Combining bioengineering and plant conservation on a Mediterranean islet
iForest - Biogeosciences and Forestry, Volume 5, Issue 6, Pages 296-305 (2012)
doi: https://doi.org/10.3832/ifor0638-005
Published: Dec 17, 2012 - Copyright © 2012 SISEF
Technical Advances
Abstract
This paper reports the results of a bioengineering intervention within the Mediterranean Basin carried out at Lampedusa Island (Strait of Sicily) on the “Spiaggia dei Conigli”, the only sand shore of all Sicilian territory where the sea turtle Caretta caretta lays its eggs every year. The erosion of the steep slope over the beach itself caused sensitive changes in the grain size of shore’s sediment and reduced the area of the beach with fine sand suitable for C. caretta oviposition. In order to reduce surface water flow and to stop erosion, several bioengineering options were adopted using only native plant species to preserve local botanical heritage and to prevent the local extinction of some species. One year after interventions, average plant establishment was about 90% and many species which were severely endangered before the action (i.e., Jacobaea maritima (L.) Pelser & Meijden subsp. bicolor (Willd.) B. Nord. & Greuter and Limoniastrum monopetalum (L.) Boiss.) are now at low risk. Micropropagation and inoculation with beneficial root microbial symbionts were successfully applied to selected species. Regular demographic and phytosociological monitoring on permanent plot areas enabled to quantify the effect of bioengineering techniques on plant percentage cover and plant survival. The combination of bioengineering, biotechnology, and agronomic practices applied on plants appears to be effective in increasing plant cover and preserving several locally endangered plant species. Results presented here suggest that erosion can be controlled without moving large quantities of soil and without planting tree species.
Keywords
Reforestation, Landscape, Protected area, Endangered plants, Lampedusa island
Introduction
The coastal areas of the Mediterranean and especially those of its islands are at great risk of environmental degradation ([1]). To counter this risk, the EU has developed a number of projects, one of which concerns the protection of the habitats suitable for the loggerhead sea turtle, Caretta caretta. Within the EU-Project LIFE03 NAT/IT/000163 “Reduction of human impact on Caretta caretta and Tursiops truncatus and their conservation in Sicily”, action C1 included bioengineering ([33]), within an area of the nature reserve “Isola di Lampedusa” ([34]). This action concerned the geomorphological and ecological restoration of the area upstream of the Spiaggia dei Conigli (beach of rabbits, hereafter indicated with the acronym SDC), one of the few Italian nesting sites of C. caretta ([43]). The work also included the reconstitution of the dirt road leading down to the beach, realized in the 1980s and maintained until 1996, which in the last few years has become a preferential path for the flow of meteoric water. A road to the beach triggered major erosion of the steep slope over the beach itself and caused sensitive changes in the grain size of shore’s sediment. This flow has swept soil and stones from the hillside between the road and beach downstream, altering the sand composition of the beach and making oviposition by sea turtles increasingly difficult ([6], [43]).
Moreover, the whole island was subject to intense degradation as a consequence of human colonisation, so that during the last 170 years local animal and plant species disappeared or underwent a severe depletion along with plant communities ([25], [37], [38], [29], [27]).
This work describes one of the first restoration projects concerning a Mediterranean beach based on the use of propagated autochthonous species, bioengineering techniques and microbial biotechnologies.
The aim of this study was to integrate the use of bioengineering techniques and biological conservation for the restoration of the SDC area and particularly: (i): to use propagation material exclusively from autochthonous plant species (herbs, grasses and shrubs) for revegetation to stop species loss and genetic pollution in Lampedusa Island; (ii): to apply agronomic techniques and seedlings inoculation with beneficial root microbial symbionts in order to enhance plant establishment; (iii): to evaluate the effect of different bioengineering techniques on the establishment rate of some key species.
Bioengineering techniques have been typically applied to mountain environments ([47], [18]), while on coastal areas they have been seldom used, and mostly on dune ecosystems ([35], [48]). In recent times, an increasing attention has been paid on the idea of combining bioengineering purposes and ex situ conservation through the propagation of autochthonous species ([42], [41]). In this work plants species were chosen on the basis of the results of previous investigations on the local vascular flora ([3], [28]) and on the semi-natural and agricultural landscape of Lampedusa ([37], [38], [26], [39]).
Beneficial soil fungi and nitrogen fixing bacteria play a crucial role in producing fundamental ecosystem services such as soil fertility, nutrient cycling and favouring plant community dynamics ([53], [49]). The diversity of bacteria and arbuscular mycorrhizal fungi (AMF) communities can decline due to habitat loss and anthropogenic disturbance and their reintroduction in the rhizosphere of greenhouse-propagated plants can help plant growth, productivity, health, and stress relief ([7]).
The success of restoration actions was assessed in terms of vegetation cover and changes in the floristic composition of plots, by comparison with unaffected areas and by monitoring plant growth and survival within the restored sample plots.
Materials and methods
Study area
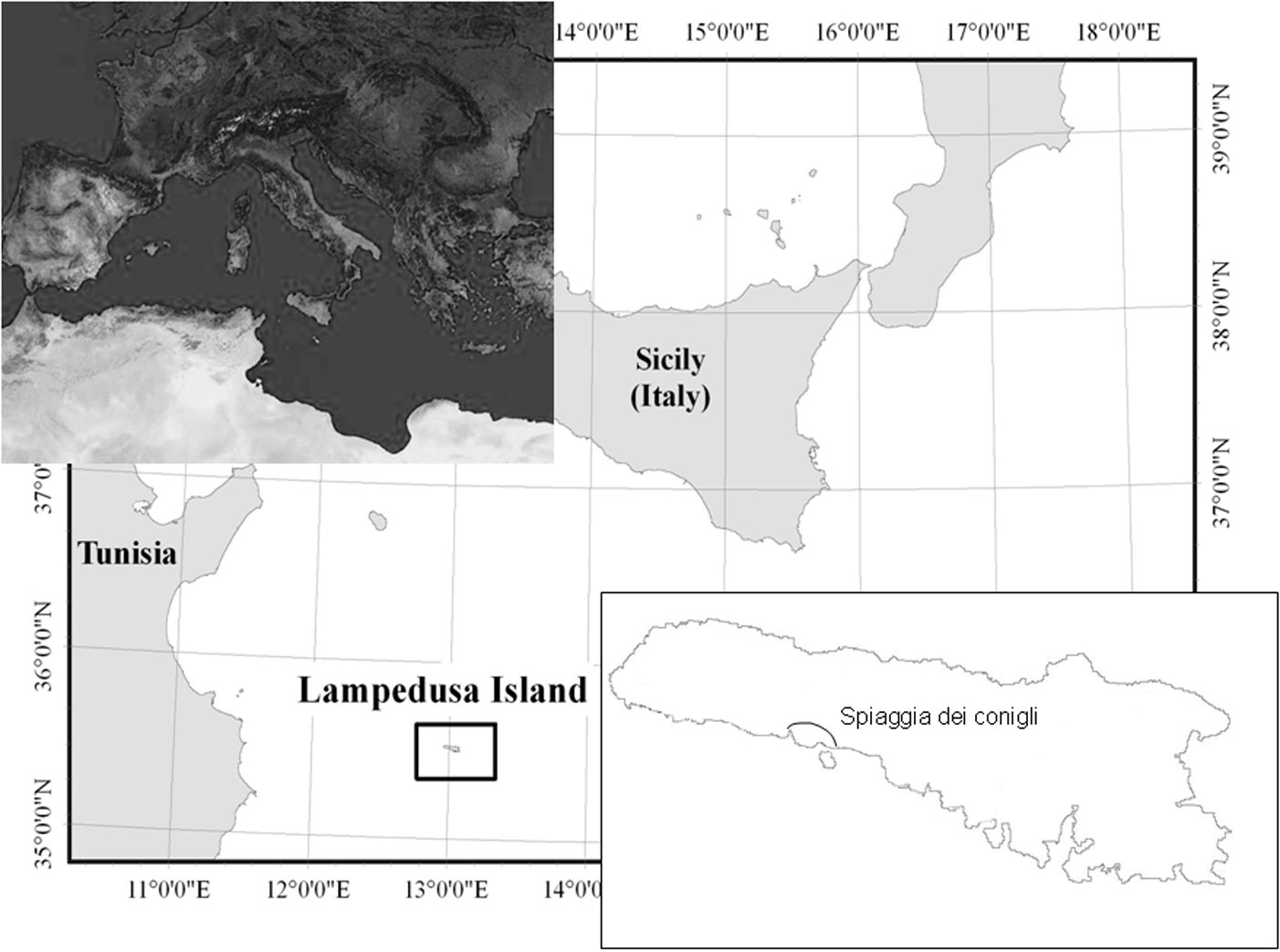
Lampedusa is the biggest island of Pelagie Archipelago (approx. 20 km2); it is located in the Strait of Sicily, some 115 km off the Tunisian coast and 195 km off Sicily (Fig. 1). The island is a triangular plateau with a quite uniform and slightly sloping surface. The southern coast is rugged, with numerous canyons, cliffs, and deep coves that often host sandy beaches the most famous of which is SDC. The average annual rainfall of Lampedusa is 321 mm with a pronounced concentration of precipitation in autumn and winter ([26]). Mean annual temperature is 19.3 °C, with an average of 13.5 °C in the coldest months (January and February) and 26.5 °C in the warmest month (August). According to Rivas-Martínez ([45]) classification, Lampedusa has a thermo-Mediterranean bio-climate, with a dry season that lasts about 6 months (from late February to late October).
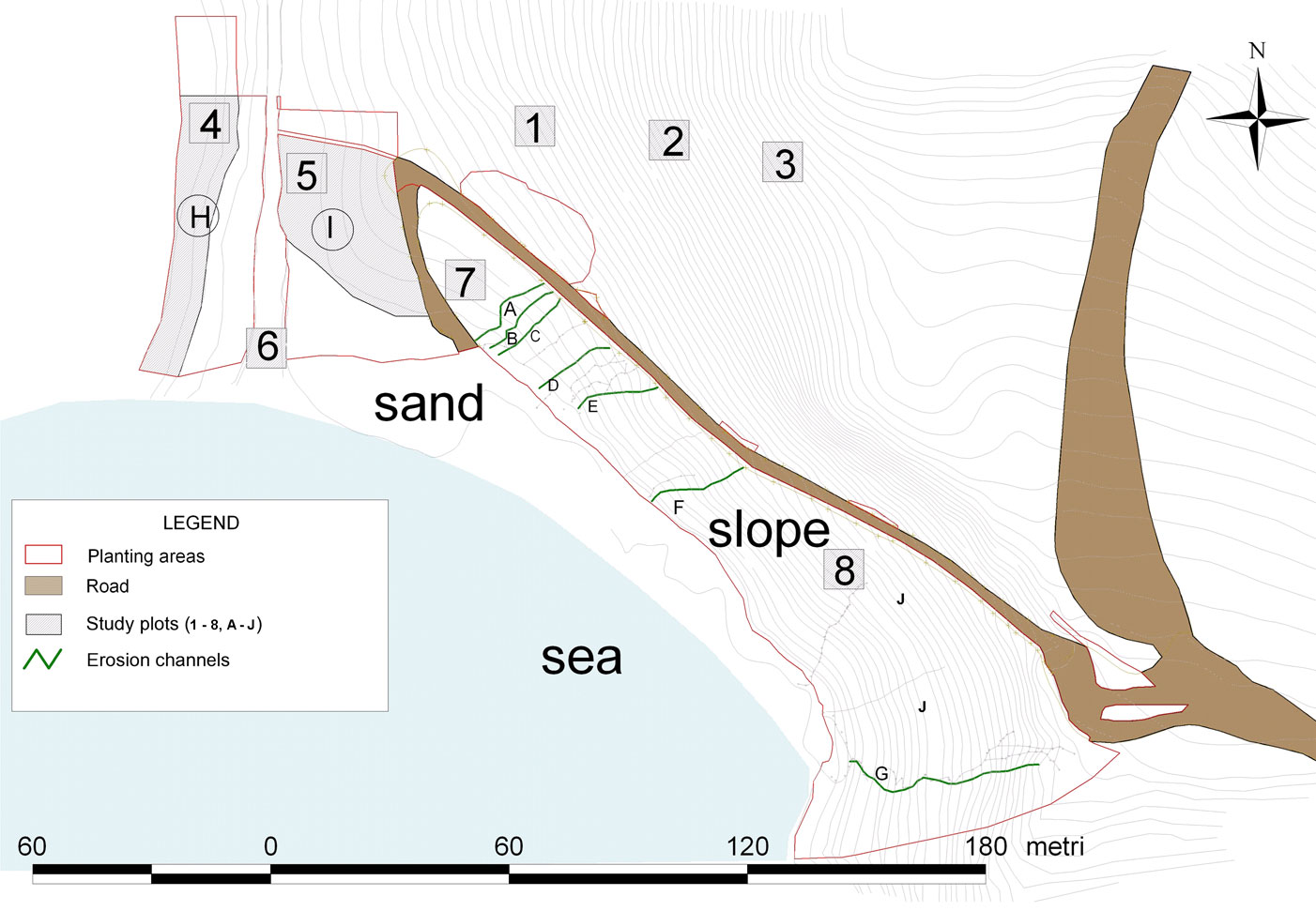
The intervention area covered about 13 000 m2 above the “Spiaggia dei Conigli” (Fig. 1 and Fig. 2). The area was subdivided on the basis of the restoration actions in seven erosion channels (A to G) that were subject to both planting and bioengineering treatments as described below, two areas (H, I) where only planting was realized (PO: Planted Only), and two areas with jute nets only (J: Jute - Fig. 2). Other three areas were only subject to bioengineering interventions without planting (BO: Bioengineering Only); finally, one area not subject to erosion nor to any intervention was considered as control for plant cover evaluation (NSE: Not Subject to Erosion).
Fig. 2 - A map of “Spiaggia dei Conigli” where bioengineering and planting were carried out in order to reduce the erosion. See the text and Tab. 5 for explanation of study plots and planting areas.
Plant propagation, pre-planting and planting techniques
Plant material for propagation was obtained from seeds, cuttings, or “clumps” (a clump included shoots, roots, and associated soil) and was collected in 2004 and 2005 on the slopes overlooking SDC and elsewhere at Lampedusa. Plant species and their mode of propagation are listed in Tab. 1. The standard abbreviation of the authors of the scientific names concerning all the plants used for bioingeneering activities are reported on Tab. 1, while the authors of the other plants have been indicated when mentioned for the first time. A portion of plant material was propagated in a specialized private nursery in Sicily, then transferred and acclimatized at lampedusa local nursery of the Regional Agency of Forests, where other species (e.g., Juniperus turbinata, Capparis spinosa subsp. rupestris and Periploca angustifolia) were also propagated. Perennial herbs and grasses (e.g., Asphodelus ramosus, Charybdis maritima and Hyparrhenia hirta) were usually propagated by use of the vegetative organs and were either directly transplanted from other sites of Lampedusa or radicated in the nursery before transplantation at SDC. Only Arbutus unedo was micropropagated in the laboratory by Legambiente (an environmentalist organization, encharged of the management of the nature reserve “Isola di Lampedusa”) at the laboratory site in nature reserve “Grotta di Carburangeli”. Several woody species (Clematis cirrhosa, Lycium intricatum, Rubus ulmifolius, Ruta chalepensis, and Teucrium fruticans) were propagated by use of rooted cuttings, which were rooted directly in the field or in the nursery before transplantation in the field. During spring 2007, some 4883 specimens belonging to 43 different taxa (Tab. 1) were transplanted in the degraded area above SDC.
Tab. 1 - Plant propagation method and establishment rate in the SDC area. The risk level of all endemic, rare and/or threatened vascular plants is reported according to IUCN classification ([13]). (EW): extinct in the wild; (CR): critically endangered; (EN): endangered; (VU): vulnerable; (LR): low risk; (DD): lack of data). A semi-quantitative evaluation of local rarity, mainly based on data from Pasta ([37]) and La Mantia et al. ([28]) is given within the fourth column: (rrr): extremely rare (n < 50 plants); (rr): very rare (50-200 plants); (r): rare (200-500 plants); (c): common; (lo): localized. For six extremely rare species the exact number of individuals is reported; (+): propagated by micropropagation; (pls): transplanted in situ. (#): the original source (clump or seed) could not be determined; (**): this species is extinct in the wild at Lampedusa; (*): native and artificially propagated individuals could not be distinguished; (^): for species propagated in small numbers, the absolute value is shown rather than the percentage.
| Taxon | Status IUCN | Used propagules | Presence before restoration | Number of specimens | Numerical increase after restoration | % plants establishment (October 2010) |
|---|---|---|---|---|---|---|
| Endemics of Lampedusa island or Pelagie Archipelago | ||||||
| Lampedusas Fleabane Chiliadenus lopadusanus Brullo |
VU | Clump (pls) | c | 10 | 0 | 0.0 |
| Lampedusas cliff pink Dianthus rupicola Biv. subsp. lopadusanus Brullo & Minissale |
- | Seed | rr | 193 | 75 | 62.5 |
| Lampedusas sea-lavender Limonium lopadusanum Brullo |
LR | Clump | c | 5 | 0 | 0^ |
| Pelagian sand lily Pancratium linosae Soldano & F. Conti |
EN | Seed | lo | 20 | 20 | 100.0 |
| Pelagian sea blite Suaeda pelagica Bartolo, Brullo & Pavone |
CR | Seed | lo | 40 | 15 | 37.5 |
| Endemic of Sicily, Sicily and Maltese Archipelago, S Italy and Sicily or central Mediterranean areas | ||||||
| Silver ragwort Jacobaea maritima (L.) Pelser & Meijden subsp. bicolor (Willd.) B. Nord. & Greuter |
LR | Seed | 0** | 26 | 11 | 50.0 |
| Other rare, endangered, or nationally protected species | ||||||
| Cliff crossworts Crucianella rupestris Guss. |
VU | Seed | c | 10 | 0 | 0.0 |
| Shrubby St Johns Wort Hypericum aegypticum L. subsp. webbii (Spach) N.K.B. Robson |
EN | Seed | c | 23 | 1 | 25.0 |
| Phoenician juniper Juniperus turbinata Guss. |
VU | Seed | r | 307 | 284 | 95.3 |
| Limoniastrum Limoniastrum monopetalum (L.) Boiss. |
VU | Seed | Rrr | 32 | 28 | 87.5 |
| Matted boxthorn Lycium intricatum Boiss. |
DD | Seed | - | 88 | 46 | 53.5 |
| Rooted cuttings (pls) | c | 10 | 1 | 10.0 | ||
| Wolfbane Periploca angustifolia Labill. |
LR | Clump | c | 5 | 0 | 0^ |
| Seed | - | 619 | 574 | 92.0 | ||
| Mediterranean Phagnalon Phagnalon saxatile (L.) Cass. subsp. saxatile |
LR | Clump | c | 9 | 3 | 37.5 |
| - | Seed | - | 5 | 3 | 2^0 | |
| Other rare or endangered species at the local level | ||||||
| Stinking bean trefoil Anagyris foetida L. |
- | Seed | 5 | 179 | 106 | 67.5 |
| Strawberry tree Arbutus unedo L.+ |
- | Plants (by micropropagation) | 1 | 24 | 22 | 91.7 |
| Carob tree Ceratonia siliqua L. |
- | Seed | rrr | 12 | 12 | 100.0 |
| Fern-leaved clematis Clematis cirrhosa L. |
- | Plants | r | 10 | 0 | 0.0 |
| - | Rooted cuttings | - | 10 | 0 | 0.0 | |
| Shrubby scorpion vetch Coronilla valentina L. subsp. glauca (L.) Batt. |
- | Seed | 6 | 31 | 15 | 55.6 |
| Mediterranean heath Erica multiflora L. subsp. multiflora |
- | Seed | rr | 10 | 0 | 0.0 |
| Myrtle Myrtus communis L. |
- | Seed | 4 | 281 | 177 | 63.4 |
| Broad-leaved Phillyrea Phillyrea latifolia L. |
- | Seed | rr | 31 | 13 | 86.7 |
| Common bramble Rubus ulmifolius Schott |
- | Rooted cuttings | 3 | 2 | 2 | 2^ |
| Other species | ||||||
| Wild asparagus Asparagus acutifolius L. |
- | Seed | - | 23 | 11 | 100.0 |
| Branched asphodel Asphodelus ramosus L. |
- | Clump (pls) | - | 105 | 77 | 79.4 |
| Mediterranean saltbush Atriplex halimus L. |
- | Seed | - | 528 | 406 | 78.1 |
| Caper Capparis spinosa L. subsp. rupestris (Sibth. & Sm.) Nyman |
- | Seed | - | 36 | 8 | 88.9 |
| Sea squill Charybdys maritima (L.) Speta |
- | Clump (pls) | - | 228 | 194 | 95.1 |
| Conehead thyme Coridothymus capitatus (L.) Rchb. f. |
- | Seed | - | 115 | 30 | 40.0 |
| Sea fennel Crithmum maritimum L. |
- | Seed | - | 163 | 92 | 56.1 |
| Tree spurge Euphorbia dendroides L. |
- | Seed | - | 358 | 180 | 57.7 |
| Pine spurge Euphorbia pinea L. |
- | Seed | - | 7 | - | n.d* |
| Horned poppy Glaucium flavum Crantz |
- | Clump # | - | 5 | - | 0.0 |
| - | Seed # | - | 23 | 18 | 72.0 | |
| Coolatai grass Hyparrhenia hirta (L.) Stapf s.l. |
- | Clump (pls) | - | 21 | 0 | 0.0 |
| - | Plants | - | 161 | 65 | 59.1 | |
| Golden samphire Limbarda crithmoides (L.) Dumort. |
- | Plants | - | 159 | 129 | 74.1 |
| - | Clump | - | 10 | 7 | 70.0 | |
| Shrubby Micromeria Micromeria fruticulosa (Bertol.) ilci |
- | Clump | - | 5 | 1 | 1^ |
| Rock Phagnalon Phagnalon rupestre (L.) DC. subsp. annoticum (Burnat) Pignatti |
- | Clump (pls) | - | 5 | 1 | 1^ |
| - | Seed | - | 5 | 0 | 0.0 | |
| Lentisk Pistacia lentiscus L. |
- | Seed | - | 441 | 376 | 87.0 |
| White Hedge-nettle Prasium majus L. |
- | Seed | - | 137 | 86 | 64.7 |
| Fringe rue Ruta chalepensis L. |
- | Rooted cuttings | - | 6 | 0 | 0.0^ |
| Saltwort Salsola oppositifolia Desf. |
- | Seed | - | 38 | 34 | 91.9 |
| Shrubby seablite Suaeda vera J.F. Gmelin |
- | Clump | - | 17 | 8 | 47.1 |
| - | Seed | - | 9 | 10 | 90.9 | |
| Shrubby Germander Teucrium fruticans L. |
- | Seed | - | 180 | 103 | 80.5 |
| - | Rooted cuttings (pls) | - | 94 | 0 | 0.0 | |
| Hairy Thymelaea Thymelaea hirsuta (L.) Endl. |
- | Clump (pls) | - | 12 | 9 | 75.0 |
Microbial inoculation
A few plant species were also inoculated in the nursery at seedling phase with beneficial microbial symbionts to improve their fitness. Anagyris foetida was inoculated with a nitrogen-fixing Mesorhizobium strain isolated in Sicily from A. foetida root nodules ([11], [10]) and with a mix of a commercial inoculum of ectomycorrhizal fungi (MICOSAT F® VO12 WP, produced by CCS Aosta s.r.l., Italy) composed of a mix of crushed mycorrhizal roots and spores of endomycorrhizal fungi and mycelia of the genus Glomus (G. mosseae, G. intraradices, G. viscosum, G. coronatum and G. caledonium) and saprotrophic fungi (Tricoderma viride). Atriplex halimus, Ceratonia siliqua, Coronilla valentina subsp. glauca, Dianthus rupicola subsp. lopadusanus were treated with the above mentioned commercial inoculum together with spores and mycelium of ectomycorrhizal fungi, such as Tuber uncinatum, Hebeloma spp., Coenococcum spp., and saprophytic fungi, Tricoderma herthianum. Inoculation on Ceratonia siliqua was carried out on 1-year old plants. The effects of microbial inoculation were analysed once in the nursery as for growth parameters and once in the field as for survival rate, and compared to uninoculated plants.
Agronomic practices and protection against herbivores
During the first year after planting, all plants were irrigated (monthly during summer, when needed during winter). In order to defend plants from rabbits, an extract of garlic (Allium sativum L. - garlic cloves soaked in water for 2 days) was applied by spraying (several garlic based repellents for rabbits are used and patented in the United States). Larger plants were protected with cylinders of green, stiff plastic net (shelters).
Bioengineering techniques
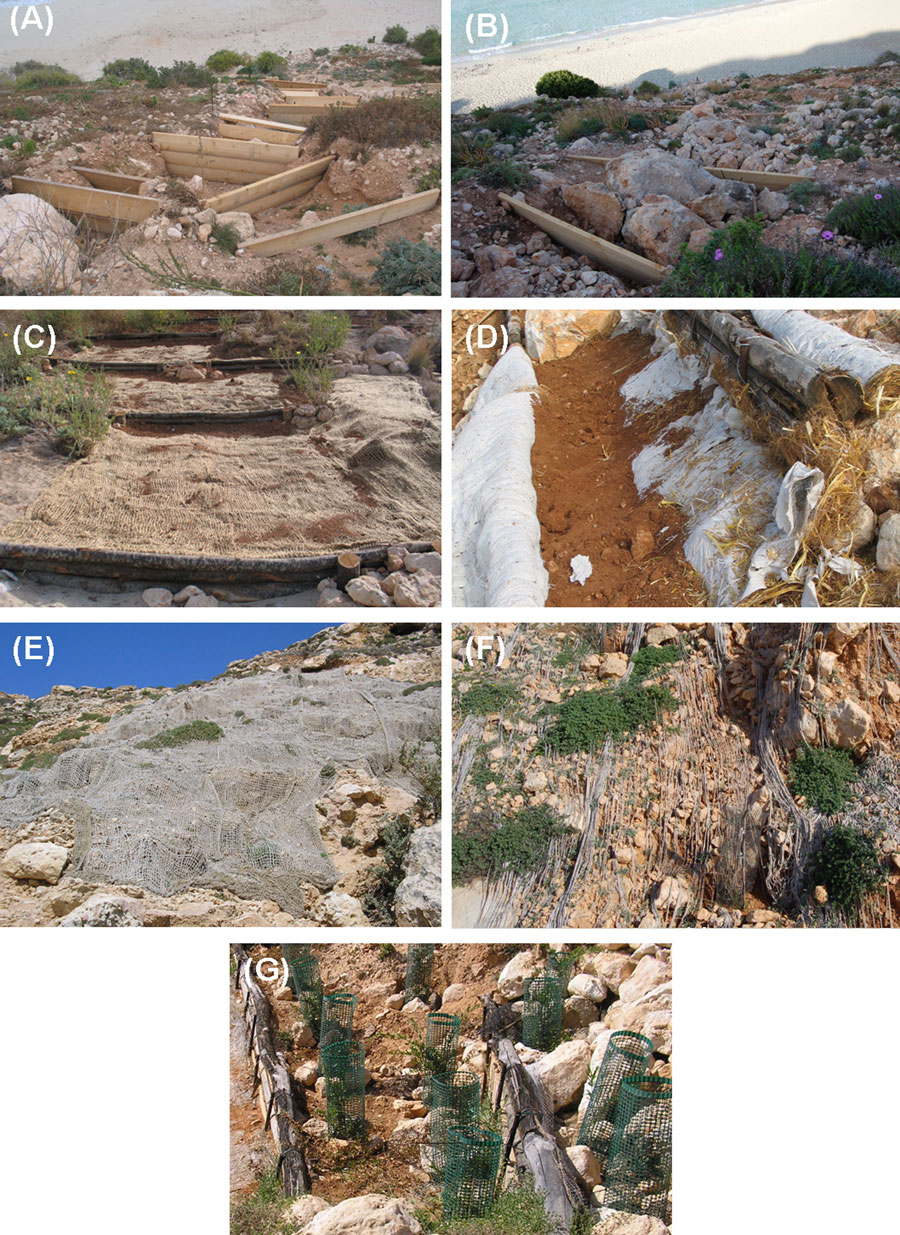
Bioengineering included the construction of 13 fences to reduce water flow and soil erosion (Fig. 3). Fences were made with chestnut boards and poles, and built up across eroded areas (A to G - Fig. 4). The fence height ranged from 0.3 to 1.5 m, depending on the depth of the eroded channel. The eroded channels above the fences were filled with various combinations of sand, soil, stones, “felt mats”, and “biomats”. In order to fill some channels, the stones previously rolled down from the slope were re-used. The felt mats consisted of wood residue in a polypropylene mesh (“Ecofelt W 200”, “Biofeltro” of Harpo S.p.A., Trieste, Italy).
Fig. 3 - Pictures of the “Spiaggia dei Conigli” before and after the restoration. The beach, the hillside, and the road during summer 2005, before intervention: note the severe erosion on the hillside (A). In 2006, bioengineering was carried out to reduce soil erosion; woody fences and stones used to reduce erosion and increase plant establishment (B-D).The hillside in winter 2010, two years after the intervention (E).
Fig. 4 - Some details of the different bioengineering techniques applied in the study area: woody fences (A), woody fences filled with stones (B), felt mats (C), vegetative pockets made of biomat (D), jute nets (F), jute nets after two years stopped erosion processes enhancing shrubs’ growth (E). A particular of the shelters to avoid rabbit grazing (G).
The biomats (“Stcmat”, “Geostuoia biodegradabile”, Harpo S.p.A.) consisted of coconut fiber. When an eroded channel was very deep, “vegetative pockets” were formed by placing a felt mat or biomat at the bottom of the channel and filling it with soil, sand, or stones. Additional details on these materials are provided by Menegazzi & Messana ([33]). Where the erosion had removed nearly all soil layer so that only stone outcrops remained, these were covered with jute nets (“Geojuta”, “Georete antierosione biodegradabile”, Harpo S.p.A.).
Three fences were placed across channels filled with soil (FeSo), six were placed across channels filled with sandy soil (FeSSo), and two were placed across channels filled with stones and soil (FeStSo). One fence was placed across a channel on the steep part of the slope, and the channel was filled with vegetative pocket containing stones [FeStVp(slope)]; another one was placed across a channel that was filled with stones, soil, and biomat (FeStSoB); another else was placed across a channel and filled with a vegetative pocket containing sand [FeVp(sand)]. In two cases, channels without fences were filled with vegetative pockets and biomats (VpB) and with vegetative pockets, biomat, and stones (VpBSt). Fig. 4 illustrates some of the techniques and materials used in order to build up fences. Plants were then transplanted inside these areas.
To reduce disturbance and costs, the bioengineering was performed without heavy equipment. Most of the work was hand-made with shovels, wheel barrows, and other simple tools.
Plant survival and assemblage dynamics monitoring activities
The area was subdivided on the basis of the restoration actions (see study area) and a different combination of selected plant species were used according to their peculiar requirements. For example, Myrtle tree was used only in upper sectors, while Crithmum maritimum was preferred near the sand shore. On the other hand, the species with a wider ecological range and used in large amounts (Periploca angustifolia, Euphorbia dendroides, Pistacia lentiscus, Juniperus turbinata and Lycium intricatum) have been used in many sectors all over the intervention areas. Plant establishment was monitored by field surveys carried out on June 2007, March 2009 and October 2010, when all individuals were checked. The survey concerned the erosion channels subject to eight different bioengineering interventions and planting (within areas A to G) and two areas (H and I) for an overall surface of about 2210 m2 (about 18% of the entire area involved in the intervention) where 332 specimens had been planted without any bioengineering (Fig. 2).The change of both floristic composition and percentage cover in areas affected and unaffected by interventions was surveyed through repeated phytosociological Relevées ([8]) in October 2006, April 2007, April 2008, April 2009. These where carried out on an area not subject to erosion adjacent to the areas of intervention (plots 1-3 NSE), on planted areas H and I without bioengineering (plots 4-5), on areas subject to bioengineering that were not planted (plots 6-8) and in all the erosion channels (A-G) distinguishing the eight different bioengineering techniques applied (Fig. 2 and Fig. 4). Classification of vascular plants was based on Pignatti ([40]), while their nomenclatural treatment mainly follows Conti et al. ([12]).
Results
Plant survival and establishment
A total of 4883 specimens of 43 different taxa were transplanted to restore the area above SDC (Tab. 1). During the first survey, just one year after plantation (June 2007), 10.5% of the plants were dead (data not shown); the highest mortality was detected for Hyparrhenia hirta, Myrtus communis, Dianthus rupicola subsp. lopadusanus, and Chiliadenus lopadusanus (transplanted as clumps - Tab. 1). Transplantation was generally more successful when plants were first grown in pots in the nursery rather than directly transferred from adjacent field sites (Tab. 1). Our results confirm that seeding is more effective than transplanting in particular for Periploca angustifolia ([17]). At the second (March 2009) and third survey (October 2010 - Tab. 1), the number of additional dead plants was nearly zero.
Either endemic (e.g., Dianthus rupicola subsp. lopadusanus, Pancratium linosae and Suaeda pelagica) or critically endangered species have been successfully propagated (Tab. 1). The most noteworthy success is represented by Jacobaea maritima subsp. Bicolor, locally extinct in the wild since ten years: some 50 plants coming from a branch collected from the last survivor now live in SDC. Thanks to the survival of planted material, it has been possible to increase the local number of individuals representing several other extremely rare species such as Anagyris foetida, Arbutus unedo, Ceratonia siliqua, Coronilla valentina subsp. glauca, Limoniastrum monopetalum, Myrtus communis, Phillyrea latifolia and Rubus ulmifolius. Moreover, ex situ propagation prevented the total extinction of Erica multiflora subsp. multiflora, intermediate sea-lavender Limonium intermedium (Guss.) Brullo and to increase the abundance of Rosemary Rosmarinus officinalis L., whose local population is currently represented by three plants only.
Once planted material survived the stress connected with the initial stages of transplanting, the plants appeared permanently established. Notwithstanding soil shallowness, slope steepness, southern aspect, the influence of salt spray and the very harsh local climate, the remarkably high survival rate was probably due to the prompt and regular cultural practices, especially irrigation. Irrigation is a practice rarely used due to lack of water, but crucial to the successful plantation in arid Mediterranean areas ([52]).
The effect of microbial inoculation with mycorrhizal fungi (and rhizobia on A. foetida) on plant establishment was high in Anagyris foetida and Coronilla valentina subsp. glauca that almost doubled their survival when inoculated, in respect to uninoculated controls (Tab. 2). Inoculation increased also the establishment of Dianthus rupicola subsp. lopadusanus and Atriplex halimus by 25%. Effects of inoculation revealed that Atriplex halimus, Coronilla valentina subsp. glauca and Dianthus rupicola subsp. lopadusanus were the more responsive to microbial inoculation, while Ceratonia siliqua and Anagyris foetida appear indifferent to microbial inoculation. Symbiotic interactions could have established with naturally occurring mycorrhizal fungi (and rhizobia in the case of A. foetida) in the soil, but no attempts (control) were made to confirm this hypothesis.
Tab. 2 - Effect of microbial inoculation on plant height and field establishment. Plants were inoculated in the nursery as described in the “Materials and Methods” section and their height was measured after 9 months of growth (after 23 months for Ceratonia siliqua). Plant establishment in the field was measured after 1 year from transplantation.
| Plant species | Plant height (cm) | Plant establishment (%) | ||
|---|---|---|---|---|
| Inoculated | Not inoculated | Inoculated | Not inoculated | |
| Anagyris foetida | 28.6 ± 1.1 | 28.6 ± 1.7 | 68 | 33 |
| Atriplex halimus | 20.0 ± 1.4 | 4.2 ± 0.5 | 82 | 61 |
| Coronilla valentina subsp. glauca | 12.1 ± 0.3 | 7.9 ± 0.9 | 58 | 27 |
| Ceratonia siliqua | 5.2 ± 0.2 | 4.8 ± 0.2 | 100 | 100 |
| Dianthus rupicola subsp. lopadusanus | 17.1 ± 0.4 | 12.1 ± 0.5 | 95 | 72 |
In the first days after planting, dozens of plants, including members of species known to be unattractive to rabbits, were bitten and their roots were in some cases undermined and damaged. Giving the name of SDC, it was not surprising that wild rabbits (Oryctolagus cuniculus L.) represented a serious threat to young plants. The garlic spray seemed to repel the rabbits only for 3 days; however, once plastic net protections were applied, the damaged plants rapidly recovered.
Effect of different bioengineering techniques on the establishment rate of some key species
The success of planting efforts was greatly affected by the applied bioengineering techniques and was strictly related to the ecology of species (Fig. 4B to E - Tab. 3). As shown in Tab. 3, survival was high for all species planted in areas not subject to biongineering techniques (PO) with the exception of Lycium intricatum whose survival was lower than 50%. Lycium intricatum is a species of dryland areas and poor soils ([51]), and is utilized in restoration of arid soils ([36]). Similarly the survival of Periploca angustifolia, a species of dryland areas ([30]), is decreased on sites where bioengineering was applied with addition of vegetative pockets and biomat. Euphorbia dendroides was not positively affected by interventions, probably because adapted to rocky and steep slopes rather than stony soils ([16], [9]).
Tab. 3 - Establishment rate of some key species. (a): areas where no environmental engineering was carried out.
| Bioengineering techniques | Code |
Periploca
angustifolia |
Euphorbia
dendroides |
Pistacia
lentiscus |
Juniperus
turbinata |
Lycium
intricatum |
Dianthus rupicola subsp. lopadusanus |
|---|---|---|---|---|---|---|---|
| Planting only (a) | PO | 88.85 | 90 | 88.55 | 96.65 | 48 | 84.6 |
| Fences plus stones and soil | FeStSo | 95.9 | 75 | 83.9 | - | 0 | 100 |
| Fences plus stones, soil, and biomat | FeStSoB | 80 | 0 | 87.5 | 50 | - | 66.7 |
| Fences plus soil | FeSo | 100 | - | 90.9 | 90 | - | 50 |
| Fences plus sandy soil | FeSSo | 100 | - | - | - | 30.8 | - |
| Fences and vegetative pockets on the sand | FeVp(sand) | 0 | - | - | - | 50 | - |
| Fences, stones, and vegetative pockets (slope) | FeStVp(slope) | 87.5 | 58.3 | 100 | 100 | 33.3 | - |
| Vegetative pockets and biomat | VpB | 36.4 | 35.7 | - | 100 | 0 | - |
| Vegetative pockets, biomat, and stones | VpBSt | - | 40 | 100 | - | - | 100 |
Dianthus rupicola subsp. lopadusanus is favoured by stones, on the contrary Juniperus turbinata reduces its survival only on stony substrates. Finally, Pistacia lentiscus shows an high survival in all conditions and confirms the excellent role played on intervention of restoration in Mediterranean countries ([32], [14])
Assemblage dynamics
As for the control areas bordering the areas of intervention, not affected by erosion and neither planted nor engineered (NSE - Fig. 2), percentage vegetation cover was relatively constant (plots 1-2) or increased (>10%, plot 3 - Tab. 4). The annual variation in vegetation cover in plots 1-3 was small but noticeable, as expected for ephemeral prairies under xeric local climate. Plots 4-5 (planted only, PO) registered the highest cover increase underlining the role played by planting. As expected, in areas subject to bioengineering only (BO, plots 6-8) the cover is constant or slightly decreasing in the last survey. Bioengineering without planting (plots 6-8) or lack of any intervention (plots 1-3) as in control areas, makes the process of vegetation recovery likely slow.
Tab. 4 - Changes in vegetation cover in sample plots and test areas before intervention (2006) and after intervention (2007 and 2009) according to phytosociological surveys. (NSE): not subject to erosion and not planted; (PO): planted only, i.e., without bioengineering; (BO): bioengineering only, i.e., without planting.
| Treatments | Plots | Total cover (%) | ||
|---|---|---|---|---|
| 2006 | 2007 | 2009 | ||
| NSE | 1 | 45 | 40 | 40 |
| 2 | 45 | 50 | 50 | |
| 3 | 65 | 90 | 100 | |
| Average | 51.7 | 60.0 | 63.3 | |
| PO | 4 | 80 | 95 | 80 |
| 5 | 50 | 80 | 100 | |
| Average | 65.0 | 87.5 | 90.0 | |
| BO | 6 | 50 | 35 | 35 |
| 7 | 80 | 90 | 65 | |
| 8 | 60 | 65 | 60 | |
| Average | 63.3 | 63.3 | 53.3 | |
In areas subject to bioengineering using fences, soil, biomat and planting, the recovery of native vegetation was substantially accelerated (Tab. 5), as demonstrated by the increase (average 45%) of the vegetation cover. A low increase in the cover of vegetation in areas with jute nets is likely due to the particularly harsh conditions caused by outcropping rock.
Tab. 5 - Changes in vegetation cover in the erosion channel (%) before intervention (2006) and after intervention (2007 and 2009) according to phytosociological surveys. The abbreviations of the Bioengineering techniques are reported in Tab. 3. Only bioengineering interventions repeated at least in two different sites are reported.
| Yrs | Bioengineering interventions | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FeSo | FeSSo | FeStSo | VpB | Jute nets | |||||||||||
| A | B | C | A | B | C | D | E | F | D | E | D | E | J1 | J2 | |
| 2006 | 15 | 55 | 45 | 15 | 55 | 45 | 10 | 5 | 3 | 10 | 5 | 10 | 5 | 5 | 7 |
| 2007 | 65 | 45 | 35 | 15 | 15 | 15 | 65 | 20 | 15 | 55 | 30 | 20 | 10 | 10 | 12 |
| 2009 | 90 | 70 | 84 | 70 | 50 | 55 | 95 | 50 | 20 | 100 | 95 | 75 | 30 | 25 | 25 |
In the other bioengineering interventions [FeStSoB,FeVp(sand), FeStVp(slope) and VpBSt], the diffuse unevenness of the area did not allow to establish any replications. However the observed vegetation cover changes between 2006 and 2009 were positive (from 3 to 55 for FeStSoB, from 15 to 70 for FeStVp(slope) and from 3 to 5 for VpBSt) . The only exception was detected in the FeVp(sand) treatment where a cover of 15 for the whole period of observations was recorded (data not shown).
The diachronic analysis of phytosociological relevés (data not shown), carried out during the 2 years following planting of the degraded area above SDC, clearly indicated that coverage increase was more sensitive among perennial species (chamaephytes and hemicryptophytes), performing better than annual species: this was the case of Pine spurge Euphorbia pinea, Mallow bindweed Convolvulus althaeoides L., Grey bird’s foot trefoil Lotus cytisoides L., and Seedhead Reichardia picroides (L.) Roth. Substantial growth was also evident for some therophytes typical of ephemeral prairies such as Mediterranean needle grass Stipa capensis Thunb., Purple false brome Trachynia distachyos (L.) Link and Prickly caterpillar Scorpiurus muricatus L., although species forming ruderal and nitrophilous assemblages were by far the most abundant; these included Purple viper’s bugloss Echium plantagineum L., Cress rocket Carrichtera annua (L.) DC., Sow thistle Sonchus oleraceus L., and the allochthonous Sorrel Oxalis pes-caprae L. The largest cover increase of species typical to more mature successional stages (i.e., grasses and woody species) occurred in the areas where biomats were used.
The regular visual monitoring activities (e.g., no stone rolled from the slope to the beach and no clay accumulation has been recorded after interventions) suggest that erosion is decreasing. Reduction of soil erosion processes is also supported by photographs (Fig. 3) clearly showing the aesthetical improvement of one of the most attractive beaches in Italy.
Discussion and conclusions
We report on a successful case-study of restoration concerning Mediterranean coastal environments that combined bioengineering, biotechnology, and agronomic practices using autochtonous herbs and shrubs. The work, implemented at Lampedusa, is one of the few carried out on Italian islands and appears to be consistent with the guidelines concerning the ecological restoration and biological conservation quoted in the briefing notes released by the Society for Ecological Restoration ([46]).
As erosion and plant diversity conservation problems are quite common along Mediterranean coasts, the strategies and approaches described in this report may be used for similar interventions in the neighbouring Mediterranean countries.
Both planting of native species and the construction of fences slowed surface water flow and soil erosion, thus enhancing plant survival and establishment. Moreover, it is not surprising that recent data on sea turtle nesting show a sensitive increase of oviposition frequency ([43]). The promising results obtained so far encourage the protection of areas that are vital for preserving biodiversity and the implementation of restoration efforts in similar environments along the Mediterranean coast.
Up to now, most of bioengineering installations carried out in Italy involved the use of heavy equipment and the movement of large amount of soil. The current study shows vice versa that restoration can be cost-effective also with an input of human work alone ([2]) by transferring to coastal habitats the same bioengineering techniques which have been proved since decades to be effective elsewhere, mostly on mountain environments ([47]).
The restoration experience carried out at SDC suggests that the use of local germplasm for restoration purposes may also succeed to stopping genetic erosion processes going on at local scales and menacing species persistence in front of biotic or abiotic environmental changes ([20]). Biodiversity loss pattern at Lampedusa shows many common features with other insular territories ([23], [15]). Thanks to this restoration project, plant diversity at Lampedusa Island has been preserved, and the individuals of rare species established on the restored site can now serve as source material for the restoration at other sites. Results are encouraging especially for the locally rare species Arbutus unedo, Myrtus communis, Anagyris foetida. As for other recent restoration interventions ([24]), they also underline the crucial importance of developing nursery farming to enhance the production chain of plants obtained by autochthonous germplasm, thus avoiding genetic pollution. Furthermore, the project clearly shows that combining restoration ecology with in situ instead of ex situ conservation may overcome many problems underlined by Piotto et al. ([41]).
The current study also increased our understanding of restoration practices to be applied within the Mediterranean realm ([50]) and confirms that a better knowledge of plant auto-ecology is essential for successful interventions ([5]).
Some plant species also benefited of microbial inoculation that improved their growth and establishment; microsymbiont inoculation confirmed to be a promising strategy for the recovery of Mediterranean soils, being not more expensive than soil amending or the replacement of dead plants. Management of indigenous plant-microbe symbioses aids restoration of desertified ecosystems and disturbed/anthropogenic soils ([44], [10]). According to field surveys, re-vegetation was successful in that native plant cover has been substantially increased and because soil erosion is now controlled. We expect that plant community should gradually evolve to include sub-shrubs typical to local garrigue communities.
The present report suggests also once again that the enhancement of plant succession does not require the use of “pioneer” species such as Aleppo pine. In fact, at Lampedusa, as in many other Mediterranean areas ([31], [4], [19]), dense artificial plantations with Pinus halepensis Mill. have caused substantial losses of species and habitat diversity ([39]). On the other hand, Aleppo pine was once present on the island as a native plant. Future plantations using local remnant germplasm should therefore be encouraged to get natural-like open woodlands and joining the advantage of tree coverage without a severe damage to local natural heritage.
The activity carried out at Lampedusa is the result of a fruitful interaction between academic research and local environmental management. This is considered a basic element supporting the restoration process of Mediterranean areas ([22]). Public has been informed about the need and the aims of the restoration effort and its success has a positive effect on people, in total agreement with the so-called “socially robust restoration strategies” ([21]). The project also allowed to control and minimize the human impact caused by people visiting the beach. By means of boards and other educational material, the public is now informed about the importance of protecting sea turtle breeding sites, plants and habitats. Bathing is regulated to minimize environmental disturbance, while still allowing visitors to enjoy one of the most beautiful beaches of Italy.
Acknowledgements
The research, partially supported by EU- Project Life03 NAT/IT7000163 “Reduction of human impact on Caretta caretta and Tursiops truncatus and their conservation in Sicily”, was promoted by the Province of Agrigento in collaboration with AGCI Pesca, CTS, Legambiente Sicilia, Telespazio, and the University of Turin. The research was partially supported by the project “Analisi dei sistemi seminaturali e degli agro-ecosistemi nei sistemi insulari mediterranei: Isola di Lampedusa e pantani di Vendicari” funded by the Regione Siciliana, Assessorato Risorse Agricole e Alimentari - Dipartimento Interventi Infrastrutturali, Area Studi e Programmazione; on this purpose we especially thank A. Drago, F. Guaitoli and G. Matranga. We are very grateful to S.A.VI.F. Vivai Piante s.r.l. (Agricultural Society and Forestry Nursery) of Caltanissetta which cared plants’ propagation and to B. Jaffee for revising the English version of the manuscript. We also thank two anonymous reviewers for their valuable comments to the manuscript and their constructive suggestions.
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Online | Gscholar
Gscholar
Gscholar
Gscholar
Online | Gscholar
Online | Gscholar
Gscholar
Gscholar
Gscholar
Online | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
M Del Signore
M Leanza
S Pasta
Dipartimento DEMETRA, Università di Palermo, v.le delle Scienze, Ed. 4, Ingr H, I-90128 Palermo (Italy)
V Billeci
A Dimarca
S Livreri Console
G Maraventano
G Nicolini
E Prazzi
F Sanguedolce
G Sorrentino
Legambiente Sicilia - Ente Gestore della Riserva Naturale “Isola di Lampedusa”, v. Tripoli 3, I-90138 Palermo (Italy)
Dipartimento di Scienze e Tecnologie Molecolari e Biomolecolari, Università di Palermo, v.le delle Scienze, Ed. 16 - I-90128 Palermo (Italy)
Corresponding author
Paper Info
Citation
La Mantia T, Messana G, Billeci V, Dimarca A, Del Signore M, Leanza M, Livreri Console S, Maraventano G, Nicolini G, Prazzi E, Quatrini P, Sanguedolce F, Sorrentino G, Pasta S (2012). Combining bioengineering and plant conservation on a Mediterranean islet. iForest 5: 296-305. - doi: 10.3832/ifor0638-005
Academic Editor
Roberto Tognetti
Paper history
Received: Nov 03, 2012
Accepted: Nov 05, 2012
First online: Dec 17, 2012
Publication Date: Dec 28, 2012
Publication Time: 1.40 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2012
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 60623
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 50992
Abstract Page Views: 3370
PDF Downloads: 4387
Citation/Reference Downloads: 20
XML Downloads: 1854
Web Metrics
Days since publication: 4815
Overall contacts: 60623
Avg. contacts per week: 88.13
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2012): 5
Average cites per year: 0.36
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Implications of ecotourism development in protected areas: a study from Rema-Kalenga Wildlife Sanctuary, Bangladesh
vol. 3, pp. 23-29 (online: 22 January 2010)
Research Articles
Reforestation and land use change in a drainage basin of southern Italy
vol. 6, pp. 175-182 (online: 08 May 2013)
Research Articles
Evaluation of urban forest landscape health: a case study of the Nanguo Peach Garden, China
vol. 13, pp. 175-184 (online: 02 May 2020)
Research Articles
How do urban dwellers react to potential landscape changes in recreation areas? A case study with particular focus on the introduction of dendromass in the Hamburg Metropolitan Region
vol. 7, pp. 423-433 (online: 19 May 2014)
Research Articles
Endangered and endemic species increase forest conservation values of species diversity based on the Shannon-Wiener index
vol. 9, pp. 469-474 (online: 02 January 2016)
Research Articles
Modeling the risk of illegal forest activity and its distribution in the southeastern region of the Sierra Madre Mountain Range, Philippines
vol. 15, pp. 63-70 (online: 21 February 2022)
Editorials
Adaptation of forest landscape to environmental changes
vol. 2, pp. 127 (online: 30 July 2009)
Research Articles
Communicating spatial planning decisions at the landscape and farm level with landscape visualization
vol. 7, pp. 434-442 (online: 19 May 2014)
Research Articles
Monitoring spatial and temporal pattern of Paneveggio forest (northern Italy) from 1859 to 2006
vol. 3, pp. 72-80 (online: 17 May 2010)
Short Communications
The Polish landscape changing due to forest policy and forest management
vol. 2, pp. 140-142 (online: 30 July 2009)
iForest Database Search
Google Scholar Search
Citing Articles
Search By Author
- T La Mantia
- G Messana
- V Billeci
- A Dimarca
- M Del Signore
- M Leanza
- S Livreri Console
- G Maraventano
- G Nicolini
- E Prazzi
- P Quatrini
- F Sanguedolce
- G Sorrentino
- S Pasta
Search By Keywords