranean oak species. However, this results does not explain why these deciduous species show less drought-induced damage than coexisting diffuse-porous evergreen oak species such as the widespread Q. ilex, which usually reaches a lower height ([17]). It could be speculated that taller oaks as Q. frainetto are more able to retrieve soil water under dry conditions than smaller and drought-vulnerable oak species as Q. ilex. At the same time too warm spring conditions seem to constrain more the radial growth of deciduous oak species as compared with evergreen oaks, since usually the former have an earlier onset of shoot and leaf development than the latter which are able to grow in summer ([70]). Nevertheless, it still remains unknown why evergreen species as Q. ilex, which are widely distributed across the western Mediterranean Basin, are vulnerable to drought-induced damage and why sub-Mediterranean deciduous species (Q. frainetto, Q. faginea) which are often found in less dry sites are so sensitive to excessively warm spring conditions. To answer both questions more attention should be paid to extreme climate events as summer dry spells and spring heat waves.
Fig. 7 - Box plots showing the correlations between individual ring-width indices (RWI) and spring mean maximum temperatures for declining and non-declining Q. frainetto trees sampled at the San Paolo (SP) site located in southern Italy (see Fig. 5). Declining trees responded more negatively to warmer conditions than non-declining trees (see statistics of the t-test in the upper inset). The horizontal lines within the boxes are the medians.
Generally, the investigation of oak decline associated with extreme climatic events as dry spells, showed how either exceptionally wide or narrow rings can be formed prior to tree death, albeit sudden growth reductions are often associated with decline ([89]). A recent study ([9]) carried out in America on a very large tree sample (more than 28.000 observations at the tree level) stated that died trees following drought events had not shown evidence of recovery within the last 10 years. Moreover, surviving trees showed a higher annual growth rate before and following the extreme events, compared to declining and dying trees.
Improving the resilience of declining oak stands through adaptive forest management
In the last years, the scientific community has shifted from focusing on causes of forest decline to the implementation of management actions aimed at recovering forest ecosystems severely affected by drought. The identification and development of suitable adaptive forest management strategies is essential to improve the resilience of declining stands, particularly if climate becomes warmer and drier. Indeed, these strategies should improve the capacity of stands to resist disturbances by quickly recovering ([34]). Thus, the regeneration establishment and ecological succession constitute the main outcome to achieve ([46]).
Currently, leaf loss or defoliation has been re-evaluated as a proxy to assess the health status of forests. Indeed, the percentage of crown defoliation was found strongly related to mortality rates in drought-prone areas ([18], [19]). Therefore, it can be hypothesized that an increasing probability of decline in oaks is associated with multiple early-warning indicators as leaf shedding, abundant dead branches, production of epicormic shoots, abnormal crown architecture, bark necrosis, presence of pathogens, low growth rates, etc. It is still a challenge translating these indicators of change in tree vigor into a useful measure or early-warning signal which managers can safely use to forecast the probability of forest decline or tree death. Consequently, different management options, within the perspective of adaptive forest management, need to be coherently considered to favor the resilience of stands in specific sites.
In particular, during an initial phase characterized by diffuse defoliation, one of the first management actions should be oriented at increasing the soil hydrologic balance at the tree level, by considering all the vegetation components: herbs, shrubs and understory vegetation. In the presence of degraded forests, soils should preserve its capacity of water retention and the microclimatic characteristics able to favor the natural degradation of organic matter and the turnover of nutrients. In fact, in extreme cases, soil nutrient deficiency (i.e., lower levels of soil bases associated with Al toxicity) may cause stress and could trigger mortality and reduced growth of oak species ([30]).
Thereafter, where site-specific conditions are favorable, controlled grazing could constitute an effective management tool for keeping low evapotranspiration rates at the soil level by reducing the presence of shrubs and grasses ([82]). Moreover, in some cases, the utility of maintaining wood pasture in traditional areas could be helpful also for reducing the fuel amount, thereby decreasing the risk of fires in forest stands at drought-prone areas ([76]).
Recent evidence in declining oak stands which were formerly coppices (e.g., Q. faginea, Q. ilex and Q. pyrenaica low forests in Spain) attributed their decline to an over-aging phase of trees, characterized by growth stagnation and a very low production of latewood ([24], [25], [23]). In mixed stands, Elliott & Swank ([33]) stated that mortality widely occurred in the small-size class individuals (< 10 cm in diameter) for all species. Moreover, Rozas & García-González ([78]) reported that trees in high competition stands showed a higher mortality risk due to the formation of earlywood vessels with small diameters.
In dense stands the crown and root competition for light, nutrients and water determine a reduced tree growth rate. Generally, an increasing evapotranspiration arises in the presence of a high Leaf Area Index (LAI) and an elevated density, with a negative effect on the hydrologic balance of stands. In particular, the hydraulic resistance of a stand (i.e., the ratio between the water resistance of a tree and the number of trees per stand) is expected to increase after thinning, due to the reduction of tree density. As a consequence, each single tree will increase its above and belowground biomass. Thus, deeper root systems could facilitate the water extraction from soil and raise the total individual tree water use, reducing the negative effects of drought. The increased water flux per stems will enhance the LAI, contributing to the progressively increased sapwood area of the stand. Furthermore, after thinning, the LAI will increase in the standing trees and the evapotranspiration rate, after an initial stabilization phase, will reach values similar as those observed before thinning.
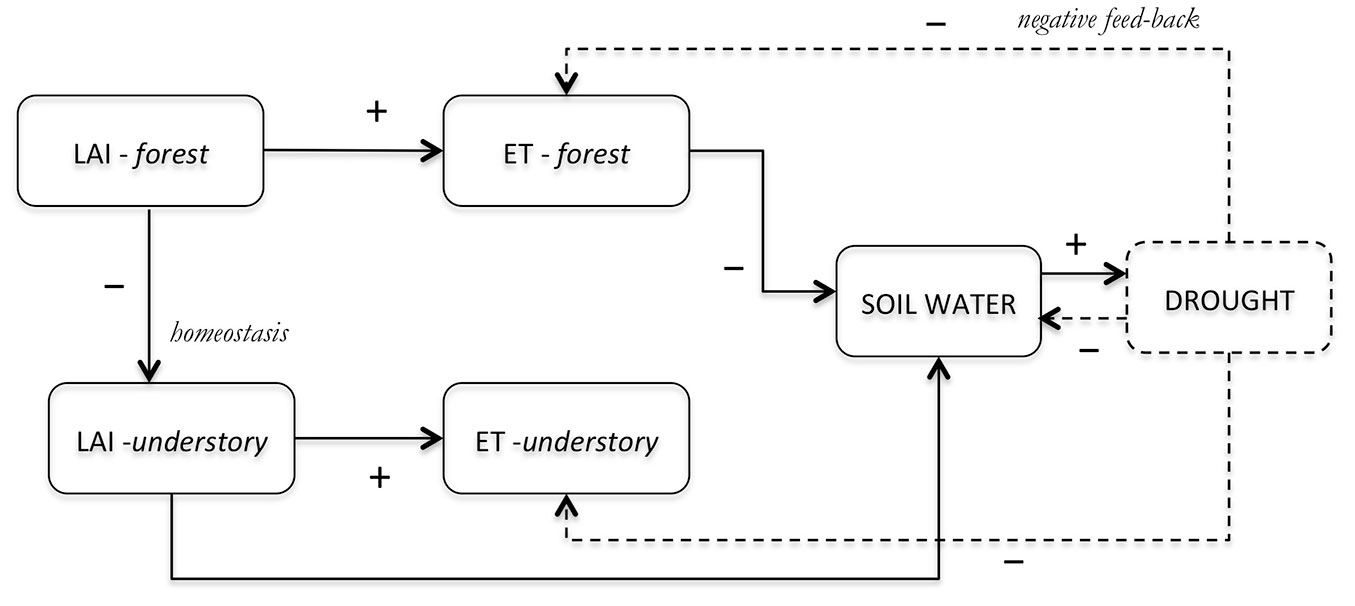
According to several studies ([77], [67]), the evapotranspiration of temperate forest ecosystems is a conservative process; this homeostasis depends on the environmental control of transpiration in trees and understory layers and on the compensation processes (negative feedback) occurring at ecosystem scale ([51]). Moreover, homeostasis is regulated by short- and long-term physiological adjustments, both strictly linked to changes in resource availability associated with tree-to-tree competition (Fig. 8).
Fig. 8 - Scheme representing the homeostasis of transpiration in a forest stand, following thinning and selection cutting. The indicators draw the flow and the symbols (+/-) report the quality of the relationship (positive or negative). In particular, by modulating the leaf area index of forests (LAI- forests), an increasing transpiration of trees (ET- forests) with the reduction of soil water content is expected, joined to a reduction of LAI and ET of understory vegetation. The possible negative feedbacks arising from drought stress are reported (dotted lines).
Management actions and silvicultural treatments, especially previous to or during dry spells, could help to regulate the equilibrium between the LAI of canopies and understory vegetation. At the stand level, sustainable harvesting aiming at reducing stand density can also result in an increased stomatal conductance that contributes to reducing drought-induced forest vulnerability ([66], [32]). Therefore, selective thinning represents an important tool for increasing the resilience of oaks in both coppices and high-forest stands showing a high level of competition. Thinning allows to reduce the intraspecific competition, thus favoring the recovery of selected trees.
Moreover, in formerly coppiced stands, long-term management actions should re-determine the optimal coppice rotation age by shortening, if necessary, the shifts of utilization in productive stands. The gradual conversion of low forests to high forests or to multi-specific coppice stands, by favoring more drought resistant species, should also be considered where the fertility and the site-specific characteristics are suitable.
Conversely, high-forest stands need different management actions, with respect to their stand-specific composition. Mono-specific high-forest stands could benefit of natural species-specific enrichment, as in mixed stands the natural regeneration of more drought resistant species is expected to contribute to the forest ecosystem equilibrium. Moreover, the establishment of different oak genotypes within stands ([87]) highly favors a successful adaptation to climatic changes (sensu [14]). In sites seriously degraded the sexual regeneration of the stands should be promoted, preventing trees from declining and losing their ability to produce viable seeds and ensuring the continuous canopy cover. Therefore, to restore heavily degraded high-forest mixed oak stands, it could be useful to anticipate the regeneration cuts. In this way, the natural regeneration will be encouraged and will replace the declining trees, while the removal of declining and dead trees will stop the spread or incidence of pest outbreaks and pathogenic fungi, also reducing the risk of fires. Moreover, even if the regeneration of native species would not be exclusively favored, mixed oak stands with high levels of diversity should also be promoted ([48]). Several studies suggest that the maintenance of a high level of tree diversity facilitates the natural adaptation processes and increases the resilience of forest ecosystems ([61], [69]).
Within forests, pure stands are more susceptible to decline and more difficult to prevent by saving their natural composition. For example, most of Italian pure oak stands reported in Fig. 2 are forest types of priority habitats for the European Union (Directive 92/43/ECC) often included in protected areas with peculiar ecological importance for biodiversity conservation (see for example Fig. 9). Some of those habitats, currently subjected to non-sustainable management, are prone to decline and need flexible management strategies promoting the resilience of oak species (sensu [42]). The loss of a single species may have profound impacts on designed protected areas, particularly when it was one of the main reasons for the designation of the reserve ([50]). Often, forestry laws tend to favor a more conservative management based on close-to-nature principles in protected natural areas. Here, specific management actions should be considered for improving the resilience of declining stands. Alongside the aforementioned management actions, in particular cases it could be useful to proceed with special protection protocols with the dissemination of locally collected seeds or by planting seedlings obtained from local germplasm, in order to preserve the species-specific stand composition.
Fig. 9 - A pure Quercus frainetto stand located in Southern Italy (Oriolo, 40° 00′ 10″ N, 16° 23′ 30″ E) showing symptoms of decline during the last three decades. The average age of trees is 140 years, the density is about 350 trees ha-1 and more than the 40% of the standing trees show a high level of decline (crown and shoot dieback, leaf shedding, growth loss and tree mortality). Selective thinning in less degraded areas and the anticipation of the regeneration cuts in heavily degraded areas should be promoted in order to stimulate the regeneration.
The proposed management actions should be addressed in selected areas, in order to evaluate the species-specific responses under different conditions. However, where results are not as successful as expected, management actions should be corrected and remodulated, following the principles of the adaptive forest management ([11]), in order to ensure the best conditions and improve the resilience of the stands.
Conclusions
In our literature search, most of studies carried out on oak decline in Italy and Spain reported drought as the main driving factor. Although this phenomenon seems generalized and strictly related to the site-specific conditions, drought and in general the ongoing climate warming are identified as the main threats to declining oak stands. Indeed, abandonment of the traditional use (periodic thinning) in the case of coppice forests, unfavorable site conditions (i.e., shallow soil, reduced fertility, steep slopes, etc.) combined with long-term drought stress seem to predispose oak stands to decline. Despite the almost full convergence of such studies on this topic, a lack of detailed information on the physiological mechanisms involved in oak decline still exists.
Declining oak trees are often characterized by a reduction of ring width, due to the absence or low production of latewood in ring-porous species. Decline is also associated to a decrease in lumen area of the widest vessels, those forming the earlywood in ring-porous species, and abundant lumen vessels filled with tyloses, indicating a potential decrease of hydraulic conductivity.
Recent studies found that smaller tree size and competition might worsen the effect of droughts on tree vigor. Thus, adaptive forest management strategies constitute essential tools for improving the resistance and the resilience of stands which show ongoing decline. In particular, the following practices could enhance the resilience of susceptible oak stands in drought-prone Mediterranean areas: (i) controlling the understory vegetation through targeted cuts and controlled grazing; (ii) reducing aboveground biomass through selective thinning; (iii) anticipating the regeneration cuts to stimulate seed dispersal and promote sexual regeneration; and (iv) shortening the rotation period of coppice stands. Furthermore, the identification of species, stands or trees prone to drought-induced decline could represent a valuable early-warning indicator to increase the attention and the sensibility at managing declining forests. Managing declining forests is a challenge but it has to be addressed to ensure the current level of ecosystem services that forest provides to humans and to assure their resilience in the near future.
Acknowledgments
This research arose from a communication held at the 10th Congress of the SISEF society (2015). The work was financially supported by the project “Alarm of forest mortality in Southern Italy” (Gorgoglione Administration, Basilicata Region, Italy). MC was supported by the PhD program from the course of Agricultural, Forest and Food Science at the University of Basilicata (Italy). Authors acknowledge three anonymous referees for their constructive comments that contributed to improve the paper. JJC acknowledges the support of the FUNDIVER project (Spanish Ministry of Economy and Competitiveness, CGL2015-69186-C2-1-R).
References
CrossRef | Gscholar
Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Online | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Michele Colangelo
Angelo Nolè
Francesco Ripullone
School of Agricultural, Forest, Food and Environmental Sciences, University of Basilicata, Potenza (Italy)
Pyrenean Institute of Ecology (IPE-CSIC), Avda. Montañana 1005, 50192 Zaragoza (Spain)
Corresponding author
Paper Info
Citation
Gentilesca T, Camarero JJ, Colangelo M, Nolè A, Ripullone F (2017). Drought-induced oak decline in the western Mediterranean region: an overview on current evidences, mechanisms and management options to improve forest resilience. iForest 10: 796-806. - doi: 10.3832/ifor2317-010
Academic Editor
Tamir Klein
Paper history
Received: Dec 15, 2016
Accepted: Jul 12, 2017
First online: Sep 25, 2017
Publication Date: Oct 31, 2017
Publication Time: 2.50 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2017
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 61428
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 47265
Abstract Page Views: 6392
PDF Downloads: 6632
Citation/Reference Downloads: 53
XML Downloads: 1086
Web Metrics
Days since publication: 3062
Overall contacts: 61428
Avg. contacts per week: 140.43
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2017): 113
Average cites per year: 12.56
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Lenticel infection in Fraxinus excelsior shoots in the context of ash dieback
vol. 12, pp. 160-165 (online: 04 March 2019)
Review Papers
Linking patterns of forest dieback to triggering climatic and weather events: an overview on Mediterranean forests
vol. 17, pp. 309-316 (online: 30 September 2024)
Technical Reports
Means of combating forest dieback - EU support for maintaining forest health and vitality
vol. 2, pp. 38-42 (online: 21 January 2009)
Review Papers
Increasing resistance and resilience of forests, a case study of Great Britain
vol. 17, pp. 69-79 (online: 21 March 2024)
Research Articles
Genetic variation of Fraxinus excelsior half-sib families in response to ash dieback disease following simulated spring frost and summer drought treatments
vol. 9, pp. 12-22 (online: 08 September 2015)
Research Articles
Contrasting holm oak provenances show different field performance but similar resilience to drought events eight years after planting in a Mediterranean environment
vol. 11, pp. 259-266 (online: 29 March 2018)
Research Articles
Temporal development of collar necroses and butt rot in association with ash dieback
vol. 10, pp. 529-536 (online: 05 May 2017)
Research Articles
Shrub facilitation of Quercus ilex and Quercus pubescens regeneration in a wooded pasture in central Sardinia (Italy)
vol. 3, pp. 16-22 (online: 22 January 2010)
Research Articles
Application of fungicides and urea for control of ash dieback
vol. 8, pp. 165-171 (online: 13 August 2014)
Short Communications
Assessing Pinus pinea L. resilience to three consecutive droughts in central-western Italian Peninsula
vol. 13, pp. 246-250 (online: 19 June 2020)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword