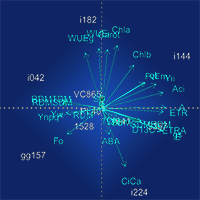
Water deficit is one of the abiotic stresses that most affects the growth and survival of Eucalyptus. Mechanisms used to tolerate water-limited environments influence the distribution of Eucalyptus species in their natural environment. Here, we take a physiological approach to pre-screen Eucalyptus plants for tolerance to drought. Ten different clones of E. urophylla and E. grandis × E. urophylla that are known to show contrasting responses to water deficit under field conditions, were grown in Clark’s nutrient solution (WW, well-watered) and with polyethylene glycol (-1.0 MPa) to simulate water deficit (WD). Clones responded differently to drought with differentiated photosynthetic limitations in drought-treated clones. Photosynthetic rates, stomatal conductance, transpiration and internal CO2 concentrations were reduced in all genotypes under stress conditions. Clone i144 had a smaller reduction in the evaluated physiological traits, also showing increased root growth in WD-treated plants. Clones 3367 and i224, thought to be moderately tolerant, also followed these patterns. Clones gg157, 1568 and 1641, all of which are moderately sensitive under field conditions, reduced most of the physiological characters evaluated. However, clone gg157 demonstrated increased root system growth, even during short periods of water stress. Clones i042 and i182 were deemed drought-susceptible, with large reductions in photosynthesis and growth, despite showing a high increase in abscisic acid content presumably as a defense mechanism. Interaction between A (photosyntetic rate), E (transpiration rate), ETR/A (electrons transport rate/photosynthetic rate) and SDM/ RDM (shoot dry matter/root dry matter) demonstrated the most significant differences between WD-treated clones and offer great potential for use as selection criterion for water deficit-tolerant genotypes.
Keywords
, , ,
Citation
Müller C, Hodecker BER, De Barros NF, Merchant A (2020). A physiological approach for pre-selection of Eucalyptus clones resistant to drought. iForest 13: 16-23. - doi: 10.3832/ifor3185-012
Academic Editor
Claudia Cocozza
Paper history
Received: Jul 04, 2019
Accepted: Nov 21, 2019
First online: Jan 15, 2020
Publication Date: Feb 29, 2020
Publication Time: 1.83 months
© SISEF - The Italian Society of Silviculture and Forest Ecology 2020
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.

Breakdown by View Type
(Waiting for server response...)
Article Usage
Total Article Views: 45571
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 36957
Abstract Page Views: 3904
PDF Downloads: 3892
Citation/Reference Downloads: 6
XML Downloads: 812
Web Metrics
Days since publication: 2211
Overall contacts: 45571
Avg. contacts per week: 144.28
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2020): 8
Average cites per year: 1.33
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
(1)
Arndt SK, Livesley SJ, Merchant A, Bleby TM, Grierson PF (2008)Quercitol and osmotic adaptation of field-grown
Eucalyptus under seasonal drought stress. Plant, Cell and Environment 31: 915-924.
CrossRef |
Gscholar
(2)
Chaves MM, Flexas J, Pinheiro C (2009)Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Annals of Botany 103: 551-560.
CrossRef |
Gscholar
(3)
Clark RB (1975)Characterization of phosphatese in intact maize roots. Journal of Agricultural and Food Chemistry 23: 458-60.
CrossRef |
Gscholar
(4)
Corrêa TR, Picoli EAT, Souza GA, Condé AS, Silva NM, Lopes-Mattos KLB, Resende MDV, Zauza EAV, Oda S (2017)Phenotypic markers in early selection for tolerance to dieback in
Eucalyptus. Industrial Crops and Products 107: 130-138.
CrossRef |
Gscholar
(5)
Demmig-Adams B, Adams WW (1996)The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends in Plant Science 1: 21-26. doi:
CrossRef |
Gscholar
(6)
Durgbanshi A, Arbona V, Pozo O, Miersch O, Sancho JV, Gómez-Cadenas A (2005)Simultaneous determination of multiple phytohormones in plant extracts by liquid chromatography-electrospray tandem mass spectrometry. Journal of Agricultural and Food Chemistry 53: 8437-8442.
CrossRef |
Gscholar
(7)
Farquhar GD, Richards RA (1984)Isotopic composition of plant carbon correlates with water use efficiency of wheat genotypes. Australian Journal of Plant Physiology 11: 539-552.
CrossRef |
Gscholar
(8)
Flexas J, Bota J, Loreto F, Cornic G, Sharkey TD (2004)Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biology 6: 269-279.
CrossRef |
Gscholar
(9)
Flexas J, Diaz-Espejo A, Galmés J, Kaldenhoff R, Medrano H, Ribas-Carbo M (2007)Rapid variations of mesophyll conductance in response to changes in CO
2 concentration around leaves. Plant, Cell and Environment 30: 1284-1298.
CrossRef |
Gscholar
(10)
Foyer C, Furbank R, Harbinson J, Horton P (1990)The mechanisms contributing to photosynthetic control of electron transport by carbon assimilation in leaves. Photosynthesis Research 25: 83-100.
CrossRef |
Gscholar
(11)
Genty B, Briantais J-M, Baker NR (1989)The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta - General Subjects 990: 87-92.
CrossRef |
Gscholar
(12)
IBA (2019)IBA Relatório 2019 [IBA Report 2019]. Indústria Brasileira de Árvores, Pöyry Consultoria em Gestão e Negócios Ltda., Brazil, pp. 79. [in Portuguese]
Online |
Gscholar
(13)
Jiang F, Hartung W (2008)Long-distance signalling of abscisic acid (ABA): the factors regulating the intensity of the ABA signal. Journal of Experimental Botany 59: 37-43.
CrossRef |
Gscholar
(14)
Lawlor DW, Tezara W (2009)Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: a critical evaluation of mechanisms and integration of processes. Annals of Botany 103: 561-579.
CrossRef |
Gscholar
(15)
Li FL, Bao WK, Wu N (2009)Effects of water stress on growth, dry matter allocation and water-use efficiency of a leguminous species,
Sophora davidii. Agroforestry Systems 77: 193-201.
CrossRef |
Gscholar
(16)
Macfarlane C, Adams MA, White D (2004)Productivity, carbon isotope discrimination and leaf traits of trees of
Eucalyptus globulus Labill. in relation to water availability. Plant, Cell and Environment 27: 1515-1524.
CrossRef |
Gscholar
(17)
Martins GS, Freitas NC, Máximo WPF, Paiva LV (2018)Gene expression in two contrasting hybrid clones of
Eucalyptus camaldulensis ×
Eucalyptus urophylla grown under water deficit conditions. Journal of Plant Physiology 229: 122-131.
CrossRef |
Gscholar
(18)
Maxwell K, Johnson GN (2000)Chlorophyll fluorescence - a pratical guide. Journal of Experimental Botany 51: 659668.
CrossRef |
Gscholar
(19)
Medrano H, Escalona JM, Bota J, Gulías J, Flexas J (2002)Regulation of photosynthesis of C3 plants in response to progressive drought: stomatal conductance as a reference parameter. Annals of Botany 89: 895-905.
CrossRef |
Gscholar
(20)
Merchant A, Tauz M, Arndt SK, Adams MA (2006)Cyclitols and carbohydrates in leaves and roots of 13
Eucalyptus species suggest contrasting physiological responses to water deficit. Plant, Cell and Environment 29: 2017-2029.
CrossRef |
Gscholar
(21)
Merchant A, Ladiges PY, Adams MA (2007)Quercitol links the physiology, taxonomy and evolution of 279 eucalypt species. Global Ecology and Biogeography 16: 810-819.
CrossRef |
Gscholar
(22)
Merchant A, Arndt SK, Rowell DM, Posch S, Callister A, Tauz M, Adams MA (2010)Seasonal changes in carbohydrates, cyclitols, and water relations of 3 field grown
Eucalyptus species from contrasting taxonomy on a common site. Annals of Forest Science 67: 104.
CrossRef |
Gscholar
(23)
Michel BE, Kaufmann MR (1973)The osmotic potential of polyethylene glycol 6000. Plant Physiology 51: 914-916.
CrossRef |
Gscholar
(24)
Mokotedi MEO, Watt MP, Pammenter NW (2010)Analysis of differences in field performance of vegetatively and seed propagated
Eucalyptus varieties II: vertical uprooting resistance. Southern Forests 72: 712-718.
CrossRef |
Gscholar
(25)
Müller C, Silveira SFDS, Daloso DM, Mendes GC, Merchant A, Kuki KN, Oliva MA, Loureiro ME, Almeida AM (2017)Ecophysiological responses to excess iron in lowland and upland rice cultivars. Chemosphere 189: 123-133.
CrossRef |
Gscholar
(26)
Murata N, Allakhverdiev SI, Nishiyama Y (2012)The mechanism of photoinhibition in vivo: Re-evaluation of the roles of catalase, α-tocopherol, non-photochemical quenching, and electron transport. Biochimica et Biophysica Acta (BBA) 1817: 1127-1133.
CrossRef |
Gscholar
(27)
Nunes FN, Barros NF, Novais RB, Silva IR, Stape JL (2016)Carbon isotope discrimination and differential drought tolerance in eucalypt clones. Scientia Forestalis 44: 895-903.
Online |
Gscholar
(28)
Parry MAJ, Flexas J, Medrano H (2005)Prospects for crop production under drought: research priorities and future directions. Annals of Applied Biology 147: 211-226.
CrossRef |
Gscholar
(29)
Pinto DS, Resende RT, Mesquita AGG, Rosado AM, Cruz CD (2014)Early selection in tests for growth traits of
Eucalyptus urophylla clones test. Scientia Forestalis 42: 251-257.
Online |
Gscholar
(30)
Roach T, Krieger-Liszkay AK (2014)Regulation of photosynthetic electron transport and photoinhibition. Current Protein and Peptide Science 15: 351-362.
CrossRef |
Gscholar
(31)
Schachtman DP, Goodger JQD (2008)Chemical root to shoot signaling under drought. Trends in Plant Sciences 13: 281-287.
CrossRef |
Gscholar
(32)
Schulze ED, Nicolle D, Boerner A, Lauerer M, Aas G, Schulze I (2014)Stable carbon and nitrogen isotope ratios of
Eucalyptus and
Acacia species along a seasonal rainfall gradient in Western Australia. Trees 28: 1125-1135.
CrossRef |
Gscholar
(33)
Silva FC, Shvaleva A, Maroco JP, Almeida MH, Chaves MM, Pereira JS (2004)Responses to water stress in two
Eucalyptus globulus clones differing in drought tolerance. Tree Physiology 24 (10): 1165-1172.
CrossRef |
Gscholar
(34)
Singh SK, Raja Reddy K (2011)Regulation of photosynthesis, fluorescence, stomatal conductance and water-use efficiency of cowpea (
Vigna unguiculata [L.] Walp.). under drought. Journal of Photochemistry and Photobiology B 105: 40-50.
CrossRef |
Gscholar
(35)
Stape JL, Binkley D, Ryan MG (2008)Production and carbon allocation in a clonal
Eucalyptus plantation with water and nutrient manipulations. Forest Ecology and Management 255: 920-930.
CrossRef |
Gscholar
(36)
Tardieu F, Simonneau T (1998)Variability among species of stomatal control under fluctuating soil water status and evaporative demand: modelling isohydric and anisohydric behaviours. Journal of Experimental Botany 49: 419-432.
CrossRef |
Gscholar
(37)
Valdés AE, Majada JP, Rodríguez A, Fernández B, Pagès M (2013)Drought tolerance acquisition in
Eucalyptus globulus (Labill.): a research on plant morphology, physiology and proteomics. Journal of Proteomics 79: 263-276.
CrossRef |
Gscholar
(38)
Warren CR, Bleby TM, Adams MA (2007)Changes in gas exchange
versus leaf solutes as a mean to cope with summer drought in
Eucalyptus marginata. Oecologia 154: 1-10.
CrossRef |
Gscholar
(39)
Warren CR, Aranda I, Cano FJ (2011)Responses to water stress of gas exchange and metabolites in
Eucalyptus and
Acacia spp. Plant, Cell and Environment 34: 1609-1629.
CrossRef |
Gscholar
(40)
Warren CR, Aranda I, Cano FJ (2012)Metabolomics demonstrates divergent responses of two
Eucalyptus species to water stress. Metabolomics 8: 186-200.
CrossRef |
Gscholar
(41)
Wellburn AR (1994)The spectral determination of chlorophylls
a and
b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. Journal of Plant Physiology 144: 307-313.
CrossRef |
Gscholar
(42)
White DA, Crombie DS, Kinal J, Battaglia M, Mcgrath JF, Mendharn DS, Walker SN (2009)Managing productivity and drought risk in
Eucalyptus globulus plantations in south-western Australia. Forest Ecology and Management 259 (1): 33-44.
CrossRef |
Gscholar
(43)
Zhou Y, Huang L, Zhang Y, Shi K, Yu J, Nogués S (2007)Chill induced decrease in capacity of RuBP carboxylation and associated H
2O
2 accumulation in cucumber leaves are alleviated by grafting onto figleaf Gourd. Annals of Botany 100: 839-848.
CrossRef |
Gscholar
(44)
Zhou SX, Medlyn BE, Prentice IC (2016)Long-term water stress leads to acclimation of drought sensitivity of photosynthetic capacity in xeric but not riparian
Eucalyptus species. Annals of Botany 117: 133-144.
CrossRef |
Gscholar