Distribution and concentration of cadmium in root tissue of Populus alba determined by scanning electron microscopy and energy-dispersive x-ray microanalysis
iForest - Biogeosciences and Forestry, Volume 1, Issue 2, Pages 96-103 (2008)
doi: https://doi.org/10.3832/ifor0458-0010096
Published: May 20, 2008 - Copyright © 2008 SISEF
Research Articles
Abstract
In a polluted environment with potentially toxic elements, plants may play a relevant role on contaminant absorption or stabilization. The aim of this study was to discriminate two poplar clones in their tolerance to high Cd concentrations (50 μM) in the growth solution and to show the potential of poplar in the cleaning-up of Cd-contaminated substrate. Chemical analyses to determine the content of heavy metals in biological samples involve their destruction by digestion with concentrated acids, preventing the localization of potentially toxic elements in situ. In contrast, scanning electron microscopy equipped with energy-dispersive x-ray microanalysis may provide information on element localization and chemical composition of biological samples. Scanning electron microscopy allows for the observation of samples in a dry or wet state, at high magnifications and good field depth with a minimum preparation, and the possibility to combine structural and analytical information by energy-dispersive x-ray microanalysis and digital images. Although, energy-dispersive x-ray microanalysis has relatively low detection limits, it is useful in establishing distribution maps of potentially toxic elements inside cells and tissues. Tissue concentration and localization of Cd (and other elements) in root tips of Populus alba clones (6K3 and 14P11) were investigated, using two different types of scanning electron microscope (ambient temperature and low temperature) both coupled with energy-dispersive x-ray microanalysis. These techniques were useful to investigate structural modifications and to identify in situ concentration and distribution of Cd in poplar roots, establishing indirect correlations between accumulation and localization of the metal. Overall, observations suggested differential patterns between 6K3 and 14P11 clones in accumulating Cd within the root profile, though overall concentration and content of Cd in the root system, determined by atomic absorption spectrophotometry, did not differ between clones. The possibility that these accumulation patterns arise from differences in uptake processes and structural properties is discussed and related to tolerance mechanisms.
Keywords
Introduction
Heavy metal accumulation in plants is often restricted to the root tissue, with only small amounts transported to the shoot ([46]). An understanding of the toxicologic and physiologic responses of Populus to heavy metals is of particular relevance when attempting to predict the impact of ions on multipurpose tree plantations. This is particularly important when studying metal effects in contaminated riparian ecosystems receiving large quantities of cation laden domestic and industrial wastes. Researches have been conducted on the use of poplar trees for the extraction (phytoremediation) or immobilization (phytostabilization) of heavy metals in polluted sites ([32], [40], [24]). In addition, Populus are currently grown for pulpwood and as a renewable energy source ([30]). However, studies of heavy metal absorption and accumulation in poplar trees are limited, as are investigations on physiological responses and the basis of poplar tolerance to these elements, and mechanisms of their detoxification ([4], [14]).
Trace elements are defined as chemicals with low concentrations in plant tissues (lower than 0.1%), independent of their toxicity or nutritional value. Heavy metals such as Cd are not essential and are also highly toxic ([25]). Cadmium is a relatively rare element with undetermined biologic function ([44]). It is considered to be one of the most hazardous trace elements in the environment because of its high toxicity to all components of aquatic communities ([53]). Cadmium has received widespread attention because of its accelerated release into the environment as the result of industrial pollution ([45]). The phytotoxicity of Cd is well known (e.g., [49]) and is manifested as inhibition of plant growth ([47]), nitrate assimilation ([22]) and photosynthesis (e.g., [8]), as well as disturbances in plant ion ([56]) and water balances ([7]). Many of these plant responses to heavy metals are a result of the inhibition of enzymatic activity caused by the binding of heavy metal ions to sulfhydryl groups in the active sites of enzymes and by substitution of essential metals ([49]).
In particular, Cd may interfere with the uptake of positively charged ions in plant roots ([55]) and with element translocation to the shoot ([26]). Plants have developed different tolerance strategies to grow on soils rich in heavy metals ([5], [6]). “Excluders” have a low uptake of trace elements, by active exclusion in the root system, even at high external concentrations in the soil solution. “Accumulators” are able to tolerate high concentrations of trace elements in their tissues, and this accumulation can be produced even at low external concentrations in the soil solution. “Indicators” have a relatively constant root uptake over a wide gradient of trace elements in the soil. Driven by the potential of their use to extract and remove (or stabilize) toxic metals from contaminated soils ([37]), there has been recent progress in our understanding of the uptake, translocation and compartmentation of heavy metals in poplars (e.g., [15], [41], [48], [11]).
Little is known about the physiology of intracellular Cd sequestration in poplars. There are different methods for determining elements distribution in plant tissues such as histochemical ([51], [42]), cell fractionation ([28]), x-ray microanalysis ([23], [50], [51]), particle-induced x-ray emission (micro-PIXE - [1]) or nuclear micro-probe technique (NMP - [2]). Although not always useful at very low metal concentrations, x-ray microanalysis provides specific signals compared to other methods ([58] et al. 2005). In the present work we used x-ray microanalysis to determine tissue localization and concentration of Cd in root tips of Populus alba clones (6K3 and 14P11).
These genotypes from contrasting environments were compared under salt stress conditions by measurement of growth, Na accumulation, and expression of PM-ATPase and V-ATPase genes in leaves of control and salt-stressed plants ([9]). The different response to salinity showed by these clones was related to a different ability in excluding Na at the root level and to a different regulation of ion transport across membranes in leaf cells, which could be the expression of adaptation to ecological conditions of original provenances. A different genetic basis was supposed to underlie the different degree of salt tolerance observed. Knowledge of Cd distribution in white poplar roots of various clones may help to understand some mechanisms of Cd accumulation and tolerance in this multipurpose tree species and to select for desirable genotypes. White poplar has rapid growth, and is naturally found on a wide range of soil types, being relatively tolerant to pollution ([29], [17]). These poplars grow on riverbanks, in south and central Europe, Western Asia and North Africa; besides, they are widely used in urban forestry. An ancillary objective was that of comparing two different scanning electron microscope techniques in describing the distribution of Cd within root tissues
Materials and methods
Plant material and growth conditions
Woody cuttings of Populus alba L. clones (6K3 and 14P11) with uniform size (20 cm in length and diameter larger than 1 cm) were collected from mother plants at IBAF-CNR (Montelibretti - Roma, Italy). Cuttings were grown in pots suitable for hydroponic culture (diameter 13-15 mm), and supplied with third strength Hoagland nutrient solution, pH 6.5. After three weeks of development in the hydroponic system, 5 homogeneous plants per genotype were selected and randomly assigned to two groups of treatment; Cd was added as CdSO4 (CdSO4·4H2O - Sigma, Saint Louis, USA) to the nutrient solution to give concentrations of 0 or 50 μM Cd. To avoid root necrosis, the solution was completely replaced twice a week and aerated by diffused air aeration systems. Pots were located inside a growth chamber with controlled temperature (25-20 °C day-night) and photosynthetic photon flux density (metal halide lamps, 300 μmol m-2 s-1), and a 14-h photoperiod and 70-80% relative humidity.
After three weeks of treatment, plants were gently removed from the solution and 20 mm long segments of primary and secondary roots were taken from each plant and immediately plunged either in liquid N2 or in a solution of 3% glutaraldehyde (0.01 mol l-1 phosphate buffer) for storage. Analyses were done by low or ambient temperature scanning electron microscopy, respectively.
Scanning electron microscope (SEM) and energy-dispersive x-ray microanalysis (EDXMA)
For low temperature SEM (Cryo-SEM) investigations frozen-hydrated root samples were mounted on an aluminium stub with Tissue-Tek. Specimens were moved to a dedicated cryo-preparation chamber (SCU 020, Bal-Tech, Balzers, Liechtenstein), freeze-fractured by a motor-driven fracturing microtome at -120 ºC, surface etched for 5 min at -80 ºC under high vacuum (lower than 2 · 10-4 Pa), sputter-coated with 10 nm of gold (measured by quartz thin-film monitor) in an argon atmosphere (lower than 2.2 · 10-2 Pa) to produce an electrically conductive surface. Specimens were then transferred into a cryo-stage (-180 °C) inside a SEM (Philips 515, Eindhoven, the Netherlands) equipped with the cryo unit. The EDXMA was performed in the cryo-SEM using an acceleration voltage of 17 kV, a take-off angle of 16.5° and a working distance (sample to final lens) of 12 mm. Spectra from 0 to 20 keV were collected at increments of 10 eV per channel with the electron beam focused on a spot area in the centre of selected cells. Background and element-specific peak spectra were analysed using the program EDAX DX-4 (EDAX, San Francisco, USA), which fully deconvolutes the spectra and allows the corrections for interference between elements. The results are presented as peak/background ratios percentage for K, Ca and Cd elements. In addition, a two-dimensional distribution pattern was recorded by scanning the area of specimens repeatedly for 30 min and integrating counts for K, Ca and Cd (most abundant elements) within their respective spectrum windows into dot maps. Therefore, the dot map collected provides only qualitative information of element distribution and was not sensitive in depicting small variations. Slow-scan images were digitised at 768x576 pixels (256 grey levels) and analysed with AnalySIS 2.1 (Soft-Imaging Software GmbH, Germany). The observation of frozen-hydrated root samples by cryo-SEM enables the real highlighting of structural aspects of root features during development. Internal root structures were examined on fracture planes obtained by freeze fracturing transversally the frozen-hydrated sample.
For ambient temperature SEM investigations (ambient-SEM), root samples were fixed in 3% glutaraldehyde solution (C5H8O2, 0.01 mol l-1 phosphate buffer) to maintain protein structures and then post-fixed for 3 h in 1% osmium tetroxide (OsO4) to fix proteins and lipids, and to improve the contrast of electron microscope pictures. Specimens were then rinsed in fresh buffer and dehydrated through a series of ascending ethanol-water solutions (20-30-40-60-80-95-100%), with a last wash in acetone for a better CO2 substitution during the dehydration procedure at a pressure of 1200 bars. Afterwards, to produce an electrically conductive surface and thus prevent charging under the electron beam, root samples were critical-point dried (critical point drier K850, Emitech, Ashford, UK) before gold (SEM) or carbon (EDXMA) sputtering (sputter coater K550, Emitech, Ashford, UK) under vacuum; carbon was used instead of metal coating to avoid interference with measured elements, while gold was used to improve conductivity and increase topographic contrast. Observations were done in a SEM (Zeiss DSM 940A, LEO Elektronenmikroskopie GMbH, Oberkochen, Germany) operating at 10 kV.
Determination of Cd content
Root samples for Cd determination were dried at 105 °C and wet digested in HNO3:HClO4 (4:1, v/v). The content of Cd was determined by atomic absorption spectrophotometry (Perkin Elmer 3300, Norwalk, USA).
Statistical analysis
The experiment was set up in a completely randomised design with five replicate plants for each treatment (n = 5). Data were averaged on a plant basis and subjected to analysis of variance (ANOVA) to test significant differences between the main effect of genotype, treatment and interaction terms. The Student’s t-test (pairwise) was used to compare Cd concentration means for the two clones. Significant effects were considered at P ≤ 0.05.
Results and Discussion
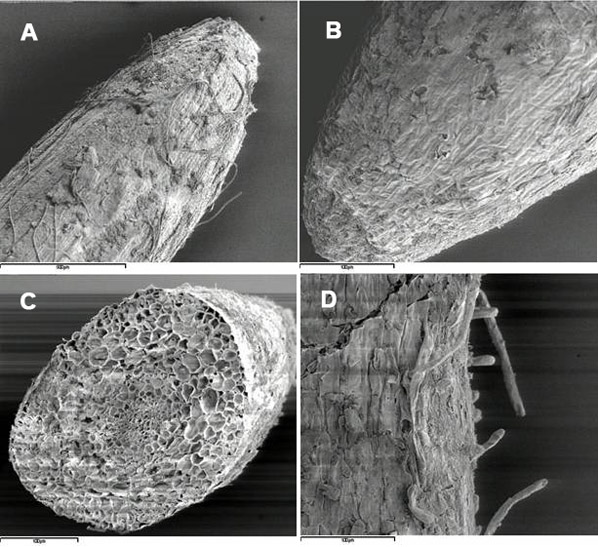
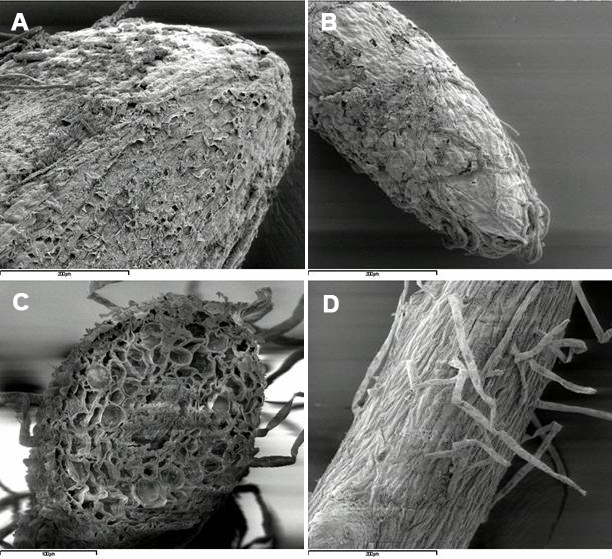
Internal and external characteristics of primary and secondary roots were not strongly affected by the treatment with Cd (Fig. 1A, B, C, D and Fig. 2A, B, C, D; ambient-SEM), despite the fact that the metal was found dispersed throughout all root tissues. Indeed, no major cell shrinkage or tissue necrosis was observed in roots of these poplar clones after the treatment. However, a specific analysis on vessel lumina associated with changes in wall strength in response to metal was not done, and possible effects of Cd on plant transport system cannot be ruled out. In plants subjected to long-term treatment with Cd, alterations of the root structure might also influence redistribution and accumulation of the metal in different root tissues and/or cell compartments ([58]).
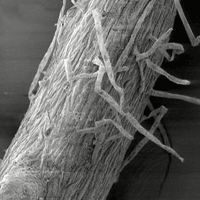
Fig. 1 - Cryo-scanning electron micrographs of poplar roots, clone 14P11: A, primary root apex (control); B, secondary root apex (treated with Cd), C primary root cross-section (treated with Cd); D, root hairs (treated with Cd).
Fig. 2 - Cryo-scanning electron micrographs of poplar roots, clone 6K3: A, primary root apex (control); B, secondary root apex (treated with Cd), C secondary root cross-section (treated with Cd); D, root hairs (treated with Cd).
In the control treatment the average concentration of Cd in root tips was below the detection limit of EDXMA. From SEM-EDXMA, only Fe and P other than Cd concentrations were affected by the treatment (Tab. 1). The presence of metal in the growing solution increased Cd and Fe concentrations in the root tissues, while decreased that of P, in both clones. Contrasting effects of the treatment on clone 6K3 and 14P11 were observed for Cu, K and S. High negative correlation of Cd concentration with K (and Ca) concentrations in roots has been observed in alfalfa seedlings ([16]), indicating competition for the same carrier and suggesting differential responses to Cd in these poplar clones mediated by K metabolism. Cadmium might have disturbed plant mineral metabolism, its toxicity inducing changes in the nutrient balance. Indeed, Cd disrupts iron (Fe) metabolism in plants ([56]). The occurrence of Cd-Fe interactions is consistent with the finding that changes in thylakoid structure are similar in Cd-treated and Fe-deficient plants ([18]) including poplar ([38], [39]). Although the mechanism underlying Cd-induced Fe deficiency in plants has not been identified, there are several possible explanations. The root Fe-deficiency-inducible enzyme Fe(III)-chelate reductase is inhibited by Cd ([3]), suggesting that Cd may directly impair Fe acquisition. Also, Cd, which usually accumulates in roots ([43], [59]), almost completely inhibits Fe translocation from roots to shoots, leading to increase root Fe concentrations in cucumber ([18]) and mung bean ([27]). Once in the apoplast, Cd may compete with other divalent metal ions such as Ca2+, Zn2+ and Fe2+ for binding sites and transport mechanisms ([13]).
Tab. 1 - Effects of Cd treatment on the concentration (SEM-EDXMA) of various elements in root tips of P. alba clones (6K3 and 14P11). Values are in weight %, peak/background (Cd in control samples was detected below the instrument threshold); data are the mean (± SE).
| Clone | Treatment | Cd | Cu | K | Fe | S | Ca | P |
|---|---|---|---|---|---|---|---|---|
| 6K3 | Cd | 1.15 (0.14) | 0.61 (0.11) | 0.70 (0.07) | 1.15 (0.13) | 2.90 (0.19) | 1.34 (0.12) | 2.93 (0.15) |
| Control | < 5 mM | 0.00 (0.00) | 0.34 (0.06) | 0.10 (0.10) | 1.49 (0.01) | 0.74 (0.18) | 3.84 (0.42) | |
| 14P11 | Cd | 0.25 (0.07) | 0.31 (0.05) | 0.12 (0.02) | 0.16 (0.04) | 0.84 (0.11) | 0.48 (0.10) | 1.27 (0.17) |
| Control | < 5 mM | 0.75 (0.25) | 0.62 (0.14) | 0.08 (0.04) | 1.97 (0.31) | 0.38 (0.05) | 3.34 (0.28) | |
| ANOVA (P) | ||||||||
| clone | - | 0.1606 | 0.3786 | 0.3028 | 0.0564 | 0.0947 | 0.0986 | 0.0545 |
| treatment | - | 0.0317 | 0.7343 | 0.6748 | 0.0326 | 0.7677 | 0.3465 | 0.0088 |
| interaction | - | 0.1606 | 0.0396 | 0.0035 | 0.0675 | 0.0073 | 0.4895 | 0.2983 |
Differences between clones in the concentration of metal and nutrients at the apex, and between 0.5 and 1 cm (median) and at 2 cm (section) from the apex of root samples were always consistent (Tab. 2; SEM-EDXMA). In general, differences between clones were evident for the position of preferential metal and nutrients accumulation, which was the apex for clone 6K3 and in the range 0.5-1 cm from the apex for clone 14P11. The potential for enormous genetic variation of poplar is promising for selecting clones of local germplasm collections with a high capacity for phytoextraction of specific heavy metals from soils or waters. These poplar clones are currently studied for determining physiological and morphological parameters in plants exposed to toxic concentrations of Cd solution, as well as, their ability to accumulate Cd in roots and to translocate it to leaves (CNR-IBAF, Montelibretti, Italy). Again, they are also under genetic analysis by selecting genes involved in the different steps of the process for Cd uptake, sequestration, compartmentalisation, and translocation, which will eventually help in identifying possible molecular descriptors of plant performance for phytoremediation (Università della Tuscia, Viterbo, Italy).
Tab. 2 - Concentrations (SEM-EDXMA) of various elements in root tips of P. alba clones (6K3 and 14P11) subjected to Cd 40μM, sampled at the apex, and between 0.5 and 1 cm (median) and at 2 cm (section) from the apex. Values are in weight %, peak/background; data are the mean (± SE).
| Clone | Position | Cd | Cu | K | Fe | S | Ca | P |
|---|---|---|---|---|---|---|---|---|
| 6K3 | apex | 1.52 (0.27) | 0.86 (0.21) | 0.90 (0.12) | 1.71 (0.22) | 2.62 (0.30) | 1.73 (0.23) | 2.96 (0.22) |
| median | 0.94 (0.19) | 0.65 (0.18) | 0.71 (0.11) | 1.09 (0.18) | 3.18 (0.38) | 1.37 (0.21) | 2.69 (0.09) | |
| section | 0.96 (0.21) | 0.27 (0.11) | 0.47 (0.12) | 0.56 (0.16) | 2.91 (0.30) | 0.85 (0.14) | 3.18 (0.42) | |
| 14P11 | apex | 0.08 (0.04) | 0.25 (0.08) | 0.07 (0.03) | 0.03 (0.02) | 0.42 (0.12) | 0.16 (0.04) | 0.59 (0.16) |
| median | 0.47 (0.15) | 0.44 (0.08) | 0.18 (0.03) | 0.32 (0.09) | 1.28 (0.17) | 0.87 (0.21) | 2.00 (0.28) | |
| section | 0.02 (0.02) | 0.06 (0.06) | 0.12 (0.07) | 0.08 (0.05) | 0.69 (0.32) | 0.18 (0.08) | 1.06 (0.42) | |
| ANOVA (P) | ||||||||
| clone | - | < 0.0001 | 0.0026 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
| position | - | 0.2566 | 0.0157 | 0.0683 | 0.0008 | 0.0090 | 0.0155 | 0.0959 |
| interaction | - | 0.0095 | 0.2038 | 0.0099 | < 0.0001 | 0.7495 | 0.0060 | 0.0042 |
Overall, the SEM-EDXMA showed a higher capacity of Cd accumulation in clone 6K3 than clone 14P11. Clonal differences in the aptitude of metal accumulation were, however, not supported by the chemical analysis (Tab. 3). These methodological discrepancies might be only apparent and due to the scattered scenery of EDXMA when mapping a localized portion of the root compared to the complete sampling of the root while performing the chemical analysis. In turn, this behaviour might highlight differences in the translocation capacity between clones; clone 14P11 being more efficient than clone 6K3 in the translocation process of metal and nutrients from roots to the stem. A delay in Cd transport through membranes inside cells might represent, according to Nedelkoska & Doran ([31]), an important defence mechanism against metal poisoning, enabling simultaneous activation of intracellular detoxification mechanisms of Cd.
Tab. 3 - Concentration and content of Cd in the root system of P. alba clones (6K3 and 14P11) determined by atomic absorption spectrophotometry. Data are the mean (± SE); t-test comparison between clones is also reported.
| Clone | Cd concentration (mg/kg) |
Cd content (mg per root) |
|---|---|---|
| 6K3 | 6089 (1068) ns | 1.91 (0.46) ns |
| 14P11 | 7964 (944) ns | 4.71 (1.18) ns |
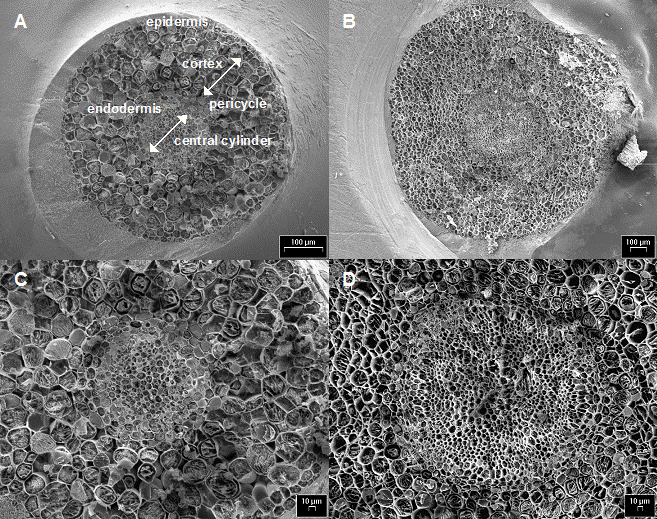
Cryo-SEM images of frozen-hydrated transversally freeze-fractured young poplar roots did not show, in both clones, any damage to the whole root structure, such as shrinkage or collapse (Fig. 3A and C). In particular, central cylinder and endodermis in roots of both clones showed perfectly preserved cells (Fig. 3B and D). Differences between treatments were found somewhat in the number of cell rows of these two tissues (data not shown). Living, constantly growing root fragments were sampled for analysis, and the preparation procedure did not cause injure of tissues. Therefore, we may conclude that the main transport system was not impaired at the root level in these poplar clones. Nevertheless, changes in cell structure might appear after longer time of exposure to Cd and at higher concentrations of the metal ([42]). Increasing amounts of Cd penetrating deeper into the root as well as inside cells may be expected in this latter case, suggesting that the cell wall might be the first barrier protecting the protoplast from Cd toxic action, and Cd binding to the cell wall might be an important mechanism of tolerance to this metal, especially at low to medium concentrations and short time of exposure (from several days up to a few weeks).
Fig. 3 - Cryo-scanning electron micrographs of poplar root cross-sections: A and C, clone 14P11; B and D clone 6K3.
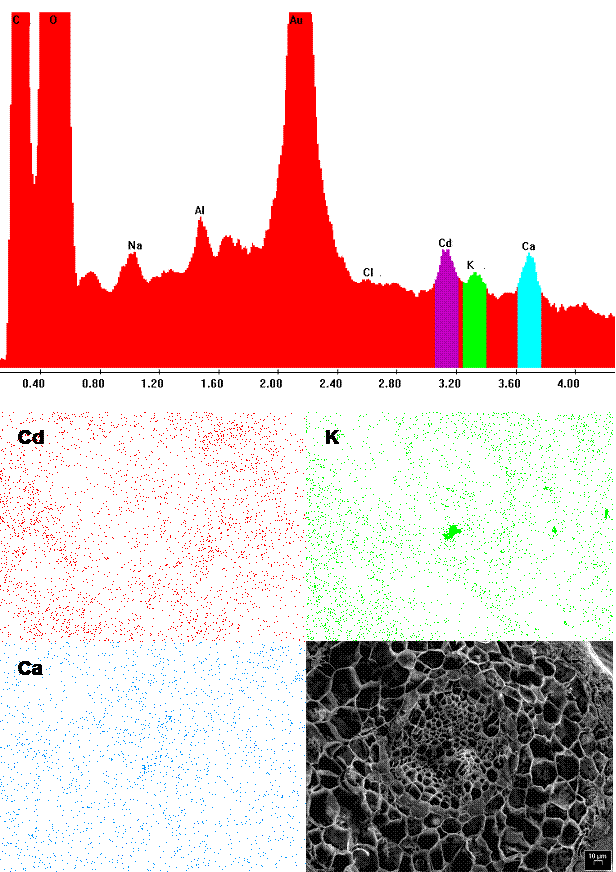
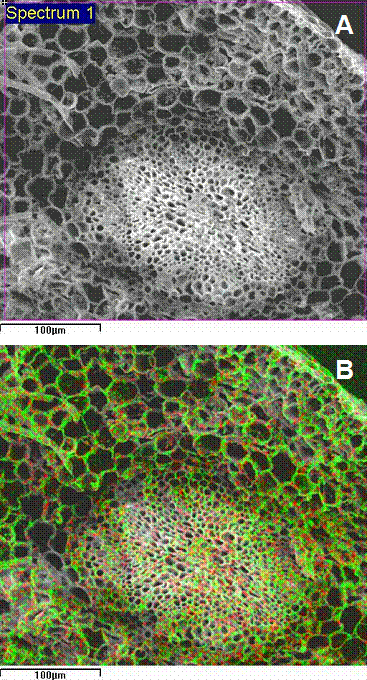
The poplar root accumulated so much Cd that the peak/background ratio was high enough for recording dot-maps of metal distribution (Fig. 4; Cryo-SEM-EDXMA). In our experiment, Cd accumulated in different root tissues - in parenchyma cells of cortex, in the endodermis, in parenchyma cells of central cylinder and in xylem vessels. However, compared with other important ions (e.g., Ca, showing a rather diffuse allocation), the distribution of Cd within the root (similar to that of K) was preferentially localized in the surroundings of central cylinder. Many studies have shown that the main site of Cd accumulation in roots is the apoplast, particularly cell walls ([58]). Khan et al. ([23]) and Lozano-Rodríguez et al. ([28]) found that in maize roots the metal was mainly detected in cell walls of cortex parenchyma, endodermis and pericycle, while much smaller amounts were found in protoplasts. These results are contradictory with those of Rauser & Ackerley ([35]) who did not find Cd in cell walls of maize and Agrostis roots. Instead, Cd was located in electron-dense granules in the cytoplasm and in vacuoles of differentiating and mature cells and in nuclei of undifferentiated cells. Similarly, Vázquez et al. ([51]) showed the presence of Cd in the vacuole and nuclei but not in cell walls, cytoplasm and plastids of bean roots.
Fig. 4 - EDXMA spectrum obtained by the scanning electron microscope and corresponding dot-map localization of Cd (red), K (green) and Ca (blue) within the root of P. alba.
Nevertheless, a privileged accumulation of Cd in cell walls of poplar roots was confirmed by ambient-SEM-EDXMA observations (Fig. 5A, B). The dot-map of metal distribution also evidenced that Cd within the root was preferentially localized in the surroundings of central cylinder. The importance of Cd binding to cell walls and the limitation of its subsequent translocation to shoots has been demonstrated for root cells of non-hyperaccumulating plants ([54], [20]) and was recently described for Thlaspi caerulescens hairy roots ([10]). Some authors suggest that in roots, the capacity to bind Cd in the cell wall has a protective action against the deleterious effect of Cd by reducing the amounts of cytosolic Cd ([28], [33], [60]). Again, Wójcik et al. ([58]) and Vögeli-Lange & Wagner ([52]) suggest that the cell wall plays a minor role in Cd retention in leaves, and the main mechanisms of Cd detoxification are located inside cells, in vacuoles. Cadmium may induce synthesis of metal binding organic substances for metal detoxification in the cytoplasm ([36], [57]). A limitation in ion binding specificity of these compounds would result in increased accumulation of the metal in the root and decreased metal translocation to the shoot ([21]), which may be clone-specific.
Fig. 5 - Ambient temperature scanning electron micrograph of a poplar fractured root cross-section (A) and corresponding dot-map localization of Cd (red) obtained by energy-dispersive x-ray microanalysis (B).
Cellular Cd is known to accumulate preferentially within vacuoles, which has often been proposed as a possible mechanism of Cd tolerance in plants. Although the role of cell walls in Cd binding and storage in plants remains controversial, Cd was found both inside vacuoles and in cell walls. This latter result was obtained by both ambient-SEM- and cryo-SEM-EDXMA observations and compartmental studies and in both clones, indicating an additional storage mechanism. Artefacts and/or Cd losses may occur during the preparation of cell fractions separated by biochemical and physical methods (epidermal strips, protoplast extraction, apoplast wash fluid, and cell wall extraction). In this sense, the cryo-SEM-EDXMA is more reliable, but lack of sensitivity and interference with other elements (e.g., K) impose cautions when using a single approach. Using EDXMA at room temperature may cause redistribution and loss of elements, which lowers the reliability of data obtained with this technique. Large losses of loosely bound Cd from roots during tissue preparation by conventional fixation for electron microscopy can be partly overcome through cryo-fixation, if the latter is fast enough to retain the original distribution of inorganic elements sufficiently for micro-analytical study ([19]). This method, without the interference of chemical fixative and with no ice crystal damage, allows opportunities to localize elements under conditions closely resembling the natural state, though estimates of metal distribution are qualitative and at a low spatial resolution, unless ultrathin cryo-sections are opportunely prepared and used for quantitative analysis at subcellular level.
Conclusions
The present work shows the potential for Cd accumulation in poplar clones. This may be of importance for soil remediation technologies based on metal extraction by plants in phytoremediation. A dissimilar depletion of Cd in the rhizosphere of these two poplar clones may occur because of differential uptake, and different Cd-induced responses may occur because of interference with other ions. In the root of these poplar clones Cd might form precipitates with other elements, such as P ([25]), in the apoplast of the rhizodermis. The diffuse presence of the metal in cortex parenchyma cells while physiological activity was not impaired points to a decreased Cd toxicity with constant Cd content in the growing solution, and in turn suggests Cd storage in minor toxic forms or in cell compartments weakly sensitive to Cd ([12]). When challenged with heavy metals, plants are known to synthesize chelators (such as phytochelatins) that are able to bind metals, thus reducing the amount of free metals in their cells ([34]). The role of phytochelatins and their precursor glutathione in shuttling Cd ions from the cytosol to the vacuole needs to be investigated in these clones. However, the possibility of a higher rate of metal transport into the vacuole through an additional sequestration mechanism deserves further studies in poplars. The results of this study demonstrated the importance of applying multiple methodologies toward a fuller understanding of cellular response to heavy-metal exposure. We believe that only through compound studies one can gain the insight needed to make predictive statements regarding the effect of metal toxicants on different poplar clones.
Acknowledgments
We acknowledge funding by MUR (PRIN 2005). We are indebted to Lucia Maiuro (CSIM, Università del Molise) for SEM analysis.
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
R Tognetti
EcoGeoFor Lab - Dipartimento di Scienze e Tecnologie per l’Ambiente e il Territorio, Università degli Studi del Molise, Contrada Fonte Lappone, I-86090 Pesche, IS (Italy)
BioLabs, Scuola Superiore Sant’Anna, v.le Rinaldo Piaggio, I-56025 Pontedera, PI (Italy)
Corresponding author
Paper Info
Citation
Cocozza C, Minnocci A, Tognetti R, Iori V, Zacchini M, Scarascia Mugnozza G (2008). Distribution and concentration of cadmium in root tissue of Populus alba determined by scanning electron microscopy and energy-dispersive x-ray microanalysis. iForest 1: 96-103. - doi: 10.3832/ifor0458-0010096
Paper history
Received: Oct 12, 2007
Accepted: Jan 16, 2008
First online: May 20, 2008
Publication Date: May 20, 2008
Publication Time: 4.17 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2008
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 59078
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 48509
Abstract Page Views: 4468
PDF Downloads: 4866
Citation/Reference Downloads: 74
XML Downloads: 1161
Web Metrics
Days since publication: 6497
Overall contacts: 59078
Avg. contacts per week: 63.65
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2008): 38
Average cites per year: 2.11
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Bioaccumulation of long-term atmospheric heavy metal pollution within the Carpathian arch: monumental trees and their leaves memoir
vol. 17, pp. 370-377 (online: 27 November 2024)
Research Articles
Heavy metal (Zn, Pb, Cd) concentration in soil and moss (Pleurozium schreberii) in the Brynica district, southern Poland
vol. 4, pp. 176-180 (online: 11 August 2011)
Review Papers
Heavy metals and woody plants - biotechnologies for phytoremediation
vol. 4, pp. 7-15 (online: 27 January 2011)
Research Articles
Variation of major elements and heavy metals occurrence in hybrid aspen (Populus tremuloides Michx. × P. tremula L.) tree rings in marginal land
vol. 13, pp. 24-32 (online: 15 January 2020)
Review Papers
Monitoring the effects of air pollution on forest condition in Europe: is crown defoliation an adequate indicator?
vol. 3, pp. 86-88 (online: 15 July 2010)
Research Articles
Temporal analysis of pollutant metals in trees of three parks in Mexico City’s Metropolitan Area
vol. 18, pp. 138-145 (online: 01 June 2025)
Technical Reports
Air pollution regulations in Turkey and harmonization with the EU legislation
vol. 4, pp. 181-185 (online: 11 August 2011)
Review Papers
A review of the performance of woody and herbaceous ornamental plants for phytoremediation in urban areas
vol. 13, pp. 139-151 (online: 14 April 2020)
Editorials
COST Action FP0903: “Research, monitoring and modelling in the study of climate change and air pollution impacts on forest ecosystems”
vol. 4, pp. 160-161 (online: 11 August 2011)
Research Articles
Can species Cedrela fissilis Vell. be used in sites contaminated with toxic aluminum and cadmium metals?
vol. 14, pp. 508-516 (online: 11 November 2021)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword