Influence of pH, nitrogen and sulphur deposition on species composition of lowland and montane coniferous communities in the Tatrzanski and Slowinski National Parks, Poland
iForest - Biogeosciences and Forestry, Volume 12, Issue 1, Pages 141-148 (2019)
doi: https://doi.org/10.3832/ifor2203-012
Published: Feb 27, 2019 - Copyright © 2019 SISEF
Research Articles
Abstract
Nitrogen and sulphur deposition is considered as a negative factor for biodiversity, usually leading to changes in species composition and structure of plant communities, and ultimately to the impoverishment of biodiversity. In this study we investigated the variation over time (2001, 2006, 2011) in species composition and structure of the understory vegetation at varying levels of sulphur and nitrogen deposition in two conifer plantations (>100 year-old) growing in different climate areas of Poland (Scots pine at the Slowinski National Park, northern seaside; Norway spruce at the Tatrzanski National Park, southern mountains). The structure of the floor vegetation at both sites changed markedly during the studied decade, as clearly confirmed by principal component analysis. Among the environmental variables analyzed (NH4+, NO3-, SO42-, pH in the throughfall and in soil solution sampled at two different depths), only nitrates were non-significantly correlated with PC axes. The results confirmed the negative effects of the concentration of both elements on undergrowth and tree recruitment in the coastal stand (Empetro nigri-Pinetum). On the other hand, in the mountain stand (Abieti-Piceetum) we observed an increase over time of nitrophilous species typical of the beech forest, which represent the natural vegetation of this area, suggesting a gradual natural restoration of the native vegetation in the long run.
Keywords
Nitrogen Deposition, Sulphur Deposition, Climatic Changes, Coniferous Communities, Biodiversity
Introduction
Most European forests have lost their primary character. Exploitation of tree stands, both deciduous on fertile soils and coniferous on poor soils, began with the intensive development of agriculture and the industrial revolution which took place at the beginning of the 19th century. Although the poor soils that support the growth of coniferous trees were less valuable for agricultural production, they provided precious raw material and fuel needed in industry. Past management reduced the proportion of natural broad-leaved forests from 66% to 33% of the forested area in Central Europe ([18]). Later, deforested areas were mostly planted using fast growing conifers (mainly Norway spruce and Scots pine), regardless of whether their soil requirements corresponded to the potential habitat. The results of this more than 100-year-old process of planting Scots pine trees in the lowlands and Norway spruce in the mountains are extensive, even-aged, monospecific stands very sensitive to snow and wind damage ([18]), pollutants (NOx and SO2), or outbreaks of pests and fungal infections ([40], [27], [17]). Currently, such plantations are subject to an extensive dieback across all Central Europe and to adverse changes in the habitat, especially these located on fertile deciduous forest sites.
Progress in the field of environmental protection helped to reduce the inflow of pollutants, but their concentration in the soil, along with the impact of climatic factors, still have an effect on the plant composition of forest communities. In Poland, the dieback of conifer forests in vast mountain areas had all features of ecological disaster and led to the hydrogeological instability in the drainage basins of the main Polish rivers ([23], [32], [12], [6], [14]). Currently, the restoration of forests with tree species consistent with the local potential vegetation has become a pressing need in many degraded areas. Recreating the natural structure and desirable species composition in degraded forests is referred to as rehabilitation, which may be based on natural succession and/or anthropogenic methods like planting, partial overstory removal, etc. ([18], [34]). Rehabilitation of degraded forests can be realized by transformation or conversion ([33]). The first approach consists in the anthropogenic restoration of the more desirable forest structure while maintaining its continuity ([25]); the latter is based on natural or artificial regeneration of the forest after the removal of damaged trees ([11]).
The deposition of nitrogen and sulphur has a great relevance for the functioning of the whole forest ecosystem at various levels. Overrun of critical loads affects soil processes (nitrification, mineralisation, acidification and decomposition of organic matter), individual trees (photosynthesis, drought tolerance, regeneration, susceptibility to pest and pathogens) and biodiversity (lichens, fungi, bryophytes, herbs and fauna). In soils poor in nitrogen its input can stimulate the growth of roots and stems, whereas in other types of soil it may clearly inhibit it. The increased N deposition may change the nutrition of trees by increasing the N concentration in leaves and/or decreasing the uptake of other nutrients, like Mg, K or P by trees and herbs ([5]). It may also reduce the microbial biomass of the ecosystem ([38]). Many observations showed an increase in the abundance of nitrophilous species in forests as N deposition increases ([5]). Herb species in deciduous forests respond to N deposition by, among others, higher growth rates of some nitrophilous species ([9]), but in oligotrophic pine forests dominated by bryophytes, lichens, and heather, the undergrowth dramatically changes and is replaced by common grasses (mostly Deschampsia flexuosa and Festuca ovina), thus significantly decreasing the understory diversity of these forests ([5]). It is generally accepted that the impact of pollutants like nitrogen and sulphur oxides on vegetation is negative, leading to habitat nitrification and to the decrease of soil pH; this entails the simplification of species composition in plant communities and have a negative impact on biodiversity ([13], [4], [7]).
The aim of this study was to assess the direction of changes in species composition and structure of forest floor at varying levels of sulphur and nitrogen deposition in two protected coniferous forests growing in different climate areas in Poland. Our objective was to disentangle the role of former land-use, habitat characteristics and the potential plant cover on the composition and structure of the plant community.
Materials and methods
Study sites
Slowinski National Park
The area is located on fixed dunes with gentle slopes (about 10°) and SW aspect in the Baltic coast at an altitude of 5 m a.s.l. The tree stand is composed of 110-year-old Scots pine trees planted in the phytocoenosis Empetro nigri-Pinetum (Libb. et Siss. 1939 n.n.) Wojt. 1964, which is a typical habitat for pine. The main diagnostic species, Empetrum nigrum, and the combination of species characteristic of this association were present from the beginning of the survey.
Tatrzanski National Park
The area is located in the lower montane zone at 1140 m a.s.l. on a 40° slope with N aspect. The tree stand is an approximately 140-year-old Norway spruce monoculture that was planted in the habitat of a fertile beech wood (Dentario glandulosae-Fagetum W. Mat 1964 ex Guzikowa et Kornas 1969), which later evolved into Abieti-Piceetum montanum Szaf., Pawl. et Kulcz. 1923 em. J. Mat. 1978. The beech did not appear in the understory until recently, but species of the class Querco-Fagetea have been present since the beginning of the study, as expected based on the potential vegetation of this habitat ([22]).
Data collection
The survey was conducted in two permanent plots (0.25 ha) established in 2001, according to the Programme Coordinating Centres ([28]), which are representative of the old spruce monocultures in the mountains (Tatrzanski NP) and in the coastal pine forest (Slowinski NP). The observations were carried out in three years (2001, 2006 and 2011). The factors investigated in the study included species composition, vegetation cover, throughfall, soil solution and climatic parameters.
Each plot was subdivided in one hundred squares of area 25 m2 (subplots) and surveyed to estimate the coverage of each vascular plant species in all layers. The assessment was made for each species according to the following scale: 1%, 5%, 10%, 20% up to 100%. This enabled detecting even small changes in the coverage of particular species.
The habitat preferences of the species in relation to soil pH (F) and the nitrogen concentration (N) were assessed based on the environmental indices of Ellenberg et al. ([8]). The names of syntaxa and syntaxonomic characteristics of the species were determined according to the “Guide to the determination of plant associations of Poland” ([21]).
To assess the load of nitrogen and sulphur reaching the forest soil, the concentration of these elements was estimated from the throughfall and not from the open field. Such an approach was adopted in other similar experiments ([13], [37], [7]). Throughfall was collected every month using ten five-litre polyethylene bottles per plot with 14.5 cm diameter funnels, which were replaced with 21 cm diameter polyethylene snow sleeve collectors in the winter time. The soil solution was collected using six ceramic cup lysimeters installed at the depth of 25 and 50 cm and sampled once a month in the spring and summer. Mixed samples of throughfall collected every month and mixed soil solution samples were taken for analyses. The ion chromatographic method was used to determine SO42- and NO3- concentrations in water (Dionex DX100®, Ion-Pac AS4A column). The concentration of NH4+ in water samples was determined using the Nessler method.
To calculate the annual loads of sulphur and nitrogen for each particular year the monthly concentrations of SO42-, NO3- and NH4+ were multiplied by the amount of throughfall and the obtained values were summed up.
Data analysis
All statistical analyses were performed using the packages “stats” ([29]) and “vegan” ([26]) in the R environment. In order to investigate the trends in vegetation changes, unconstrained unimodal Detrended Correspondence Analysis (DCA) was used. Since the lengths of axes were relatively short in this ordination, linear Principal Components Analysis (PCA) was conducted instead of DCA for both study plots ([16]). The significance of changes in vegetation among particular years was assessed using the “envfit” function implemented in the “vegan” package. The differences among centroids of sample scores of subplots along the first two PCA axes were tested by 999 permutations. Vegetation of a specific year was considered as a single group of vegetation data in PCA ordination. The particular years were treated as factors (2001, 2006, 2011). The value of centroids, statistics of the R2 values (goodness-of-fit) and p-value were obtained using the function “enfit”.
The “envfit” function of the package “vegan” was also applied to explore the relationships between the distribution of points (representing the subplots in the PCA ordination space) and the soil variables (nitrogen, ammonia, sulphates and pH). The impact of these variables on vegetation was assessed by plotting the direction of variation of each variable in the PCA biplots. The significance of the influence of these factors was tested by 999 permutations.
The same permutational test based on vector fitting was used to examine the impact of the soil variables (nitrogen, ammonia, sulphates and pH) on vegetation during the study. Differences in the frequency of selected species among study years were analysed using the log-likehood test (G-test). The frequency of each species in each plot was calculated as the proportion of subplots where each species occurs. Since the obtained data deviated from the normal distribution, non-parametric tests were used. The significance of changes in species cover among the sampling years was tested using the Friedman’s rank test followed by the Conover’s test for pair-wise comparisons or the Wilcoxon’s paired test in the case the species was absent in the first years of the study ([31]).
Meteorological data (average monthly temperatures and monthly precipitation) were collected for the period of 1951-2011 and analysed for particular decades in order to identify possible trends of climate changes in both areas.
Results
Slowinski NP
The forest stand in the Slowinski NP is formed by Scots pine planted in the habitat of coastal pine woodland, Empetro nigri-Pinetum, about 110 years ago. There was no bush layer in the plot. The species composition of the floor vegetation and especially the presence of species characterising this association - Empetrum nigrum - still indicate that the natural plant composition was present at the beginning of the survey. Despite the changes in the abundance and frequency of individual species in the understory, 13 species of vascular plants and 3 species of bryophytes were recorded over the ten years of observations. The undergrowth was primarily composed of species preferring acidic soils poor in nitrogen (index F = 1-2; index N = 1-3 - Tab. 1).
Tab. 1 - Changes in floor vegetation of Empetro nigri-Pinetum phytocoenosis at the Slowinski National Park (5 m a.s.l.; pine monoculture approx. 110 years old, planted in pine forest habitat). (index R=1-3): species characteristic of acidic soils; (index N=1-3): species of soils poor in nitrogen; (V-P): class of coniferous forests - Vaccinio-Piceetea; (Q-F): class of deciduous forests - Querco-Fagetea; (A): tree layer; (C): herb layer; (D): moss layer. Asterisks near the value in the frequency columns indicate that this differs significantly from the remaining values after G-test, whereas asterisks in the cover data columns indicate significant differences after Friedman’s or paired Wilcoxon’s tests (*: p<0.05; **: p<0.01; ***: p<0.001). Different letters near the value in the same row indicate significant differences (p<0.05) after Conover’s test.
| Class | Species / Layer | Frequency [%] | Cover [%] (Range / Mode) | ||||
|---|---|---|---|---|---|---|---|
| 2001 | 2006 | 2011 | 2001 | 2006 | 2011 | ||
| V-P | Pinus sylvestris / A | 99 | 99 | 100 | 20-80/50 | 10-70/60 | 10-70/50 |
| V-P | Pinus sylvestris / C | 5 | 1 | - | 1/1 | 10/10 | - |
| V-P | Vaccinium myrtillus R=2,N=3 | 100 | 100 | 100 | 10-90/60 b | 20-90/70 a | 5-80/50 b *** |
| V-P | Vaccinium vitis-idaea R=2,N=1 | 100 | 100 | 98 | 1-30/20 a | 5-20/10 b | 1-20/5 c *** |
| - | Deschampsia flexuosa R=2,N=3 | 98 | 100 | 100 | 1-40/20 b | 10-70/20 a | 5-50/10 c *** |
| V-P | Melampyrum pratense R=3,N=2 | 98 | 100 | 100 | 1-30/20 b | 10-90/20 a | 1-30/10 b *** |
| - | Quercus robur | 38 | 17 | 23 | 5-10/5 | 5-10/10 | 1-10/10 |
| - | Calluna vulgaris R=1,N=1 | 23 | 18 | 23 | 1-30/10 | 5-30/10 | 5-20/20 |
| V-P | Empetrum nigrum N=2 | 17 ** | 6 | 4 | 1-20/1 a | 5-20/5 b | 5-10/5 b *** |
| - | Quercus petraea | 14 | 18 | 20 | 1-10/10 | 5-10/10 | 1-10/10 |
| V-P | Picea abies | 12 | 9 | 12 | 1-30/5 | 5-30/20 | 10-40/10 |
| Q-F | Fagus sylvatica | 6 | 4 | 10 | 1-30/30 b | 10-30/30 b | 1-40/30 a ** |
| - | Betula pendula | 4 | 3 | - | 1-10/5 | 10/10 | - |
| - | Sorbus aucuparia | 1 | 1 | 2 | 5/5 | 10/10 | 1-10/10 |
| V-P | Luzula sylvatica | - | - | 1 | - | - | 5/5 |
| V-P | Dicranum scoparium / D | 100 | 100 | 100 | 100 | 100 | 100 |
| - | Dicranella heteromalla | ||||||
| V-P | Hylocomium splendens | ||||||
The most noticeable changes in species composition through time (2001 to 2011) at the Slowinski NP site were: (i) the progressive disappearance of Pinus sylvestris seedlings in the herb layer; (ii) the replacement of Quercus robur with Q. petraea in the understory; (iii) the increasing share (6% to 10%) of beech seedlings; and (iv) the significant decrease in the characteristic species Empetrum nigrum (17% to 4% - Tab. 1).
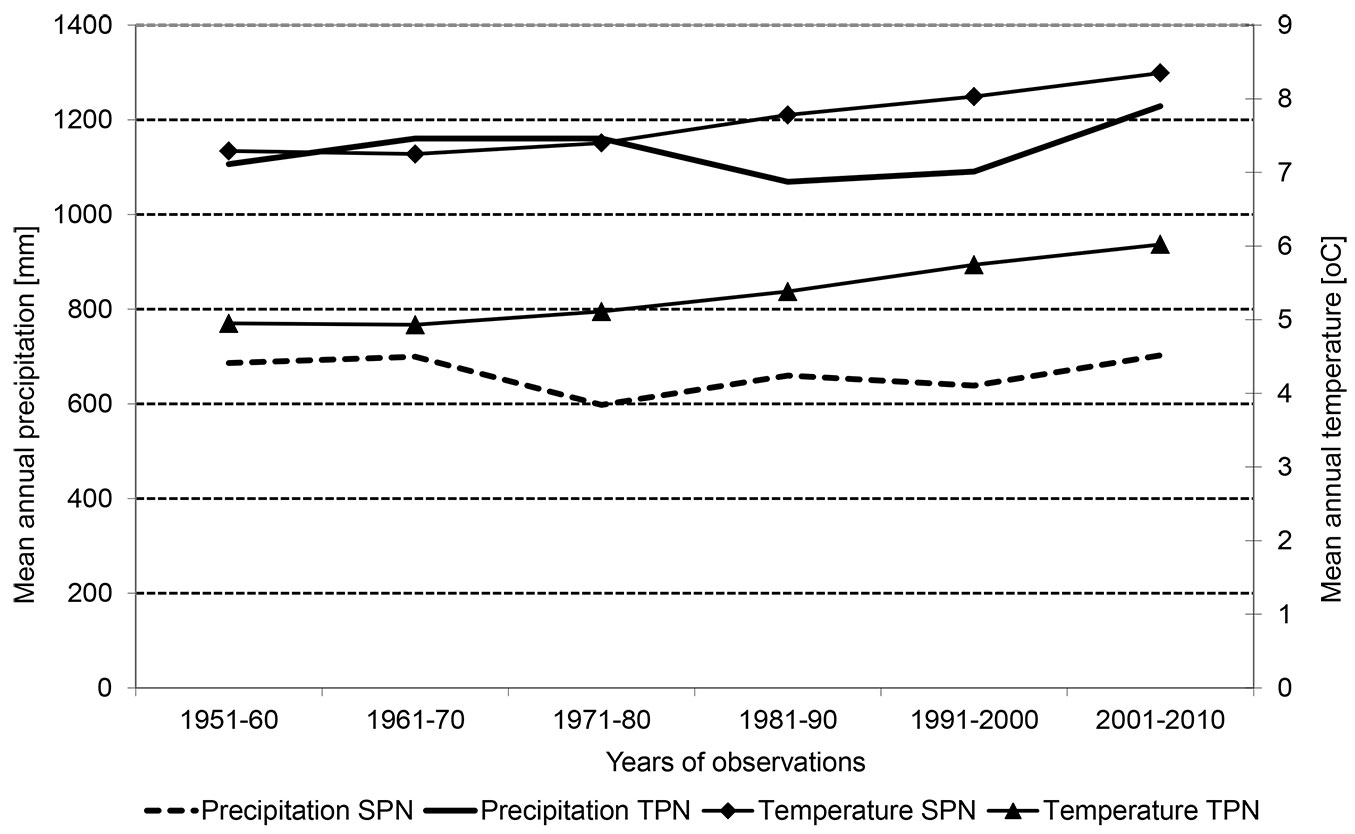
An increase in the average annual temperature of 1 °C has been recorded in the analysed area since the 1960s, while the average precipitation has remained at the same level (Fig. 1).
Fig. 1 - Mean annual precipitation and temperature in decades during the period of 60 years in two analysed areas. (SPN): Slowinski National Park; (TPN): Tatrzanski National Park.
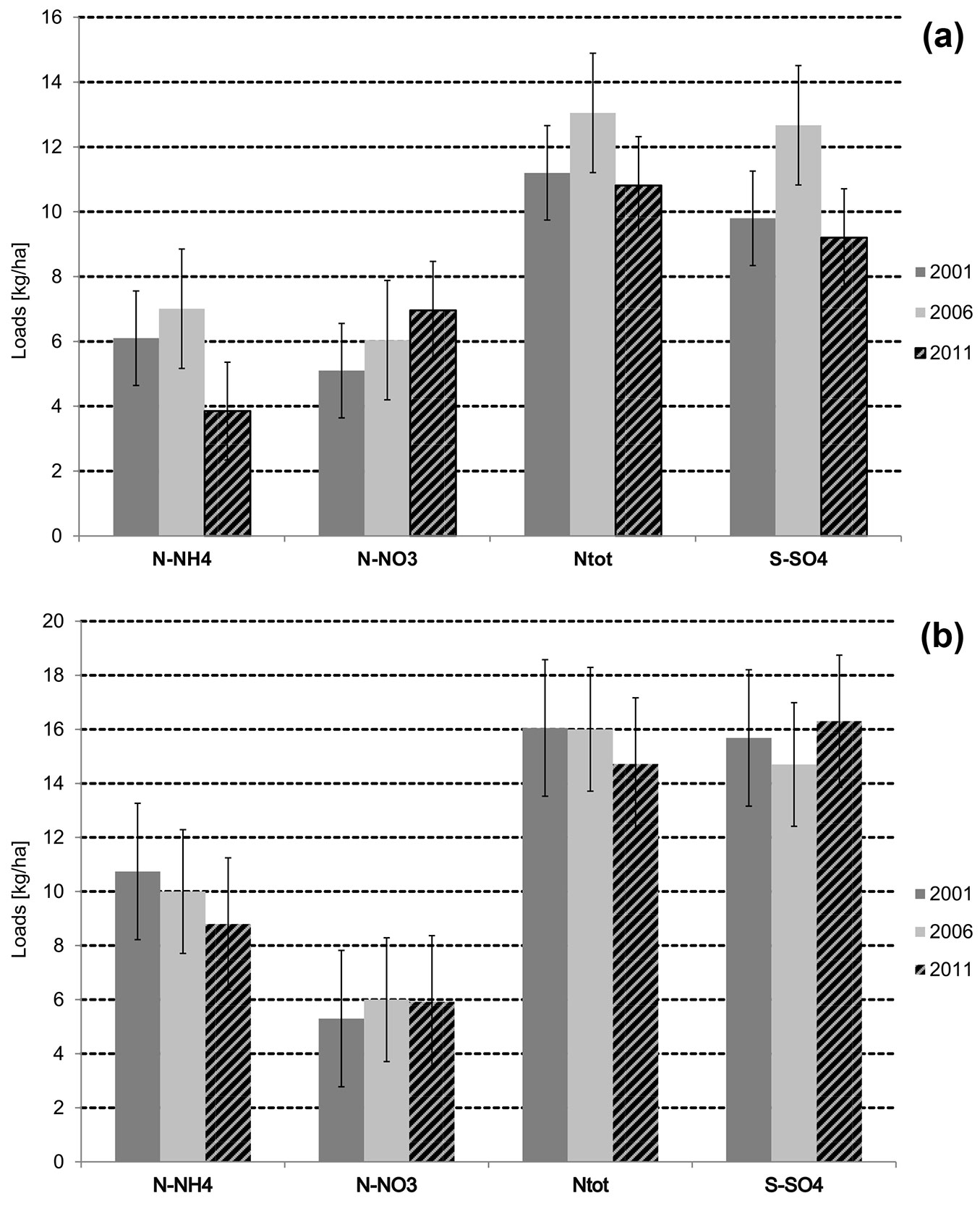
The input of total nitrogen into the forest soil in 2006 estimated by throughfall analysis increased to over 13 kg ha-1 (Fig. 2a), while it declined to a lower level in 2011. A similar pattern was observed for the sulphur load, which was somewhat lower than the nitrogen load in each investigated year and ranged from 9.2-12.6 kg ha-1 (Fig. 2a).
Fig. 2 - Comparison of annual nitrogen and sulphur loads in the throughfall in the Slowinski National Park (a) and in the Tatrzanski National Park (b), in 2001-2011.
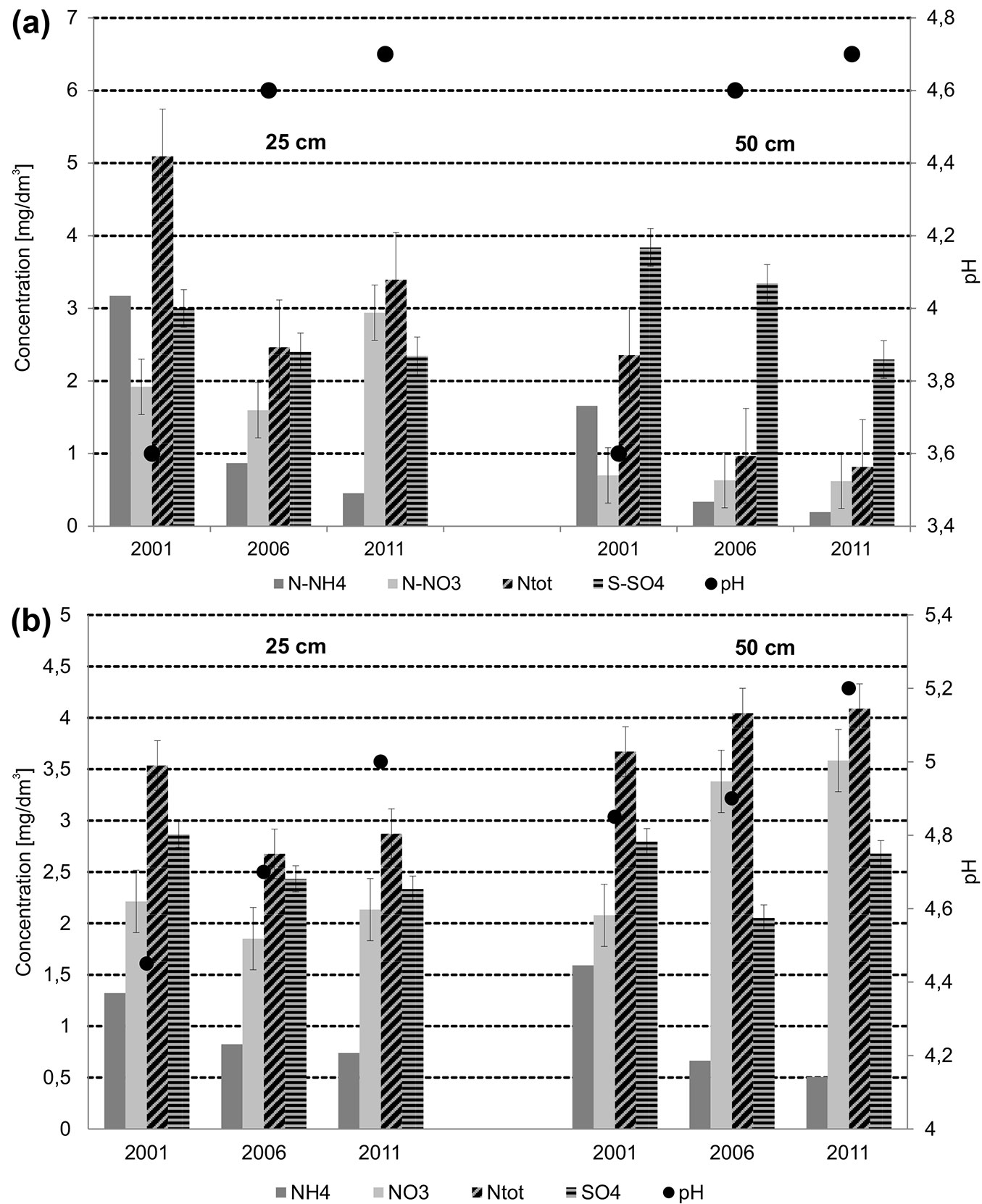
During the study period a decrease in the concentration of N and S in soil solutions from both shallow and deep layers was observed. The most pronounced decrease in concentrations was found for N-NH4+. These decreasing values are reflected by the increase of pH values in the soil solution from both layers (Fig. 3a).
Fig. 3 - Concentrations of N and S compounds and pH values in the soil solution at two depths in the Slowinski National Park (a) and in the Tatrzanski National Park (b), in 2001-2011.
Tatrzanski NP
The Tatrzanski site is a 140 year-old monoculture of Norway spruce planted in the habitat of beech forest. The ratio of the number of species representing the class Vaccinio-Piceetea to the species of Querco-Fagetea, changed from 7:6 to 7:11 and 7:10 in 2001, 2006 and 2011, respectively. Beech occurred as a single seedling only in one observation season (Tab. 2).
Tab. 2 - Changes in floor vegetation of coniferous phytocoenosis (Abieti-Piceetum) at the Tatrzanski National Park (1140 m a.s.l.; spruce monoculture approx. 140 years old, planted in the habitat of fertile beech forest Dentario glandulosae-Fagetum). (Index N=7-8): species of soils rich in nitrogen; (index N = 1-3): species of soils poor in nitrogen; (index R = 7-8): species of alkaline soils; (index R = 1-3): species of acidic soils; (V-P): class of coniferous forests - Vaccinio-Piceetea; (Q-F): class of deciduous forests - Querco-Fagetea; (M-A): class of subalpine and montane tall-herbs - Mulgedio-Aconitetea; (A): tree layer; (C): herb layer; (D): moss layer. Asterisks near the value in the frequency columns indicate that this differs significantly from the remaining values after G-test, whereas asterisks in the cover data columns indicate significant differences after Friedman’s or paired Wilcoxon’s tests (*: p<0.05; **: p<0.01; ***: p<0.001). Different letters near the value in the same row indicate significant differences (p<0.05) after Conover’s test.
| Class | Species / Layer | Frequency [%] | Cover [%] (Range / Mode) | ||||
|---|---|---|---|---|---|---|---|
| 2001 | 2006 | 2011 | 2001 | 2006 | 2011 | ||
| V-P | Picea abies / A | 100 | 100 | 95 | 5-90 / 70 | 5-90 / 60 | 5-80 / 50 |
| V-P | Picea abies / C | 3 | - | - | 1 | - | - |
| - | Oxalis acetosella | 100 | 100 | 100 | 20-90 / 40 | 20-80 / 30 | 20-90 / 50 |
| - | Athyrium filix-femina | 99 | 100 | 98 | 10-70 / 30 | 10-60 / 30 | 10-70 / 30 |
| V-P | Dryopteris dilatata N=7 | 98 | 99 | 100 | 10-60 / 30 | 10-50 / 30 | 5-60 / 20 |
| Q-F | Prenanthes purpurea | 92 ** | 98 | 100 | 5-50 / 20 c | 10-40 / 20 b | 5-60 / 30 a *** |
| - | Rubus idaeus | 66 | 78 | 77 | 5-50 / 10 | 5-50 / 10 | 5-50 / 20 |
| V-P | Luzula sylvatica | 43 | 40 | 43 | 1-20 / 10 | 1-10 / 10 | 5-70 / 10 |
| - | Sorbus aucuparia | 37 | 42 | 41 | 1-10 / 10 | 1-10 / 10 | 5-30 / 10 |
| Q-F | Dryopteris filix-mas | 25 | 26 | 47 ** | 5-20 / 10 b | 5-20 / 10 b | 5-40 / 20 a *** |
| V-P | Homogyne alpina N=2 | 20 | 19 | 28 ** | 1-20 / 5 | 5-10 / 10 | 1-10 / 20 * |
| - | Petasites albus | 16 | 15 | 18 | 1-20 / 10 | 5-20 / 10 | 5-20 / 10 |
| - | Sambucus racemosa N=8 | 16 | 21 | 28 | 5-20 / 10 b | 5-20 / 10 b | 10-50 / 20 a *** |
| - | Gentiana asclepiadea N=2,R=7 | 14 | 15 | 41 | 1-10 / 10 b | 5-10 / 10 b | 5-30 / 10 a *** |
| Q-F | Stellaria nemorum N=7 | 14 | 18 | 52 ** | 1-10 / 10 | 1-10 / 10 | 5-20 / 5 *** |
| Q-F | Galeobdolon luteum | 12 | 17 | 27 * | 1-10 / 5 | 5-10/5 | 5-20 / 10 *** |
| M-A | Polygonatum verticillatum | 8 | 9 | 9 | 1-10 / 10 | 5-10 / 10 | 5-10 / 10 |
| M-A | Doronicum austriacum R=7,N=7 | 8 | 12 | 11 | 5-10 / 5 | 1-10 / 5 | 1-20 / 10 |
| V-P | Vaccinium myrtillus R=2,N=3 | 8 | 8 | 7 | 5-10 / 10 | 5-10 / 5 | 5-10 / 10 |
| - | Cardamine trifolia R=8,N=7 | 6 | 7 | 8 | 5-10 / 5 b | 5-10 b | 5-20 / 10 a * |
| - | Mycelis muralis | 4 | 4 | 6 | 10 / 10 | 5-10 / 10 | 5-10 / 10 |
| V-P | Calamagrostis villosa R=2,N=2 | 4 | 1 | 2 | 1-10 / 5 | 5 | 5-10 |
| M-A | Calamagrostis arundinacea. | 3 | 3 | 6 | 1-5 / 1 b | 1-5 / 5 b | 5-20 / 10 a |
| M-A | Senecio nemorensis | 3 | 4 | 5 | 5 / 5 | 1-5 / 5 | 5-10 / 10 |
| Q-F | Luzula luzuloides R=3 | 2 | 4 | 5 | 1 / 1 | 1-5 / 1 | 5-10 / 5 |
| Q-F | Lonicera xylosteum R=7 | 2 | 3 | 7 | 10-20 / 20 b | 10-20 / 10 b | 5-30 / 20 a ** |
| - | Hieracium murorum | 2 | 2 | 4 | 5-10 | 10 / 10 | 1-10 / 10 |
| - | Carex pilulifera R=3,N=3 | 1 | 1 | - | 1 / 1 | 1 / 1 | - |
| M-A | Streptopus amplexifolius | 1 | 1 | 4 | 5 / 5 | 5 / 5 | 5-10 / 10 |
| - | Soldanella carpatica | 1 | 2 | 4 | 10 / 10 | 5-10 | 5-10 / 5 |
| M-A | Veratrum lobelianum | 1 | - | 1 | 5 / 5 | - | 20 |
| Q-F | Fagus sylvatica | - | 1 | - | - | 1 / 1 | - |
| - | Gymnocarpium dryopteris | - | 2 | 3 | - | 1-10 | 5-10 / 5 |
| - | Phegopteris connectilis | - | 12 | 38 | - | 1-10 / 10 | 1-20 / 5 |
| Q-F | Actaea spicata N=7 | - | 6 | 4 * | - | 1-10 / 5 | 5-10 / 10 |
| Q-F | Phyteuma spicatum | - | 1 | 3 | - | 5 / 5 | 5-10 / 10 |
| M-A | Adenostyles alliariae N=8 | - | 2 | 15 *** | - | 5-10 | 10-80 / 60 *** |
| Q-F | Acer pseudoplatanus N=7 | - | 4 | 4 * | - | 10 / 10 | 10-30 / 10 |
| Q-F | Dentaria glandulosa R=7,N=7 | - | 1 | 3 | - | 5 | 1-10 / 10 |
| V-P | Plagiothecium undulatum / D | 100 | 100 | 90 | 10-100 | 10-90 | 10-80 |
| - | Polytrichum attenuatum | ||||||
The death of a number of spruces in the central part of the plot significantly altered the light conditions in the forest floor, and likely affected the composition and structure of the herb layer. Indeed, we recorded an increase in the percentage of species having higher light requirements (index L = 7), such as Gentiana asclepiadea, Rubus idaeus, and Veratrum lobelianum. Among the most acidophilic species (index R = 2) only the frequency of Vaccinium myrtillus remained constant. Species requiring soils with higher pH (index R = 7-8), such as Gentiana asclepiadea and Galeobdolon luteum, significantly increased their share over time (Tab. 2). Others, like Cardamine trifolia and Lonicera xylosteum, also increased their frequency, albeit to a lesser extent. Among the species that do not tolerate a higher content of nitrogen in soil (index N = 2) only Gentiana asclepiadea and Homogyne alpina significantly increased their share in the undergrowth. Other species of the same group, such as Vaccinium myrtillus, remained stable, whereas Calamagrostis villosa reduced slightly its share. Moreover, species that tolerate a higher nitrogen content in soil (index N = 7-8), such as Sambucus racemosa, Stellaria nemorum, Cardamine trifolia and Adenostyles alliariae significantly increased their share in the phytocoenosis. In the entire studied period an increase in the number of vascular plant species from 30 to 37 was observed (Tab. 2).
A remarkable change in the undergrowth composition occurred in 2011, likely related with an increase of the soil pH (Fig. 3b). The subplots from 2011 were the most diverse in terms of species composition, while subplots from 2001 and 2006 were much closer to each other.
Sulphur and ammonia were significantly correlated and their content was higher in subplots from 2001 and 2006. As mentioned above, the significant change in the light conditions in the plot, a marked increase in precipitation (after decreasing for 20 years) and the increase in the average annual temperature of 1 °C also could have significantly affected the present structure of floor vegetation at the Tatrzanski NP site (Fig. 1).
Changes in the input of nitrogen and sulphur were slightly different in this plot compared with the Slowinski NP site. Total nitrogen concentration in the throughfall did not vary in 2001 and 2006, and slightly declined from 16 to 15 kg ha-1 in 2011. Over the whole investigated period the annual loads of the total nitrogen and sulphur in the Tatrzanski NP site were at a similar level and ranged from 15 to 16 kg ha-1 and from 15 to 16.5 kg ha-1, respectively (Fig. 2b).
In the analysed period, N concentration in the soil solution decreased in the shallow layer and increased in the deeper layer, while S concentration decreased in both layers. The most pronounced decrease in concentrations was observed for N-NH4+. In the soil solution of both layers a systematic increase in the pH value from 2001 to 2011 was recorded (Fig. 3b).
Relationship between environmental factors and species composition
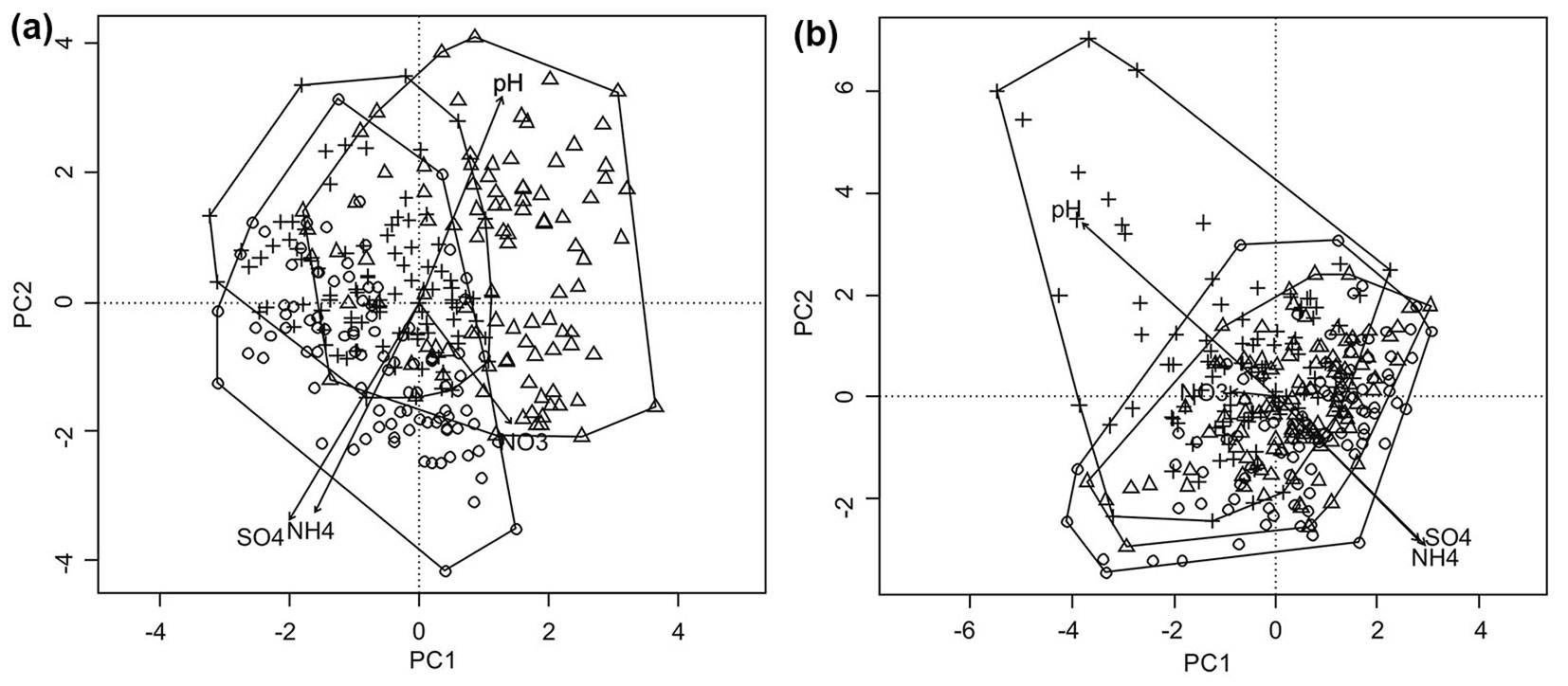
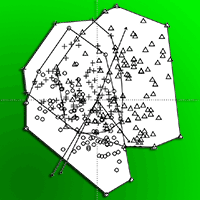
The structure of the floor vegetation at both sites changed markedly during the studied decade (Fig. 4). Indeed, the distance between subplots in Tatrzanski NP from 2001 and those from 2011 as well as subplots in Slowinski NP from 2006 is well reflected by their separation along the first axis of PCA.
Fig. 4 - Ordination diagram of subplots (100 squares) along the first two axes of Principal Components Analysis (PCA) based on species cover on permanent plots in (a) the Slowinski National Park and (b) in the Tatrzanski National Park, over the period 2001-2011. (Circles): 2001; (triangles): 2006; (crosses): 2011.
The higher contents of ammonia and sulphates were significantly associated with the ordination of subplots from 2001, while subplots from 2006 in case of the Slowinski NP and 2011 in the Tatrzanski NP were characterised by a significantly higher pH (Fig. 3a, Fig. 3b). The significance of changes among the years is reported in Tab. 3. Among the variables tested (NH4+, NO3-, SO42-, pH, year), only nitrates were non-significantly correlated with the obtained ordination (Fig. 3a, Fig. 4a, Tab. 3).
Tab. 3 - Results of vector fitting of time as a factor (2001, 2006, 2011) and environmental variables in Principal Components Analysis of vegetation in the studied plots. (ns): non significant.
| Site | Variable | PC1 | PC2 | R2 | Prob. |
|---|---|---|---|---|---|
| Tatrzanski NP | NH4+ | 0.7048 | -0.70934 | 0.1569 | 0.001 |
| NO3- | -0.9959 | 0.09024 | 0.0071 | ns | |
| SO42- | 0.7095 | -0.70473 | 0.1681 | 0.001 | |
| pH | -0.7428 | 0.66951 | 0.2574 | 0.001 | |
| year | -0.7392 | 0.67349 | 0.2485 | 0.001 | |
| Slowinski NP | NH4+ | -0.44113 | -0.89744 | 0.1898 | 0.001 |
| NO3- | 0.60251 | -0.79811 | 0.0811 | 0.001 | |
| SO42- | -0.51134 | -0.85938 | 0.2211 | 0.001 | |
| pH | 0.37709 | 0.92618 | 0.1676 | 0.001 | |
| year | 0.01124 | 0.99994 | 0.1022 | 0.001 |
The subplots from the Slowinski NP in 2001 were characterised by a higher content of NH4+ and SO42-. A greater influence of pH on the structure of the floor vegetation was observed in 2006, whereas ammonia changed across the years (Fig. 4a). In the Tatrzanski NP the situation was different and the greatest impact of pH was noted in 2011, whereas vegetation changes seems to be associated to the higher content of ammonia and sulphates in 2001 and 2006 (Fig. 4a).
Discussion
The choice of assessing nitrogen and sulphur deposition by throughfall analysis may seem disputable due to the differences between the content of individual elements in throughfall and the precipitation collected in the open field. At low N deposition, the loads of N-NO3 and N-NH4 were found to be lower in throughfall than in the open field due to nitrogen uptake from the canopy ([10]). However, the throughfall N deposition is generally higher than the open field N deposition, because the former also includes the dry deposition of particles on tree canopies ([3], [36], [10]). Such regularity was also found in a dozen spruce stands in southern Poland ([35]). Therefore, in order to determine the real input of nitrogen to the forest floor the throughfall analysis was chosen in this study.
In the last few decades, climate change and the deposition of air pollutants have significantly affected the structure of vegetation all over the globe. Generally, the observed effects of these changes have been considered as negative, in terms of simplification of the plant community structure, decrease in biodiversity, reduction in the distribution ranges of plants or changes the habitat of stenotypic species, which are the most sensitive to any environmental changes due to their low adaptive potential ([15], [30], [1], [20], [19], [24], [2]).
In our study the aforementioned effects of climate change and deposition of air pollutants were more complex and differed between the two examined areas. At the Slowinski NP site we recorded the disappearance of species characterising the coastal coniferous forest Empetro nigri-Pinetum, while other species preferring fertile habitats appeared during the investigated decade (2001-2011). Such change in the typical composition of this plant community has been reported for many areas in Europe ([4], [7]) and could be due to the interplay among several factors. Research on the Danish coastal dune heaths showed a linear increase in the share of Empetrum nigrum increasing the concentration of nitrogen in the soil ([24]), although other studies did not reveal such a dependence ([39]). Furthermore, a decrease through time in the frequency of Quercus robur (a species with a continental distribution range), and at the same time an increase in the share of Q. petraea (a species with an Atlantic distribution range) were observed in the undergrowth at the Slowinski NP site. Although the observation period was too short to draw far-reaching conclusions, the possibility exists that a slight increase in global warming may foster species typical of warmer climates and disadvantage those more adapted to colder climates. Therefore, we can conclude that the combined effect of climate change and the deposition of air pollutants at the Slowinski NP have a negative effect on local biodiversity.
The results of the studies carried out in the mountain plot of the Tatrzanski National Park were different. Across all Western Carpathians a dramatic reduction of the vitality of Norway spruce plantations in fertile habitats at lower altitudes occurred, and restoration activities aimed at the establishment of the native vegetation are taking place in many areas. Although the situation is similar to that in the Tatra Mts. (southern Poland), deforestation at Tatrzanski did not occur as it was taken under protection as a national park. Here, the impoverishment of the habitat through the long-term planting of Norway spruce resulted in the natural elimination of many undestory species typical of the beech forest (Dentario glandulosae-Fagetum), which constitute the potential vegetation in this area. In this case, the nitrogen enrichment of the habitat due to atmospheric deposition may favour the return of nitrophilous species that are typical of the fertile deciduous forests. This is clearly indicated by the changes in the undergrowth that we observed in this study, and in this context the effect of N deposition on local biodiversity would be positive. Indeed, the appearance of Acer pseudoplatanus and species of the class of subalpine tall herb (Mulgedio-Aconitetea) suggest the incipient conversion of this phytocoenosis into the rich habitat Natura 2000 code 9140 (Central European subalpine and mountain beech forests of sycamore), which is supported by the topography (steeply inclined slope). Nonetheless, the presence of beech seedlings in the undergrowth has been observed only sporadically in the study area, and this could hamper the natural restoration of the native beech forest. Any support for the phytocoenosis (e.g., beech planting), could enhance the biodiversity of this area and restore the potential vegetation which is consistent with the natural habitat.
Conclusions
The variation over time of soil acidity (pH) seems to have the greatest impact on species composition and structure of the forest floor at both sites. However, a significant increase in the share of extreme nitrophilous species was also observed over the analysed period. Not all species considered as bioindicators (e.g., Vaccinium myrtillus or Calluna vulgaris) showed a clear relationship with the analysed environmental parameters. However, all the diagnostic species for a given phytocoenosis or for higher syntaxonomic units showed the most significant variation.
In the case of the Scots pine stand (Slowinski NP), the floristic changes recorded over 10 years suggest the gradual disappearance of the main diagnostic species for this plant community (Empetro nigri-Pinetum). Contrastingly, in the case of the Norway spruce stand (Tatrzanski NP) planted in the habitat of the fertile beech wood (Dentario glandulosae-Fagetum), the floristic changes recorded suggest the occurrence of a gradual restoration in the long term of the native vegetation of the area (beech forest).
Further studies are needed in order to better elucidate the impact of changes in soil pH or the deposition of nitrogen and sulphur on the understory vegetation, in the light of the history of land-use and the potential vegetation of the habitat.
Acknowledgments
This study was co-financed under the European Union Life+ project “EnvEurope - Environmental quality and pressures assessment across Europe: the LTER network as an integrated and shared system for ecosystem monitoring” (⇒ http://www.enveurope.eu/), LIFE08 ENV/IT/000399. We would like to thank Mrs Michele Simmons and Mrs Miroslawa Cyrana-Szram for proofing the English version of the text.
References
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
University of Silesia, Department of Ecology, 28 Jagiellonska St., 40-032 Katowice (Poland)
Institute for Ecology of Industrial Areas, 6 Kossutha St., 40-844 Katowice (Poland)
University of Bielsko-Biala, Department of Ecology and Nature Conservation, 2 Willowa St., 43-309 Bielsko-Biala (Poland)
Corresponding author
Paper Info
Citation
Uzieblo AK, Staszewski T, Chmura D (2019). Influence of pH, nitrogen and sulphur deposition on species composition of lowland and montane coniferous communities in the Tatrzanski and Slowinski National Parks, Poland. iForest 12: 141-148. - doi: 10.3832/ifor2203-012
Academic Editor
Giustino Tonon
Paper history
Received: Aug 21, 2016
Accepted: Jan 12, 2019
First online: Feb 27, 2019
Publication Date: Feb 28, 2019
Publication Time: 1.53 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2019
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 44643
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 37654
Abstract Page Views: 3346
PDF Downloads: 2801
Citation/Reference Downloads: 6
XML Downloads: 836
Web Metrics
Days since publication: 2558
Overall contacts: 44643
Avg. contacts per week: 122.17
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
(No citations were found up to date. Please come back later)
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Deposition measurements and critical loads calculations: monitoring data, results and perspective
vol. 2, pp. 11-14 (online: 21 January 2009)
Research Articles
Nitrogen deposition and its impact on forest ecosystems in the Czech Republic - change in soil chemistry and ground vegetation
vol. 10, pp. 48-54 (online: 29 June 2016)
Research Articles
Effects of arbuscular mycorrhizal fungi on microbial activity and nutrient release are sensitive to acid deposition during litter decomposition in a subtropical Cinnamomum camphora forest
vol. 16, pp. 314-324 (online: 13 November 2023)
Short Communications
QA/QC activities and ecological monitoring in the Acid Deposition Monitoring Network in East Asia (EANET)
vol. 2, pp. 26-29 (online: 21 January 2009)
Short Communications
An approach to measuring biodiversity and its use in analysing the effect of nitrogen deposition on woodland butterfly populations in the Netherlands
vol. 2, pp. 46-48 (online: 21 January 2009)
Research Articles
Acid atmospheric deposition in a forested mountain catchment
vol. 10, pp. 680-686 (online: 17 July 2017)
Research Articles
Response of soil bacterial communities to nitrogen and phosphorus additions in an age-sequence of subtropical forests
vol. 14, pp. 71-79 (online: 11 February 2021)
Short Communications
The importance of forest type when incorporating forest edge deposition in the evaluation of critical load exceedance
vol. 2, pp. 43-45 (online: 21 January 2009)
Research Articles
Long-term monitoring of air pollution effects on selected forest ecosystems in the Bucegi-Piatra Craiului and Retezat Mountains, southern Carpathians (Romania)
vol. 4, pp. 49-60 (online: 05 April 2011)
Review Papers
Monitoring the effects of air pollution on forest condition in Europe: is crown defoliation an adequate indicator?
vol. 3, pp. 86-88 (online: 15 July 2010)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword