Radial growing, cambial activity, and phenology of tree species in a subtropical forest in Southern Brazil
iForest - Biogeosciences and Forestry, Volume 18, Issue 5, Pages 252-258 (2025)
doi: https://doi.org/10.3832/ifor4642-018
Published: Sep 22, 2025 - Copyright © 2025 SISEF
Research Articles
Abstract
Understanding the factors that affect cambial activity can provide historical documentation of events influencing plant growth and development. This study aimed to link cambial activity and diameter increment of native and exotic species in a floodplain forest in SE Brazil with phenological and meteorological data to answer the following questions: Is species diameter growth influenced by meteorological variables and phenological behavior? Is cambial activity seasonal, with active and inactive periods? Is cell differentiation for initial wood formation triggered by any phenological phase? Diameter increment was measured with dendrometer tapes, cambial activity through vascular cambium samples collection, and phenological behavior through the intensity index. Vegetative structures (mature leaves, senescent leaves, and buds) and reproductive structures (anthesis, immature fruit, and mature fruit) were monitored on a monthly basis. The relationship between meteorological variables and phenophases that lead ro diameter increment was analyzed using multiple linear regression. Additionally, logistic regressions was employed to examine the connection between phenophase variables and cambial activity. The results indicate that the duration of the growing season differs among species, ranging from seven to nine months. Gymnanthes klotzschiana and Blepharocalyx salicifolius showed their highest rates of radial increment from November to March, while Hovenia dulcis and Ligustrum lucidum, from August to April. The increase in diameter for these species is positively influenced by specific phenophases. For G. klotzschiana, these include the presence of mature leaves, senescent leaves, and immature fruits, while for H. dulcis, the presence of mature leaves, senescent leaves, and mature fruits. Moreover, cambial activity is seasonal, with both active and inactive periods occurring mainly in the spring and summer in the four species studied. Different phenological signals were identified as triggers for the initial wood formation, namely, the presence of senescent leaves in G. klotzschiana, budding in H. dulcis, and mature fruits and senescent leaves in L. lucidum. No relationship was found between phenophases and cambial activity or inactivity periods in B. salicifolius.
Keywords
Foodplain, Seasonality, Phenophases, Meteorological Variables, Environmental Monitoring, Ecophysiology
Introduction
Tree growth dynamics are important for the annual wood formation and carbon fixation flux. The factors that control tree growth are diverse, ranging from genotype to the environment where they grow ([17], [34]).
The relationship between climatic factors and tree growth is complex and varies according to the region and species studied. In tropical and subtropical areas, seasonal variation in precipitation and temperature, along with photoperiod, help explain the cambial activation of tree trunks ([7]). Conversely, in temperate and boreal forests, growth is influenced by specific cycles of temperature and light ([22]).
The relationship between dormancy and cambial activity varies among different species. For example, in tropical trees, vascular cambium activity can be observed throughout the year ([30]), or may be limited to a brief season. This leads to distinct periods of elevated and reduced cambial activity, excluding periods of dormancy ([6], [10], [14]). Periodicity is therefore a direct response to environmental variations ([4]) and can affect other plant processes, such as phenological behavior, leaf renewal, flowering, and fruiting.
Tree growth periodicity can be assessed by measuring radial increments year-round with dendrometer tapes, a non-destructive method that avoids material shredding ([16]). However, analyzing cambium samples can help determine whether variations in growth phases are attributed to cellular differentiation or simply to the expansion or contraction of bark in response to dry or rainy conditions ([3], [35]).
Previous studies have investigated the link between phenological processes and cambial activity in tropical forests ([31], [36]). However, integrated approaches aimed to compare radial growth and phenology across multiple time scales can provide a better understanding of how climate influences these characteristics in tropical trees ([37]).
Understanding the factors that influence cambial formation can reveal events affecting plant growth. In this context, the goal of this study was to analyze the relationship between cambial activity, diameter increment, phenological phases, and meteorological data in several tropical tree species. The study aimed to answer the following questions: (i) is the diameter increment of the species influenced by meteorological variables and their phenological behavior? (ii) Is cambial activity seasonal, with defined activity and inactivity periods? (iii) Is cellular differentiation for initial wood formation triggered by any phenological phase?
Materials and methods
The study area is a floodplain located by the Rio Barigui, in the municipality of Araucária, Paraná, Brazil (25° 34′ S, 49° 20′ W), at an average altitude of 900 m above sea level. According to the Köppen classification, the region has a humid subtropical mesothermal climate (Cfb). The annual mean rainfall is 1300-1500 mm, with no dry season and frequent frost in the winter ([2]). The study area is a fragment of Alluvial Araucaria Forest within the Brazilian Atlantic Forest.
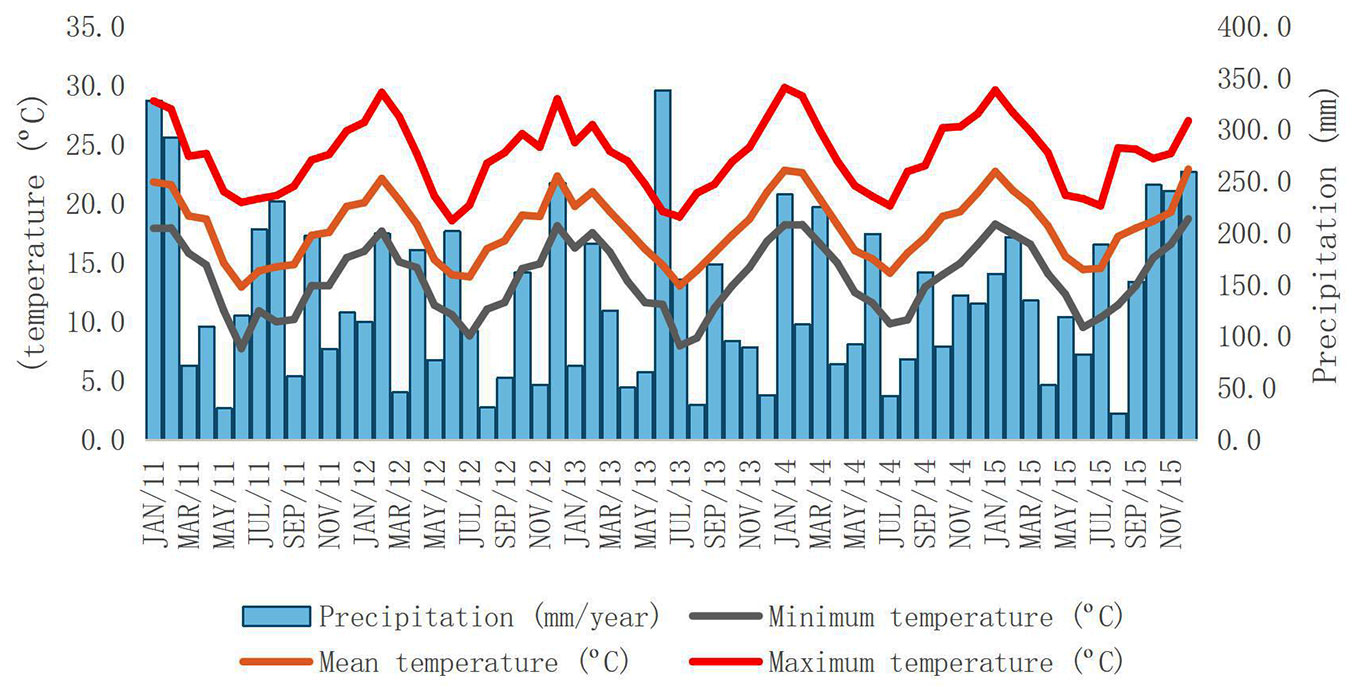
Based on meteorological data from a SIMEPAR station (Meteorological System in Paraná), located 35 km away from the study area, the annual average precipitation was 1629 mm in 2011-2012, 1555 mm in 2012-2013, 1414 mm in 2013-2014, 1537 mm in 2014-2015, and 2035 mm in 2015-2016. The monthly average of minimum, mean, and maximum temperature had variations of approximately 1 °C over the period 2001-2016. The lowest mean temperature was recorded in June 2016 (6.9 °C) and July 2013 (7.9 °C), and the highest temperature was recorded in January 2014 and January 2015, respectively, 29.8 °C and 29.6 °C. The mean annual temperature was 17.8 °C (Fig. 1).
Fig. 1 - Climate chart, data provided by a SIMEPAR station. Average monthly temperature (minimum, average, maximum - lines) and monthly rainfall values (blue bars) between 2011 and 2016.
Four tree species (two native and two exotic) representative of the Alluvial Mixed Ombrophilous Forest were considered in this study, namely Gymnanthes klotzschiana Müll.Arg., Blepharocalyx salicifolius (Kunth) O.Berg, Hovenia dulcis Thunb., and Ligustrum lucidum W.T.Aiton (Tab. 1).
Tab. 1 - Species, family, and number of individuals monitored during the study.
| Scientific name | Family | Origin | No. trees |
Dispersal syndrome |
|---|---|---|---|---|
| Gymnanthes klotzschiana Müll.Arg. | Euphorbiaceae | native | 12 | Zoochory |
| Blepharocalyx salicifolius (Kunth) O.Berg | Myrtaceae | native | 10 | Zoochory |
| Hovenia dulcis Thunb. | Rhamnaceae | exotic | 8 | Zoochory |
| Ligustrum lucidum W.T.Aiton | Oleaceae | exotic | 8 | Zoochory |
Phenological observations were carried out monthly from August 2011 to July 2016, monitoring the vegetative structures (mature and senescent leaves, and buds) and reproductive structures (anthesis, immature and mature fruits) of four tree species . Flowers and fallen fruits were also considered as indicators of phenological activities of the local species, using the Intensity Index ([26]) with adaptations.
The phenophases were classified in four intensity categories, calculated as (eqn. 1):
where ∑Fournier is the sum of the Fournier categories for each individual and N is the number of individuals in the sample. The Fournier intensity categories were: (1) absence of phenophase (flowering or fruiting), (2) presence of phenophase from 1 to 25%, (3) presence of phenophase from 26 to 50%, (4) presence of phenophase from 51 to 75% and (5) presence of phenophase from 76 to 100%. The averages of the variables over the period 2011-2016 were considered in the analysis.
For each individual tree, the circumference at breast height (1.30 m) was measured monthly throughout the study period, using stainless steel dendrometer tapes with an accuracy of ± 0.20 mm. The circumference increment was converted later into diameter increment.
For assessing the cambial activity, wood samples were collected from two individuals of each species in September, November, and December 2014, and in January, March, April, and May 2015, using the methodology proposed by Amano ([3]). Approximately 2 to 3 cm of bark and secondary xylem were collected using a cordless electric drill, followed by the use of a Pressler’s auger at 1.30 m above the ground. To prevent pathogen attacks in the removed area, copper sulfate and calcium oxide were applied in a 1:1 ratio. Wood samples were then fixed in FAA (formaldehyde-glacial acetic acid-ethyl alcohol 50%) until processing. Subsequently, the wood material was embedded, and slides were prepared for histological analysis. The active cambium was recognized by the presence of mitotic figures or phragmoplasts in the cells, or by the presence of tangential walls recently formed.
The relationship between meteorological and phenological variables with diameter increment was analyzed by multiple linear regression, given the continuous nature of the variable. Multiple regression analysis and logistic regression return the probability of occurrence of the evaluated event based on the selected independent variables. The relationship between phenological variables and cambial activity was examined using a logistic regression, due to the dichotomous nature of the cambial activity variable (0-1). The assumptions of normality, homoscedasticity, and independence of residuals were verified for all variables before analysis.
To address the multicollinearity problem of meteorological and phenophase variables, Principal Component Analysis (PCA) was applied, which resulted in a set of statistically orthogonal variables explaining the highest proportion of the total variance in the dataset through linear combinations of the original variables. Both meteorological variables, such as precipitation, minimum, mean, and maximum temperature, and photoperiod were subjected to PCA, as well as phenophase variables, and the main components were extracted. The criterion of latent roots (Kaiser) was chosen to determine the minimum number of axes to be considered, with eigenvalues > 1. The extracted principal components were then used as independent or explanatory variables in regression techniques. The stepwise method of variable selection was used to identify the significant components for the generated regression models. All analyses were performed using the Statgraphics Centurion XV program.
Results
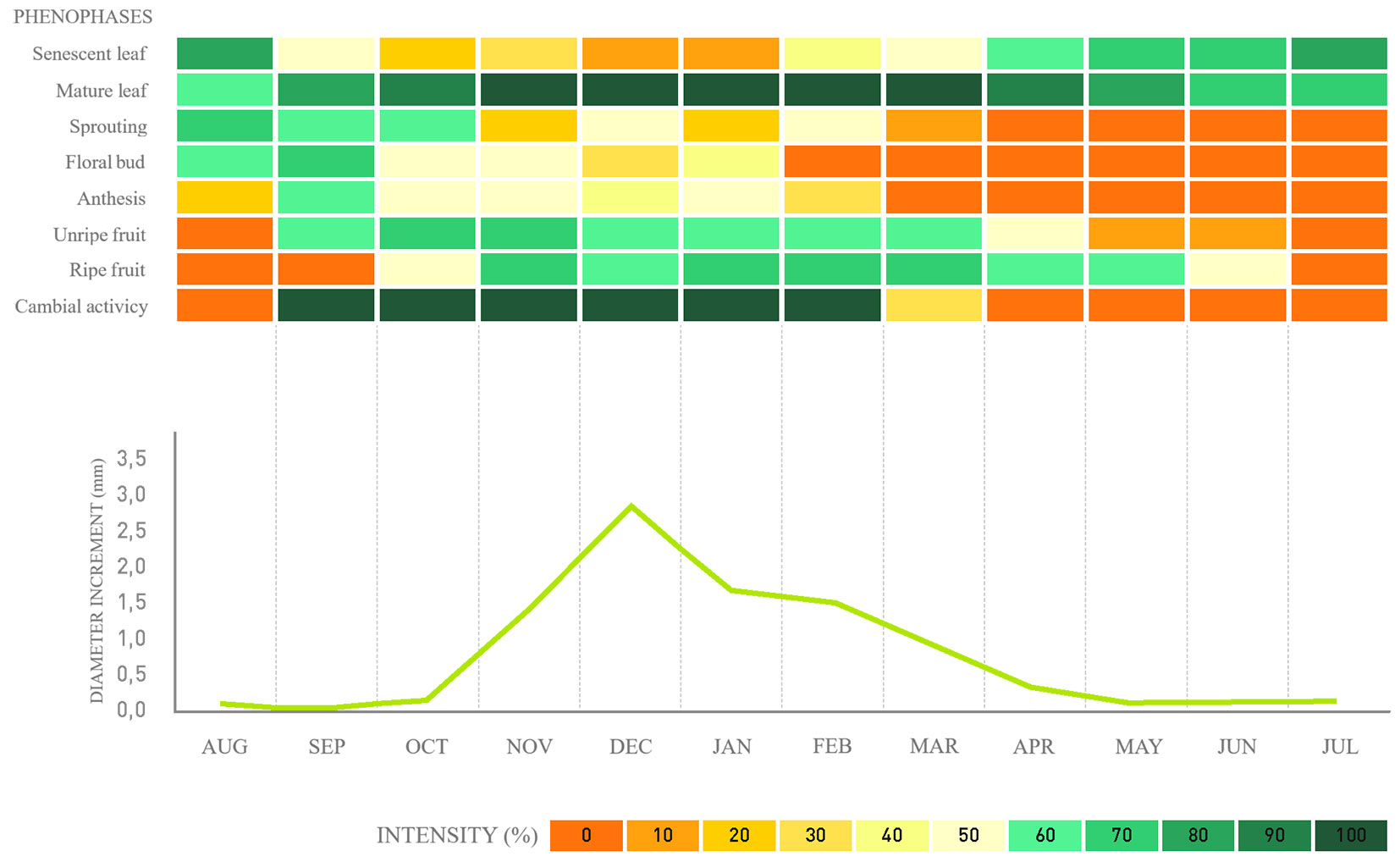
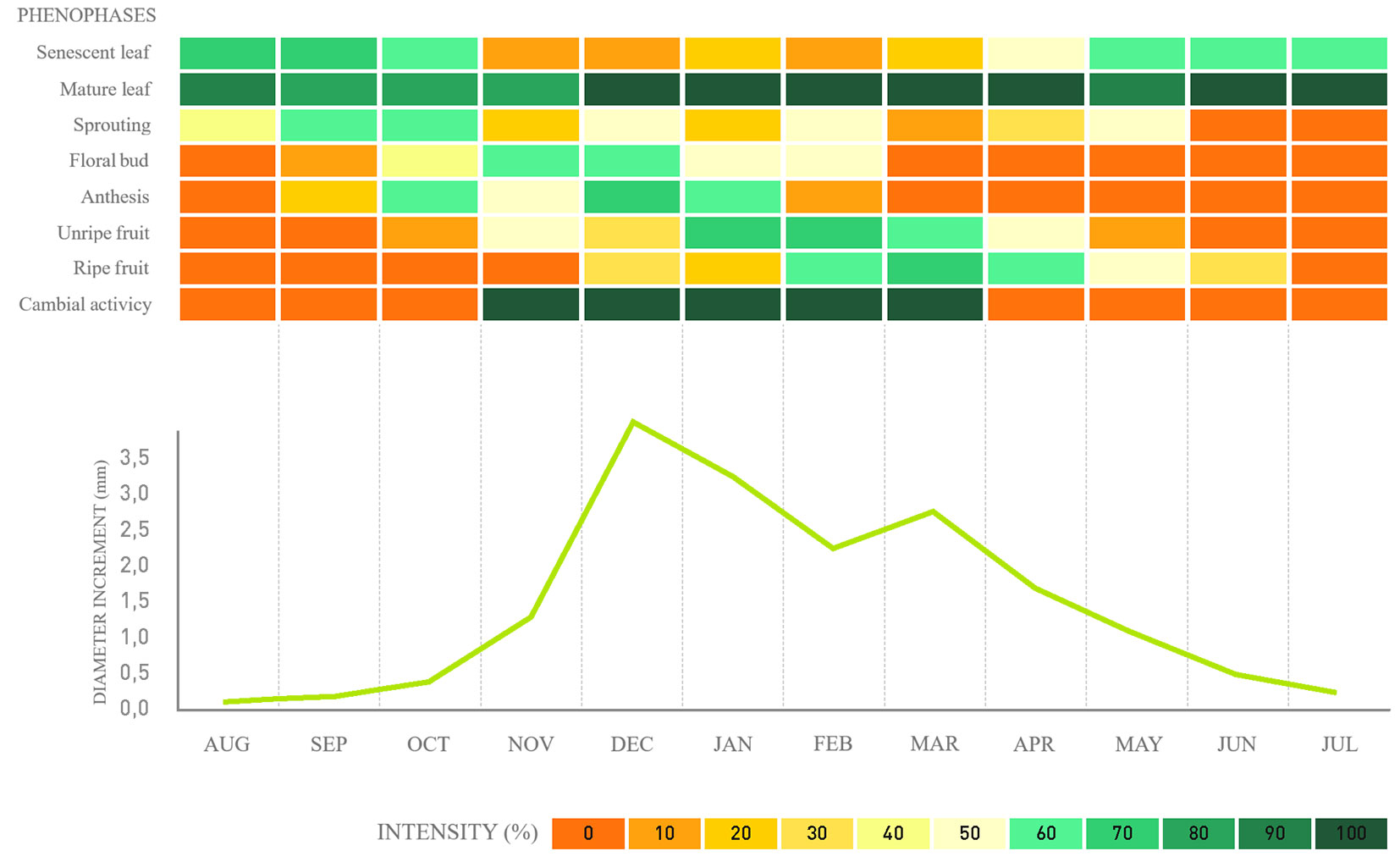
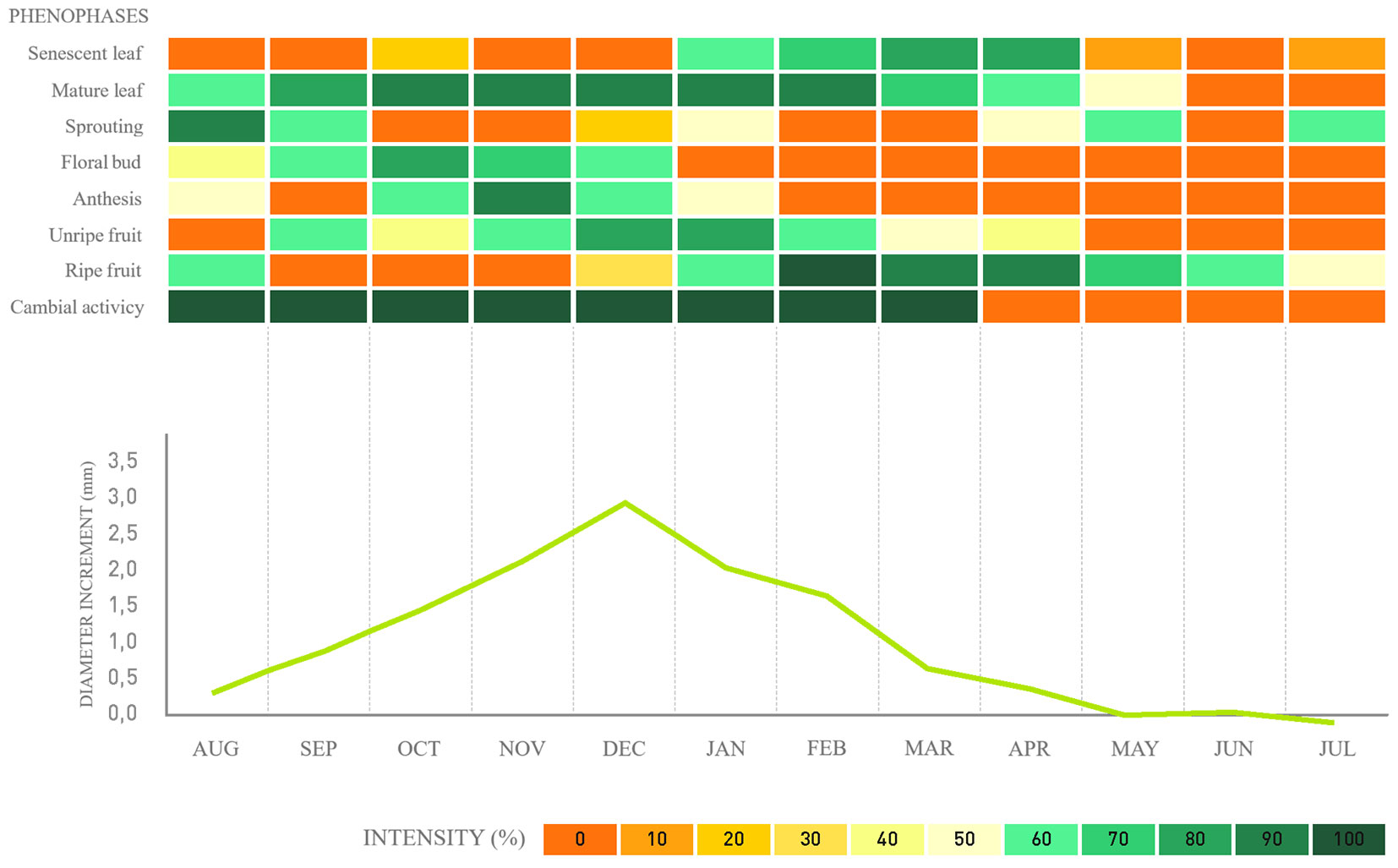
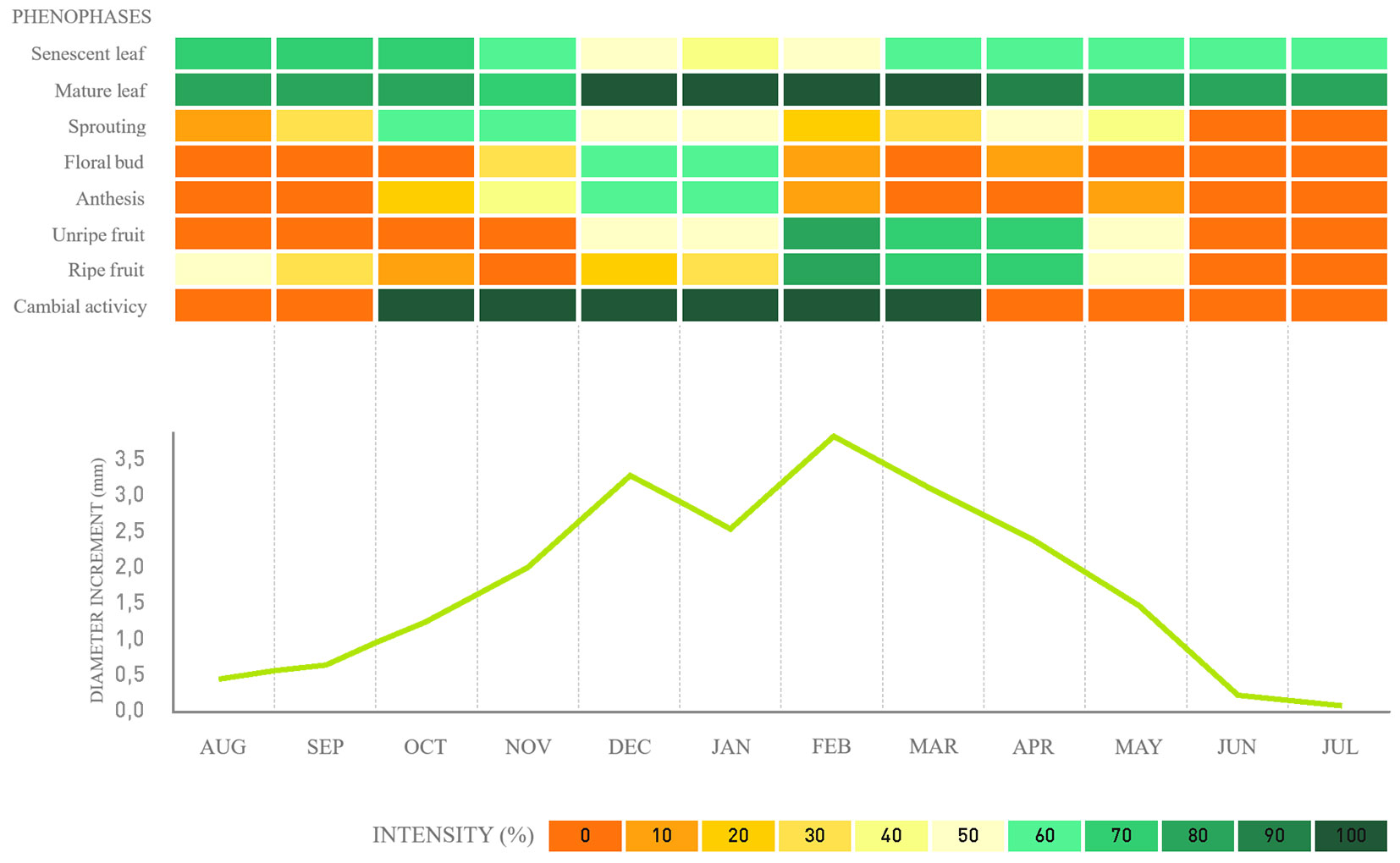
The results of phenological behavior, cambial activity, and diameter increment from the assessed species are shown in Fig. 2, Fig. 3, Fig. 4, and Fig. 5. Each figure displays the specific phenophase period, the stages of cambial activity and inactivity, and the average monthly diameter increment. The length of the growing season varied by species, with diameter increasing from seven to nine months per year; for example, G. klotzschiana and B. salicifolius have their highest rates of diameter increment from November to March, while H. dulcis and L. lucidum, from August to April.
Fig. 2 - Phenology, cambial activity, and monthly average diameter increment for Gymnanthes klotzschiana in a fragment of alluvial mixed ombrophilous forest, Paraná, Brazil.
Fig. 3 - Phenology, cambial activity, and monthly average diameter increment for Blepharocalyx salicifolius in a fragment of alluvial mixed ombrophilous forest, Paraná, Brazil.
Fig. 4 - Phenology, cambial activity, and monthly average diameter increment for Hovenia dulcis in a fragment of alluvial mixed ombrophilous forest, Paraná, Brazil.
Fig. 5 - Phenology, cambial activity, and monthly average diameter increment for Ligustrum lucidum in a fragment of alluvial mixed ombrophilous forest, Paraná, Brazil.
Regarding cambial activity, the results confirm that the species exhibits defined cambial activity and inactivity periods. For the native species, G. klotzschiana and B. salicifolius, the activity periods span over seven to four months, respectively (Fig. 2, Fig. 3), followed by a reduction in the last month until complete cambial inactivity. For the exotic species H. dulcis and L. lucidum, cambial activity lasts from seven to six months, respectively (Fig. 4, Fig. 5). We found that cambial activity occurs in all four species during summer, from December to March, which corresponds with the region’s highest precipitation period.
The principal component analysis (PCA) conducted using meteorological variables revealed that the first component (PC1) accounted for 76.9% of the total variation in the dataset. PC1 showed significant loadings for the mean, minimum, and maximum temperatures, followed by the photoperiod, while precipitation had a relatively minor impact. From the selected components, the main original variables were extracted based on the absolute values of the loadings. Tab. 2reports the loadings of the original variables for phenophase and species.
Tab. 2 - Correlation coefficients of variables with the main principal components (PC).
| Species | PC | Senescent Leaf |
Mature Leaf |
Leaf Budding |
Floral bud |
Anthesis | Immature Fruit |
Mature Fruit |
|---|---|---|---|---|---|---|---|---|
| Gymnanthes klotzschiana | I | 0.828719 | -0.783637 | -0.788494 | -0.777363 | -0.869576 | -0.34504 | -0.0799873 |
| II | 0.543153 | -0.58716 | 0.510923 | 0.593681 | 0.431977 | -0.78841 | -0.72171 | |
| III | - | - | - | - | - | - | - | |
| Blepharocalyx salicifolius | I | 0.522803 | -0.94014 | 0.801213 | 0.086753 | -0.25724 | -0.59243 | -0.47834 |
| II | -0.53165 | 0.087073 | 0.074903 | 0.730494 | 0.752091 | -0.30426 | -0.5219 | |
| III | -0.3782 | -0.28897 | 0.535586 | 0.584574 | -0.25508 | 0.656449 | 0.481861 | |
| Ligustrum lucidum | I | 0.85945 | -0.71331 | 0.395758 | -0.32302 | -0.77723 | -0.66521 | 0.359238 |
| II | -0.38634 | -0.45616 | 0.850678 | 0.680567 | 0.12379 | -0.40788 | -0.79409 | |
| III | 0.120171 | -0.49766 | 0.216342 | -0.60672 | 0.446034 | 0.466419 | -0.23086 | |
| Hovenia dulcis | I | 0.714382 | -0.7403 | 0 | -0.75715 | -0.5652 | -0.21352 | 0.787827 |
| II | -1 | -1 | 1 | 0 | 0 | 0 | 0 | |
| III | -0.34799 | 0.161152 | 0.07029 | -0.52329 | -0.53592 | 0.846996 | -0.21561 |
In the analysis of phenological data for G. klotzschiana, two main components of variation were identified by PCA, accounting for 70.0% of the total variation. Component I contributed 38.1% to the variance (eigenvalue = 2.67), while Component II contributed 29.5% (eigenvalue = 2.24). Regarding B. salicifolius, three components were extracted, which together accounted for 67.0% of the total data variation, with 30%, 22.6% and 14.9% contribution for components I, II, and III, respectively (eigenvalues = 2.10, 1.58, and 1.04).
For the exotic species H. dulcis, three components of variation were also extracted, which together accounted for 74.8% of the total vatiation. In this case, Component I, Component II, and Component III contributed 33.7%, 29.8%, and 17% to the variance, respectively (eigenvalues = 2.36, 17.2, and 1.20, respectively). Finally, for the species L. lucidum, two components of variation were extracted from the data, which together accounted for 70.0% of the total data variation. Component I contributed 34.0% to the variance (eigenvalue = 2.38), while component II contributed 29.0% (eigenvalue = 2.03).
The multiple regression analysis of diameter increment for G. klotzschiana based on PC scores revealed that Component I of meteorological variables was significant to the model, followed by Component I of the phenophase variables, which mainly reflects the mature leaves, senescent leaves, and immature fruit phenophases (Tab. 2). The model provided an adjusted coefficient of determination (R2-adj) of 0.58, indicating that the two selected principal components explain 58% of the variation in the species’ diameter increment.
Regarding B. salicifolius, the regression analysis revealed a significant effect only for the first component of PCA based on meteorological variables. In this case, the R2-adj was 0.42, implying that 42% of the species diameter increment can be explained by the variables that compose the selected component, namely, mean temperature, minimum temperature, maximum temperature, and photoperiod, in order of decreasing weight.
Regarding the diameter increment in H. dulcis, the regression analysis indicated a significant effect of the meteorological variables, Component I of phenophase variables (reflecting mature leaves, mature fruits, and senescent leaves), and Component III of phenophase variables (mainly reflecting immature fruit). The model including these three components explained 71% of the diameter increment variation in H. dulcis (R2-adj = 0.71).
To explain the diameter increment in L. lucidum, only Component I from the PC analysis based on the meteorological variables was included in the model. The resulting adjusted R² was 0.55, indicating that the selected component explained 55% of the diameter increment variation in the species. The results of the logistic regression analysis, considering only the main components of the phenophase variables, are reported in Tab. 3.
Tab. 3 - Logistic regression analysis of cambial activity as a function of the main components of phenological variables. (ns): not significant.
| Species | PC Component |
Prob. | R²-adj |
|---|---|---|---|
| Gymnanthes klotzschiana | I | 0.0001 | 0.7546 |
| II | ns | ||
| Blepharocalyx salicifolius | I | ns | 0 |
| II | |||
| Hovenia dulcis | I | 0.0042 | 0.5345 |
| II | 0.0015 | ||
| Ligustrum lucidum | I | 0.0067 | 0.6393 |
| II | 0.0002 |
Regarding cambial activity in G. klotzschiana, only Component I showed a significant effect, accounting for 75.46% of the variation in the data. As for H. dulcis and L. lucidum, two components has a significant effect on cambial activity, explaining 53.45% and 63.93% of the total variation, respectively. However, no significant relationship was identified between the main components of phenological variables and cambial activity in B. salicifolius.
The probability of occurrence of cambial activity in G. klotzschiana resulted inversely related to the main phenophase variables correlated to Component I, namely, bud and anthesis. Therefore, it can be hypothesized that the probability of occurrence of cambial activity in G. klotzschiana decreases as the intensity of these phenophases increases. The opposite relationship was observed for senescent leaves, as this variable is directly related to Component I, indicating high cambial activity when the intensity of this phenophase increases.
As for H. dulcis, the probability of occurrence of cambial activity was associated with two main components. In this case, Component I showed a direct relationship with the senescent leaf phenophase and an inverse relationship with the anthesis. On the other hand, Component II was positively correlated with the sprouting phenophase.
For the species L. lucidum, Component I showed a direct relationship with the probability of occurrence of cambial activity, primarily reflecting the mature fruit and senescent leaf phenophases. While Component II showed a direct correlation with the probability of occurrence of cambial activity, predominantly represented by the sprouting phenophase, which is also directly related to Component II.
Discussion
Development patterns in tree species refer to specific ways in which wood characteristics and the growth process evolve over time. These patterns encompass cambial activity, which involves the seasonal and cyclical processes of cambial growth. During this process, the cambium layer produces new cells that contribute to the increase in tree diameter. Cambial activity varies among species, and it is influenced by a combination of environmental factors, such as climate and soil conditions, and phenological events, such as seasonal changes in temperature and moisture. The results obtained in this study contribute to understanding the growth and development patterns of species in subtropical forests. By monitoring changes in diameter throughout the seasons, a dynamic view of intra-annual growth can be obtained ([32]) and the plant response to periodic variations in temperature and precipitation can be assessed. We found that meteorological variables have a positive effect on radial increase, the main drivers of diameter increment in the evaluated species being represented by mean temperature, minimum temperature, maximum temperature, and photoperiod. Complex interactions between environmental and genetic factors specific to each species can influence radial growth and phenology ([12], [13]). Nevertheless, approximately 50% of the diameter increment of the studied species is dependent on meteorological conditions (except precipitation), which results in distinct growth rates among species. Also, ambient temperature directly or indirectly regulates phenological behavior of species ([25]).
Previous studies indicate that precipitation is the main factor driving species growth in tropical regions, leading to the resumption of radial growth and the occurrence of many phenophases ([33], [23], [8], [11]). In contrast, our findings indicate that precipitation is not the main factor influencing diameter growth or the timing of phenological phases of the species examined. Indeed, precipitation was the only meteorological variable that was not significant in the PCA. In a specific environment where climatic seasonality is less pronounced, such as in subtropical forests, other factors may explain diameter increment such as soil type ([11]). Moreover, the phenological behavior of species could also be affected by selective biotic pressure such as herbivores, predators, pollinators, and dispersers ([38], [1]). Finally, it is also necessary to consider that the study area is located in an alluvial area, where water is not a limiting factor, even under possible deficit conditions.
Growth and reproduction of plants occur regularly when conditions are suitable, requiring them to synchronize their activities with favorable weather ([19]). This adaptation results in intra-annual variations in flowering, fruiting, sprouting, diameter growth, and cambial activity, mainly related to ambient temperature ([28]).
Several phenophases contributed to explaining part of the radial growth in G. klotzschiana and H. dulcis. For both species, the selected main component reflected the occurrence of mature leaves, senescent leaves, and immature fruit, also including mature fruit for the exotic species. For both species, a significant relationship was found between diameter increment and the presence of mature and senescent leaves. This indicates that the period during which the leaves are senescent is associated with the lowest diameter increment. Conversely, the time when mature leaves are most abundant corresponds to the highest diameter increment, ultimately leading to a peak in cambial activity.
In a study using logistic regression, a lower diameter increment has been reported after leaf fall. However, leaf shedding was not related to reproductive phenology ([29]). The relationship between diameter increment and the occurrence of immature fruit, which was not identified in other studies, may provide new insights into the plant hormonal balance during metabolic activities. This aspect was not evaluated in this study but warrants consideration as a topic for future research.
The species examined in this study typically experience a phase of reduced growth, marked by a lack of activity in the cambium. According to Breitsprecher & Bethel ([9]), while a few species around the world grow continuously, most enter periods of dormancy that help them withstand unfavorable weather conditions.
The discrepancy between the period of radial growth and cambial activity may stem from the measurement technique, as dendrometer tapes track changes in tree circumference. Radial expansion or contraction of trunks can be influenced by precipitation ([21]). Fritts ([18]) also noted that a decrease in diameter could result from bark contraction as a response to low moisture content at the time of measurement.
Regarding the seasonality of cambial activity, we found that different species exhibit both inactive and active cambium periods, which are not uniform throughout the year, and these periods are determined by the interaction of internal and external factors ([24], [20]). In G. klotzschiana, which is a perennial species with annual leaf renewal ([26]), the cambial activity period coincided with the senescent leaves phenophase. The gradual replacement of senescent leaves by new ones (bud break) triggers growth resumption through hormonal factors, such as indole-3-acetic acid (IAA or auxin), released during the bud break phenophase ([5]). Likewise, cambial activity is associated to the occurrence of mature leaves, and coincides with the highest intensity of the phenophase. Thus, leaf phenology is a good indicator of cambial activity in tree species ([27]).
For the exotic species H. dulcis, bud break is a key phenological signal, typical of deciduous trees, which triggers the reduction of metabolic activity to survive unfavorable conditions, particularly during cold winter periods. Low temperatures at the beginning of spring can impact the initiation of cambial cell division and xylem differentiation in trees. Conversely, higher temperatures at the end of winter can stimulate an early cambial reactivation ([15]).
In this study, no phenophase was associated with the period of cambial activity/inactivity in B. salicifolius, indicating that cambium could be influenced by different external or internal conditions. Further research on how these annual processes happen can provide insights into the species’ life cycle; however, it is important to emphasize that the factors that regulate radial growth may be distinct from those that reduce or trigger the resumption of cambial activity.
Conclusion
Meteorological variables, such as average temperature, minimum temperature, maximum temperature, and photoperiod, significantly influenced the growth in diameter of the four monitored species. Unexpectedly, no influence of precipitation on diametric growth was detected.
The influence of phenophases also accounts for some of the diametric growth seen in two species. For Gymnanthes klotzschiana, the relevant phenophases include mature leaves, senescent leaves, and immature fruits. For Hovenia dulcis, the significant phenophases are mature leaves, senescent leaves, and ripe fruits.
Cambial activity is seasonal, with periods of activity and inactivity in all four species; notably, cambial activity is concentrated mainly in the spring and summer.
Different phenological signals triggering the formation of initial wood were identified in distinct species. For Gymnanthes klotzschiana, senescent leaf; in Hovenia dulcis, bud break; for Ligustrum lucidum, ripe fruit and senescent leaves. No relationship was detected between the evaluated phenophases and the cambial activity and inactivity periods in Blepharocalyx salicifolius.
References
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
CrossRef | Gscholar
Authors’ Info
Authors’ Affiliation
Postgraduate Program in Forestry and Environmental Sciences, Federal University of Mato Grosso, Cuiabá, Mato Grosso (Brazil)
Larissa da Silva Bueno dos Santos 0009-0009-7988-8412
Faculty of Forestry Engineering, Federal University of Mato Grosso, Cuiabá, Mato Grosso (Brazil)
Franklin Galvão 0000-0002-1425-1607
Bruno Palka Miranda 0000-0002-4349-005X
Carlos Vellozo Roderjan 0000-0002-1872-5355
Federal University of Paraná, Curitiba, Paraná (Brazil)
Pontifical Catholic University of Paraná, Curitiba, Paraná (Brazil)
Corresponding author
Paper Info
Citation
Milani JEF, Neto AA, Bueno dos Santos LS, Amano E, Galvão F, Miranda BP, Kersten RA, Roderjan CV (2025). Radial growing, cambial activity, and phenology of tree species in a subtropical forest in Southern Brazil. iForest 18: 252-258. - doi: 10.3832/ifor4642-018
Academic Editor
Claudia Cocozza
Paper history
Received: May 17, 2024
Accepted: Apr 03, 2025
First online: Sep 22, 2025
Publication Date: Oct 31, 2025
Publication Time: 5.73 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2025
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 2805
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 1181
Abstract Page Views: 850
PDF Downloads: 705
Citation/Reference Downloads: 2
XML Downloads: 67
Web Metrics
Days since publication: 158
Overall contacts: 2805
Avg. contacts per week: 124.27
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
(No citations were found up to date. Please come back later)
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Links between phenology and ecophysiology in a European beech forest
vol. 8, pp. 438-447 (online: 15 December 2014)
Research Articles
Essential environmental variables to include in a stratified sampling design for a national-level invasive alien tree survey
vol. 12, pp. 418-426 (online: 01 September 2019)
Review Papers
Biodiversity assessment in forests - from genetic diversity to landscape diversity
vol. 2, pp. 1-3 (online: 21 January 2009)
Research Articles
Hearing nature’s heartbeat: towards large-scale real-time forest monitoring network in Italy
vol. 18, pp. 202-211 (online: 09 August 2025)
Research Articles
Background, main results and conclusions from a test phase for biodiversity assessments on intensive forest monitoring plots in Europe
vol. 2, pp. 67-74 (online: 18 March 2009)
Book Reviews
National forest inventories: contributions to forest biodiversity assessments (2010)
vol. 4, pp. 250-251 (online: 05 November 2011)
Research Articles
Long-term monitoring of air pollution effects on selected forest ecosystems in the Bucegi-Piatra Craiului and Retezat Mountains, southern Carpathians (Romania)
vol. 4, pp. 49-60 (online: 05 April 2011)
Research Articles
Tree growth, wood and bark water content of 28 Amazonian tree species in response to variations in rainfall and wood density
vol. 9, pp. 445-451 (online: 16 January 2016)
Short Communications
QA/QC activities and ecological monitoring in the Acid Deposition Monitoring Network in East Asia (EANET)
vol. 2, pp. 26-29 (online: 21 January 2009)
Technical Advances
A meta-database comparison from various European Research and Monitoring Networks dedicated to forest sites
vol. 6, pp. 1-9 (online: 14 January 2013)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword