The spread of the non-native pine tortoise scale Toumeyella parvicornis (Hemiptera: Coccidae) in Europe: a major threat to Pinus pinea in Southern Italy
iForest - Biogeosciences and Forestry, Volume 11, Issue 5, Pages 628-634 (2018)
doi: https://doi.org/10.3832/ifor2864-011
Published: Oct 04, 2018 - Copyright © 2018 SISEF
Research Articles
Abstract
Invasive pests are considered a major threat to biodiversity, conservation and agriculture. The Italian peninsula is a major site of intensive commercial exchange and transport of plants and goods, being consequently one of the European countries most invaded by alien insects. Hemiptera Coccomorpha are the largest group of non-native species recorded in Europe. For example, in the last 70 years more than 50 scale insect species have been accidentally introduced into Italy, 50% of which are now well established. This study was conducted to investigate the biology and the damage of the non-native pine tortoise scale Toumeyella parvicornis Cockerell (Hemiptera: Coccidae) accidentally introduced a few years ago into southern Italy. T. parvicornis is multivoltine in the invaded territories, being able to complete at least three generations per year, overwintering in the adult female stage. Oviposition periods during 2015-2017 surveys occurred from late April to end of May, from July to first half of August, and from mid-September to November. Fecundity was positively correlated to body size of gravid females and varied among the generations. Investigations on natural control by autochthonous species showed a seasonal activity of Metaphycus flavus (Hymenoptera: Encyrtidae), parasitizing mainly immature male individuals. The morpho-molecular approach confirms the hypothesis of an ongoing shift of parasitoid populations from other indigenous soft scales to the invasive one. Unfortunately, the low level of natural control was ineffective in hampering the spread of T. parvicornis, and preventing the dieback of local pine species, Pinus pinea, as observed in all invaded areas.
Keywords
Invasive Pest, Europe, Toumeyella parvicornis, Life History, Pinus pinea, Natural Control
Introduction
The Italian peninsula, in the centre of the Mediterranean basin, is a major site of commercial exchange and transport of plants and goods. Therefore, Italy is one of the European countries most invaded by alien insect species ([39], [40]). The accidental introduction of exotic insect species has become quite a common event given the extreme difficulty in controlling incoming plant material at the borders. A constant linear trend of accidental introduction was found in the Italian peninsula since 1945 ([37], [22]) due to the wide range of climatic conditions allowing the acclimation of tropical and subtropical species in most of its regions. This scenario is further exacerbated by global warming directly enhancing the survival of several potential pests and indirectly affecting the trophic relationships between the phytophagous insects ([1]), their host plants and their natural antagonists ([23]). Finally, the altered conditions of urban zones, characterized by severe air pollution, warming, and disruption of biological control, favour the rapid adaptation of invasive species to new areas ([31], [10]). Invasive pests are considered a major threat to biodiversity, conservation and agriculture ([11]) and, among these, scale insects (Hemiptera: Coccomorpha) form the largest group of alien insects arrived in Europe in the last 70 years, with more than 50 species recorded for Italy, of which 50% are now well established ([30]).
The genus Toumeyella Cockerell (Hemiptera: Coccidae: Myzolecaninae) currently includes 18 species mostly distributed in the Nearctic and Neotropical Regions ([26], [13]). Among these, 4 species are associated with conifers belonging to the genus Pinus in the Nearctic region: T. parvicornis Cockerell, the pine tortoise scale (PTS); T. pini King, the striped pine scale; T. pinicola Ferris, the irregular pine scale; and T. virginiana Williams and Kosztarab, the Virginia pine scale ([48]).
T. parvicornis was originally described from Florida (USA) on Pinus palustris and P. taeda. The species is highly polyphagous, being recorded on 20 species of Pinus, among which P. banksiana and P. sylvestris are considered as preferred hosts ([43], [28], [6]). The morphology of the different instars of T. parvicornis has been described in details ([49], [32], [43]) with two different morphs known, one living along pine twig axes (twig or bark form) and one on pine needles (leaf form - [26], [6]). Climatic conditions and host plant species both influence the presence of either form along latitudinal gradients. For example, the leaf form is dominant in the southern part of United States on P. caribaea, P. elliotti, P. palustris, and P. taeda, whilst the twig or bark form occur in the northern parts of its distribution area across the Canada-USA border ([6]). Phenology and life history of PTS in the native areas of Canada and United States have been investigated without continuity due to the occasional pest status reached mainly in young pine plantation, seed orchards and Christmas tree farms ([36], [38], [27], [6]). The scale was reported to be univoltine in Canada and across Northern USA ([27], [9]), bivoltine in Virginia ([49]) and multivoltine in Georgia and Florida ([6]). Large populations of the pest can produce abundant honeydew, reduce tree growth, cause decline and dieback of susceptible host species.
PTS achieved the status of invasive species only in the new millennium when it was initially recorded in Puerto Rico ([42]), and in Turks and Caicos a couple of years later ([28]). In a short time, PTS invaded the pine forests on the Turks and Caicos Islands killing more than 90% endemic Caicos pine trees ([28], [29]). In 2014, PTS was found scattered over a large area in Campania (Southern Italy), particularly in urban zones ([14]) where pine trees represent a distinctive element of the Mediterranean landscape.
The aims of the present study were to: (1) report the distribution of PTS in southern Italy; (2) characterize its biology and particularly its fecundity; (3) assess host plant susceptibility; and (4) characterize autochthonous antagonists.
Materials and methods
Life history of PTS
Scale colonies were sampled weekly during 2015-2017 in: (i) one urban zone in Naples, located at Mostra d’Oltremare Park (40° 49′ 37″ N, 14° 11′ 13″ E - 48 m a.s.l.) hosting more than 300 pines belonging to the Italian stone pine Pinus pinea; (ii) two pinewoods located in the Vesuvio National Park, the municipalities of Ercolano (40° 49′ 19″ N, 14° 23′ 02″ E - 384 m a.s.l.) and Torre del Greco (40° 47′ 32″ N, 14° 25′ 22″ E - 295 m a.s.l.). In each sampling site twenty twig terminals (10 cm) were collected randomly on different trees. Sampled material was examined with a stereomicroscope in the laboratory at the Department of Agricultural Sciences, University of Naples in Portici, Italy to assess the number of scale insects settled on the bark of a twig and on 10 couples of pine needles (selected at random from each twig). The average densities of crawlers, immatures (female and male) and adults (females and emerged males) were recorded.
Fecundity of females was determined during 2016-2017 by collecting and dissecting 50 gravid females at the beginning of the reproductive period of each generation as determined by the first eggs or crawlers found on ventral surface of female adults. Climatic data (temperature, relative humidity, rainfall) were provided by the regional weather database.
Host plants
The invaded territory consisted mainly of reforested areas, isolated plants or groups used as ornamental trees in urban zones and along Mediterranean coastal landscape. A list of Pinus spp. infested by PTS was completed. To assess host plant preference by PTS, 6 three-year-old saplings (height 60 cm) of P. halepensis, P. nigra, P. nigra var. laricio, P. pinea, P. pinaster, and P. sylvestris were reared in pots at laboratory conditions (temperature, T = 25 °C; relative humidity, RH = 60-70%) from July 2016 to March 2017 to complete three generations of the pest. Each sapling was artificially infested with crawlers emerging from 20 PTS gravid females chosen from field collected samples. The survival rate on the different pine species was assessed after 8 months.
Natural control
PTS samples showing signs of parasitization were isolated in glass vials plugged with cotton wool, kept in the laboratory at room temperature, and checked daily until the emergence of parasitoids. Emerged insects were killed in absolute ethanol and stored at -20 °C until characterization. The activity of one parasitoid species, collected for the first time in September 2015 in the Vesuvio National Park, was followed until July 2017 when a large crown fire destroyed the main monitored forested area. For this species sex-ratio and parasitization rate were calculated. Furthermore, the longevity of the parasitoid was assessed for 20 females and 20 males fed with water/honey solution (50:50) at 25 °C, 65% RH, 18/6 light/dark photoperiod. Finally, after characterization, the origin of parasitoid’s population was assessed by comparing the specimens collected on PTS with those reared from other soft scale hosts distributed on different plant species in the sampled areas.
Parasitoid characterization
A combined approach was used for the correct identification of the parasitoid collected and to understand the origin of its populations.
Molecular analysis
Before card mounting, whole specimens were subjected to total DNA extraction following a non-destructive protocol using a Chelex® 100 Resin (Biorad, Hercules, CA, USA) according to Cascone et al. ([4]). Three individuals were placed into homogenisation solution containing 5 ml of Proteinase K (20 mg ml-1) and 80 ml of 5 % Chelex, incubated at 55 °C for 60 min, then for 10 min at 99 °C and centrifuged for 5 min at 13.000 rpm. We sequenced mitochondrial Cytochrome Oxidase 1 (COI) and the internal transcribed spacer (ITS2) region obtained by PCR performed in 15 μl volumes of distilled water containing: 1X GoTaq™ buffer (Promega, Madison, WI, USA) , 2.5mM of each dNTP, 10 μM of both forward and reverse primer, 1 unit of GoTaq™ DNA Polymerase and 1μl of template DNA. COI amplifications were achieved using a Biorad thermocycler Mycycler® (Biorad) programmed for: 1 min at 94 °C, followed by 40 cycles of 30 s at 94 °C, 90 s at 48 °C, and 60 s at 72 °C, and a final step of 7 min at 72 °C ([18]). Gene was amplified using the following primer combinations: LCO/HCO ([12]), MlepF1/HymR ([21], [20]) and HCOextb/ C1J2195 ([44], [41]). PCR fragments were sequenced directly in both direction at Macrogen (Korea) using Big-Dye Terminator ver. 3.1. The obtained chromatograms were edited and assembled in Chromas ver. 2.6.4 (Technelysium, South Brisbane, Queensland, Australia). The sequences were deposited in GenBank under accession numbers MG946790-MG946792. Similarly, to amplify and to sequence the ITS2 region, thermocycler was programmed as following: 3 min at 94 °C, followed by 33 cycles of 45 s at 94 °C, 45 s at 53 °C, and 45 s at 72 °C, and a final step of 5 min at 72 °C. ITS2Fw and ITS2revb primer were used ([45]). The sequences were deposited in GenBank under accession numbers MG946793-MG946795.
Morphological analysis
Card and slide mounted material of the parasitoid were prepared following Noyes ([34]). The specimens were compared with a long series of individuals authoritatively identified and deposited at the Natural History Museum, London (UK).
Statistical analysis
The relationship between the fecundity and the combined body size of the females and egg load was determined through Pearson’s correlation and linear regression. Analysis of variance (ANOVAs) was used to determine differences in both adult female size and the chorionated egg load in four subsequent generations during 2016-2017. Differences in treatment means were tested using post-hoc multiple comparisons Tukey HSD test at α=0.05. All data satisfying conditions of normality and homoscedasticity, both untransformed or after appropriate transformation, were analyzed by the software Statgraphics Plus® (Statgraphics Technologies Inc., The Plains, VA, USA).
Results
Life history of PTS
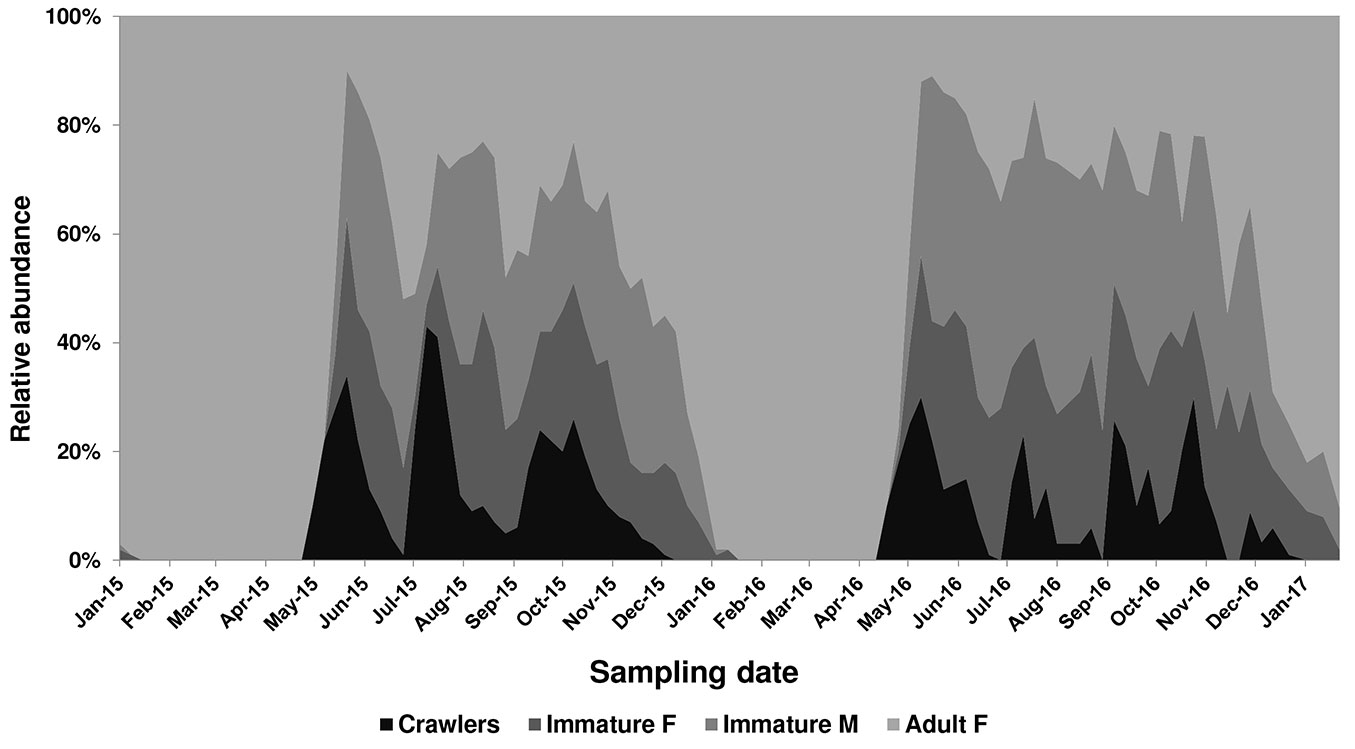
PTS distribution and life history in 2015-2016 are summarized in Fig. 1 and Fig. 2. Unless otherwise specified, life history refers to scale population sampled in the urban zone.
Fig. 2 - Average abundance of Toumeyella parvicornis life stages on Pinus pinea in Italy (Campania) from 2015 to 2017.
In Campania PTS completed at least three generations, partially overlapping, on Italian stone pine P. pinea (Fig. 2) with little differences observed among sampled sites. An early emergence (5-7 days) was recorded in 2015-2016 on urban pines in Naples in respect to pine forests on Mt. Vesuvius. The minimum time gap between two subsequent ovipositions (May-July and July-September) was on average 65 ± 3.46 days (n=200). Adult females of the third generation were recorded from the second half of November to the end of December, 73 ± 5.61 days (n=200) after the first egg hatch. The species overwinters as fecundated females mainly on twigs (bark form: 92.15%, n=26.370) and rarely on pine leaves (leaf form: 7.85%, n=207). In the following generations, the bark form represented 100% of adult females, mainly due to the high mortality of immature females settled on pine leaves during summer and autumn. Conversely, male instars were scattered on both pine leaves and twig axes, where they usually formed clusters around female stages.
The species is clearly ovoviviparous: females laid 20-25 eggs daily and hatching of the crawlers occurred in a few hours. Oviposition period lasted 35-40 days. Eggs of the first generation of the year were recorded from the second half of April to end of May. The following oviposition periods occurred from July to early August and from mid-September to November. First crawlers were recorded on May 5th in 2015 and April 24th in 2016. Mild winter and early spring temperatures during 2015-2016 led to an earlier emergence of crawlers (=12 days) in 2016 in respect to 2015.
After eclosion, the crawlers dispersed on pine shoots and settled along twig terminals or on leaves nearby. The peak density of first instars varied from year to year. In 2015 three clear peaks were recorded in mid-May, mid-July and from end of September to mid-October (Fig. 2). Conversely, during 2016 first instar presence was recorded over a longer period due to longer overlapping of subsequent generations. Sexual dimorphism became evident during the third week after the settlement of the crawlers, when immature females and males can be readily separated by their shape, oval and convex in the former, much more elongate in the latter. Adult male flight peaks were clearly distinguishable in 2015, but not in 2016 when a larger overlapping of subsequent generations occurred. Males of the first generation appeared in June 15th in 2015 and a week earlier in 2016, with flight curves peaking in the first week of July and in the last decade of June, respectively. Adult male flight peaks of the following summer and autumn generations were recorded in the second half of August and beginning of November during 2015 and in mid-August and end of October in 2016. Huge amounts of honeydew were produced during feeding activity of late instars.
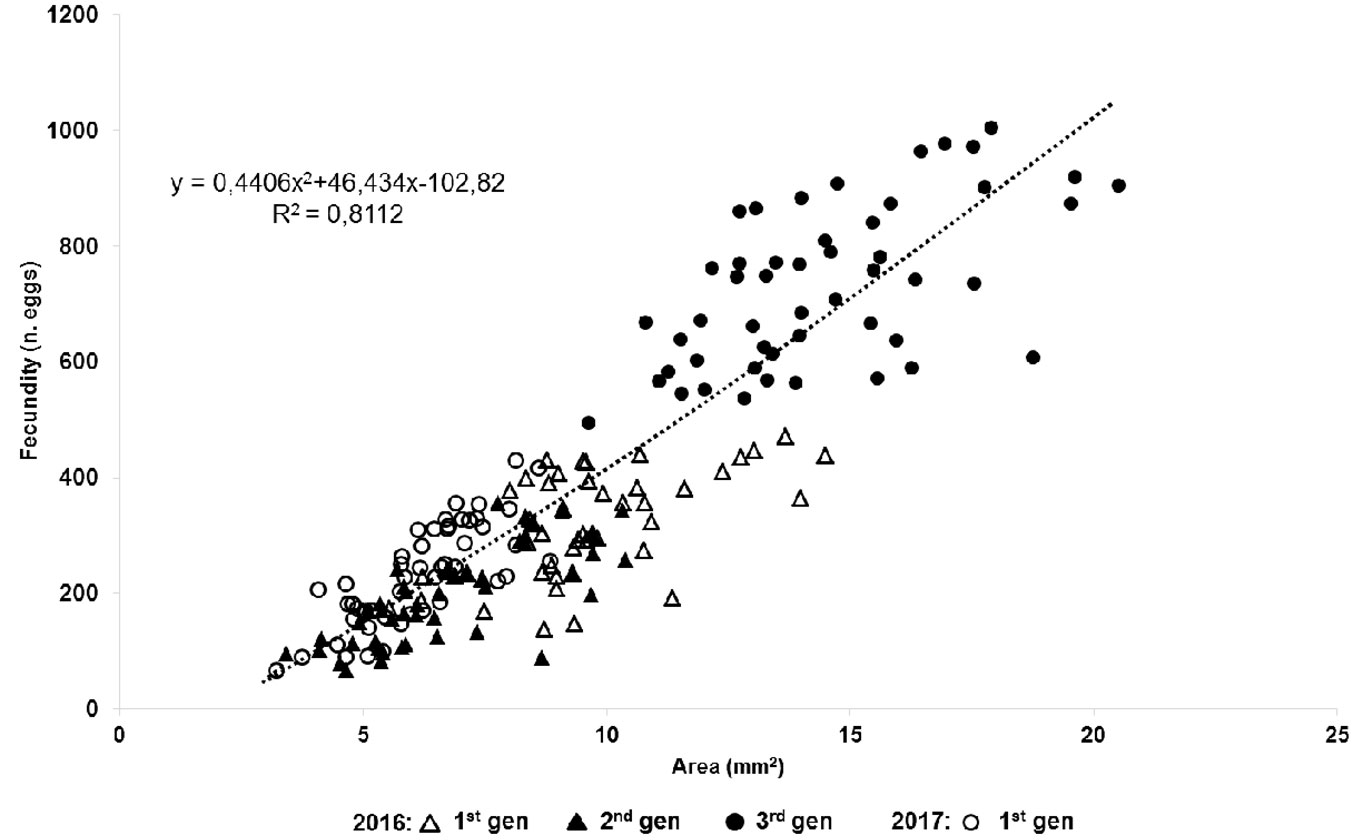
After mating, the female’s body greatly increased in size with the last molt, ovary development and reproduction. The females began to produce eggs in their ovaries 5-7 days after reaching the adult stage. Adult female densities on P. pinea reached 10 females cm-1 of twig in the overwintering population (average density: 5.51 ± 2.17 females cm-1, n=100 twigs). We determined that the fecundity of PTS (Fig. 3) correlated significantly to female body size (p<0.05). Pearson’s correlation coefficient varied from 0.59 (overwintering generation 2016-2017) to 0.78 (spring generation 2017). Linear regression analysis corroborated the results of Pearson’s correlation analysis.
Fig. 3 - Linear correlation between female size and fecundity of four consecutive generations of the Italian population of Toumeyella parvicornis for the years 2016-2017.
The number of eggs laid per female varied among the generations and ranged between 198.82 ± 11.61 for the summer generation 2016 and 730.36 ± 19.51 for the overwintering generation 2016-2017. The highest recorded fecundity was 1014 eggs female-1 and the lowest 90 eggs female-1. The average number of eggs laid by female of the sampled generations differed significantly (p< 0.01).
Host plants
In the invaded area, P. pinea resulted the most distributed species, followed by P. pinaster, P. halepensis and P. nigra. Few other pine species used as ornamental trees have been recorded. The most susceptible host plant resulted P. pinea followed by P. pinaster and P. nigra. No signs of PTS infestation were found on a dozen P. halepensis and a few P. roxburghii trees. To date, strong decline and dieback in field was recorded only for the P. pinea, due to the impressive populations of PTS hosted on a single tree.
These results were confirmed by laboratory tests on P. pinea where all saplings died after 8 months and three generation of infestation by PTS. Four pine species reared at the same conditions, namely P. pinaster, P. nigra, P. nigra var. laricio and P. sylvestris, survived and showed lower levels of infestations. Finally, PTS was unable to infest Aleppo pine saplings in laboratory.
Natural control
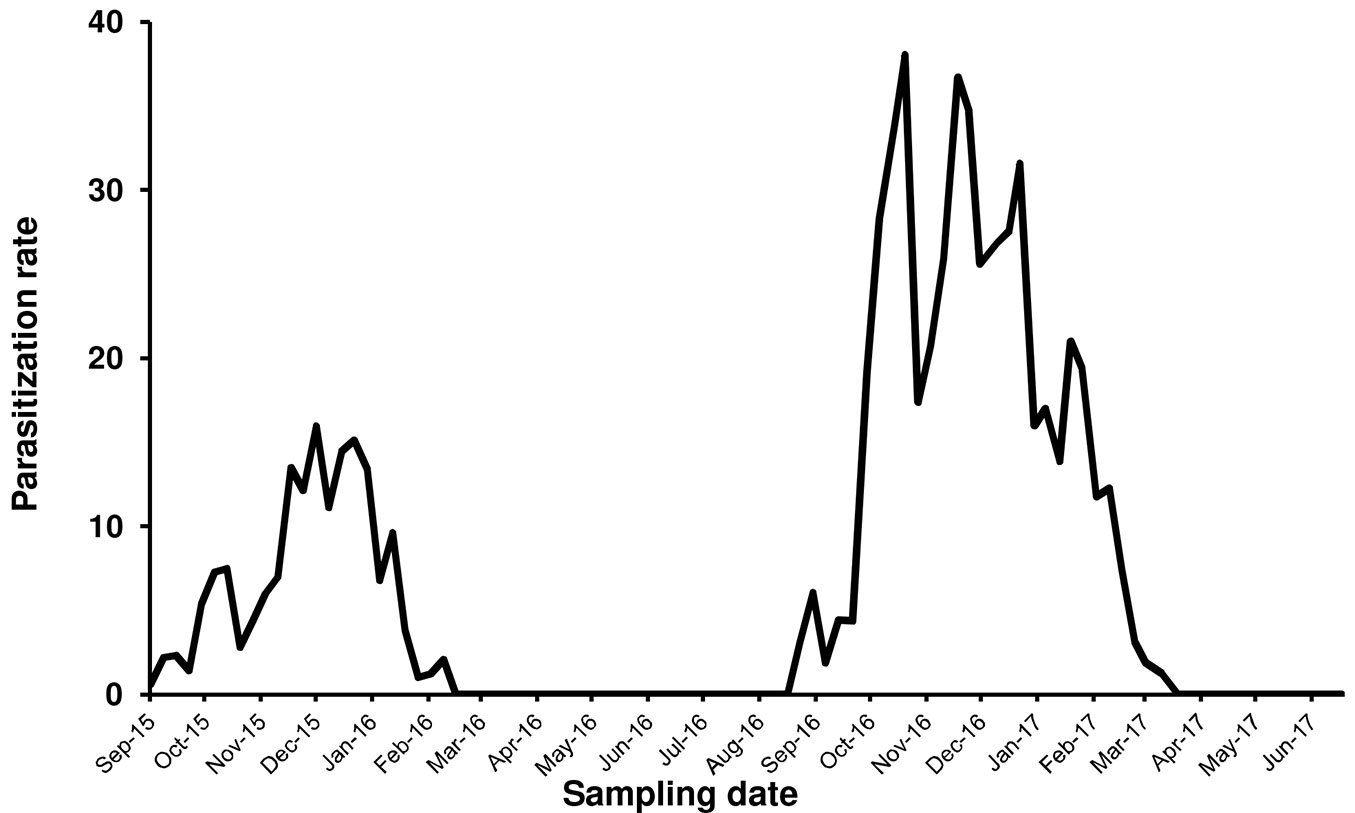
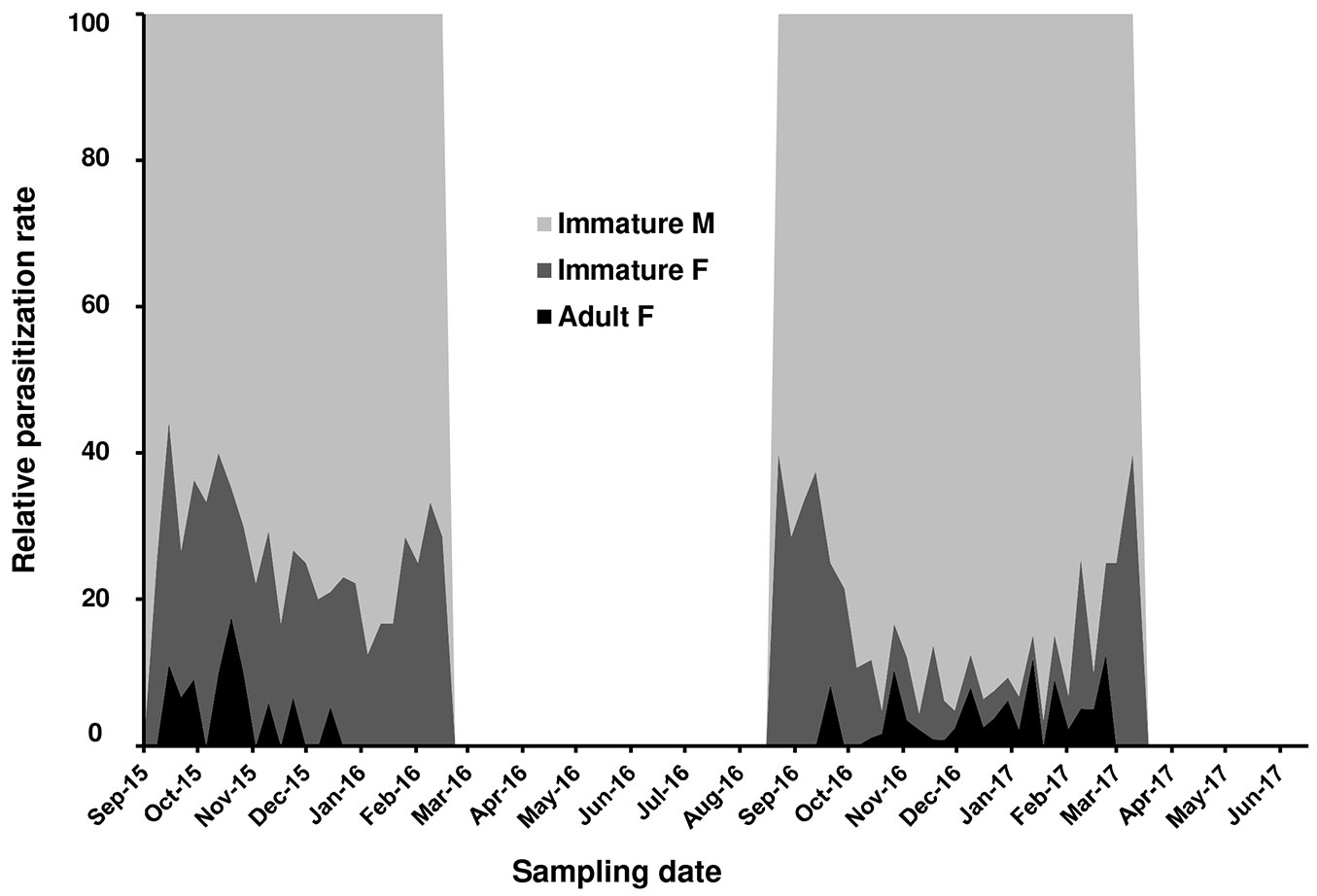
Metaphycus flavus Howard, a polyphagous parasitoid known to attack several scale insects species, emerged from PTS samples collected in the Vesuvio National Park. The first parasitized scale instar was recorded in September 2015. Fig. 4 summarized the parasitization trend during 2015-2017. Parasitization rate peaked from November to January in 2015-2016 (=18%) and 2016-2017 (=38%). Parasitized samples revealed that male instars were preferred over female ones (range: 60-90% - Fig. 5). Parasitizion rates showed a seasonal activity of M. flavus, concentrated during autumn and winter months. Living larval instars of the parasitoid were found in dissected scales until February in 2016 and March in 2017. The samples collected from March to September 2016 and during late spring 2017 showed only a scattered presence of emergence holes.
Fig. 4 - Level of parasitization realized by Metaphycus flavus on Toumeyella parvicornis in Italy (Campania).
Fig. 5 - Composition of the population of Toumeyella parvicornis parasitized by Metaphycus flavus in Italy.
Sex-ratio of the encyrtid parasitoid recorded from October to December 2016 was strongly male-biased (1.0 M : 0.53 F - n=270 M : 141 F). Longevity of emerged M. flavus specimens fed in laboratory with diluted honey reached 12.4 ± 3.2 days for males and 15.3 ± 6.3 for females.
Parasitoid characterization
Molecular analysis confirmed that M. flavus collected on PTS belongs to an autochthonous population attacking different soft scales infesting citrus trees neighboring sampled pine trees. ITS2 sequences of parasitoid specimens from PTS exactly match with those of M. flavus emerged from the brown soft scale, Coccus hesperidum.
Discussion
When colonizing a new area, an invasive pest finds virtually no barriers to its expansion. Plants that are not evolved with the pest in its native area need time to develop a viable form of resistance. Similarly, local antagonists need time to adapt to the new host. These considerations only partially apply to PTS in Campania. Whilst Italian stone pine was highly susceptible to the scale insect, local populations of an autochthonous parasitoid, adapted rapidly to the new pest. PTS is considered a polyphagous species: the ScaleNet database ([13]) reports only 8 of the 20 species listed as PTS hosts in the literature ([43], [28], [6]). P. pinaster and P. nigra var. laricio have been now added to the host list. Few data are available on the susceptibility of the different species and on host plant preference. First data about decline and dieback of a pine species caused by severe PTS infestation were observed by Malumphy et al. ([28]) in Turks and Caicos Islands. In southern Italy the heavy infestations on Italian stone pine are likely to evolve in a similar way, also favored by the local climatic conditions. P. pinea resulted extremely susceptible and unable to bear the impressive scale populations recorded without extreme consequences. In urban zones the damage consisted in the production of sticky honeydew and sooty mould layers covering all surfaces and artefacts under pine crowns. In the worst cases, large populations of the invasive scale lead to decline and dieback of most infested pines. Other than the clear preference for the Italian stone pine, the maritime pine P. pinaster was a valid host plant, showing consistent infestations even though neither decline or dieback were recorded so far. To date, Aleppo pine may be considered a resistant host. Studies of terpene concentration and related resistance mechanisms in threatened pine species should be carried out in the absence of valid control options. For example, the volatile profiles of Pinus caribaea var. bahamensis were reported to be valid indicators of susceptibility towards PTS and predictors of environmental stresses ([15], [16]). The same approach should be considered to characterize the interactions established between PTS and P. pinea in Italy.
Several aspects of PTS life history in Campania confirmed previous investigations carried out in USA and Turks and Caicos Islands ([28], [6]). Similarly, PTS behaviour here recorded is consistent with what was reported for the Southern range of the native area on Nearctic pines ([9], [6], [2]). The noteworthy environmental plasticity of the species is exemplified by the variable number of generations completed per year coupled with the acceptance of new host species ([28]). In Campania, three main periods of oviposition were recorded on P. pinea. Our survey (2015-2017) evidenced the ability of the invasive scale to spread across new territories. From the first official record ([14]), in a few months PTS colonized large areas and undermined a possible eradication program. To date over 2000 km2 are interested by heavy infestations favored by the large distribution of the highly susceptible P. pinea. The widespread presence of aged stone pines regularly planted along highways and other main routes of communication, allowed PTS to complete this rapid invasion. Active and passive dispersal behavior proved to be extremely successful. Crawlers spread from infested trees to adjacent pines where branches intertwine, but can also be carried by wind for longer distances, as already demonstrated in the Caribbean and North America ([38], [29]). Indeed, airborne dispersal of T. parvicornis crawlers has been reported to occur at a distance up to 4.8 km ([38]), rendering virtually impossible the protection of uninfested trees.
Our results confirm that the body size of gravid females of PTS is a valid parameter to predict their fecundity. The significant correlation between body size and fecundity has been reported for PTS by Rabkin & Le Jeune ([38]) in Canada and for soft scales of the genus Parthenolecanium spp. by Camacho et al. ([3]). The fertility values of PTS in Italy are in line with those recorded in North America ([38], [6]). Whilst egg load of univoltine populations of PTS reaches 500 eggs per female ([38]), in Southern Italy (Campania) it varied among generations. The highest values were recorded in the overwintering generation, resulting up to 2-3 times higher than those of spring or summer ones. Apart from body size, fecundity could also depend on different factors including environmental conditions, population density and host plant (Camacho et al. ([3]).
Earlier than expected, it took only a couple of years after PTS introduction in Campania to find the first presence of the autochthonous parasitoid M. flavus attacking its colonies. This species is a common parasitoid of preimaginal stages of soft scales including C. hesperidum, the citricola scale C. pseudomagnoliarum, the black scale Saissetia oleae and is considered cosmopolitan ([17], [35]). The environmental conditions and the habitat composition in the Vesuvian area, with citrus and olive trees together with spontaneous shrubs, resulted particularly favorable to M. flavus. Indeed, this species has been collected regularly in this area since 1970s ([47], [17]). M. flavus is included in the list of the parasitoid complex of PTS in North America, along with a few species of Coccophagus (Aphelinidae) and Microterys fuscicornis Howard (Encyrtidae) ([9], [28], [6]). Unfortunately, few data are available about the level of natural control realized by these parasitoids in the native area of the pest ([36], [6]), as recently confirmed by Myartseva et al. ([33]) in relation to New World parasitoids of Toumeyella scales. The parasitization rate by M. flavus in Campania reached higher values only during the second year of observations, indicating a possible positive trend. The period of parasitoid activity was strictly limited to September-February in both years of observations, in accordance with the presence of susceptible host stages. However, the level of parasitization resulted unable to control the scale due to a clear “sexual” preference ([24]). Indeed, male preimaginal stages were by far preferred over immature and adult females (Fig. 5). Furthermore, the simplification of the host population during winter and the presence of adult females only from the end of December to the beginning of April, did not allow the parasitoid to maintain a continuous relationship with its new host. As a consequence, M. flavus disappeared at the end of each winter season from the sampled areas, both in 2016 and 2017. A new colonization process started again during summer of 2016 with parasitoids coming from other plant-scale systems. Indeed, only 5 months later new signs of parasitization were found on the new generation of PTS. The identification of the parasitoids collected posed the question whether they were introduced with the invasive host or they belonged to local populations that adapted to it. The molecular characterization of M. flavus populations collected from different hosts in the Vesuvian area, confirmed the second hypothesis. The arrival of the invasive pest represented a large reproductive opportunity for the Vesuvian population of M. flavus but considering the biological features of both species, it is likely that a continuous passage from local scale species to PTS will happen at the beginning of the season every year. The strong male-biased sex ratio of M. flavus collected on PTS possibly indicated an incomplete adaptation to the new host and this aspect is worthy to be investigated in depth. Unfortunately, in July 2017 a devastating crown fire destroyed the monitored forested areas making it impossible to complete these observations. The difficulty to implement sustainable control options against PTS makes the absence of efficient natural control even worse.
PTS has the potential to become a key factor for the survival of P. pinea in the newly invaded territories in Campania and in the whole Mediterranean area. Apart from the direct damage caused by the alien scale, the weakened trees may become more susceptible to pine bark beetles including the harmful Tomicus destruens Wollaston, with serious impact for urban, coastal and forest landscapes. Moreover the pest may be positively influenced by the heat island effect, and can thus develop faster or become more active in new urban zones earlier than in rural areas, as reported in previous studies on other soft scales ([31], [3]). It is likely that the impact of PTS on Italian and Mediterranean pine trees will dramatically increase in the next few years, prompting the development of classical biological control programmes based on the selection and introduction of natural enemies from the native area. Adequate risk assessment protocols should then evaluate the possible threats linked to the release of any exotic natural enemy ([7], [46], [5]). Recent studies discussing the rates of success of biological control programmes realized from late 19th century suggest that this could represent the only feasible approach to control PTS in all invaded areas ([8], [19], [25]).
Acknowledgements
Individual contributions: APG coordinated the project; APG and EG developed the research; GJ, ER, SS were the principal investigators for the grant; AF analyzed data; APG, EG and PC identified parasitoid insects; APG, EG and AF wrote the paper. The authors declare that they have no conflict of interest.
This research was partly supported by the Regione Campania, 2015-2016 Plan of Phytosanitary Action, URCOFI project (III/2016 grant n. B25E12000260002). The authors wish to thank Dr. Luigi Zagaria for his helpful assistance at the Mostra d’Oltremare Park in Naples.
References
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Online | Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Alessandro Foscari
Giovanni Jesu
Silvano Somma
Dipartimento di Agraria, Università degli Studi di Napoli “Federico II”, v. Università 100, I-80055 Portici, Napoli (Italy)
Pasquale Cascone
Emilio Guerrieri
CNR, Istituto per la Protezione Sostenibile delle Piante (IPSP), v. Università 133, I-80055, Portici (Italy)
Department of Life Sciences The Natural History Museum, London (UK)
Corresponding author
Paper Info
Citation
Garonna AP, Foscari A, Russo E, Jesu G, Somma S, Cascone P, Guerrieri E (2018). The spread of the non-native pine tortoise scale Toumeyella parvicornis (Hemiptera: Coccidae) in Europe: a major threat to Pinus pinea in Southern Italy. iForest 11: 628-634. - doi: 10.3832/ifor2864-011
Academic Editor
Massimo Faccoli
Paper history
Received: May 24, 2018
Accepted: Jul 13, 2018
First online: Oct 04, 2018
Publication Date: Oct 31, 2018
Publication Time: 2.77 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2018
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 49365
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 40077
Abstract Page Views: 4234
PDF Downloads: 4002
Citation/Reference Downloads: 7
XML Downloads: 1045
Web Metrics
Days since publication: 2677
Overall contacts: 49365
Avg. contacts per week: 129.08
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2018): 18
Average cites per year: 2.25
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Decline in commercial pine nut and kernel yield in Mediterranean stone pine (Pinus pinea L.) in Spain
vol. 13, pp. 251-260 (online: 03 July 2020)
Research Articles
Hemlock woolly adelgid niche models from the invasive eastern North American range with projections to native ranges and future climates
vol. 12, pp. 149-159 (online: 04 March 2019)
Technical Notes
Stem-injection of herbicide for control of Ailanthus altissima (Mill.) Swingle: a practical source of power for drilling holes in stems
vol. 6, pp. 123-126 (online: 05 March 2013)
Research Articles
Stand structure and regeneration of Cedrus libani (A. Rich) in Tannourine Cedar Forest Reserve (Lebanon) affected by cedar web-spinning sawfly (Cephalcia tannourinensis, Hymenoptera: Pamphiliidae).
vol. 11, pp. 300-307 (online: 13 April 2018)
Research Articles
Spread intensity and invasiveness of sycamore maple (Acer pseudoplatanus L.) in Lithuanian forests
vol. 8, pp. 693-699 (online: 19 March 2015)
Research Articles
Distribution and abundance of the alien Xylosandrus germanus and other ambrosia beetles (Coleoptera: Curculionidae, Scolytinae) in different forest stands in central Slovenia
vol. 12, pp. 451-458 (online: 29 September 2019)
Short Communications
Detection and quantification of the air inoculum of Caliciopsis pinea in a plantation of Pinus radiata in Italy
vol. 12, pp. 193-198 (online: 10 April 2019)
Research Articles
Assessing escapes from short rotation plantations of the invasive tree species Robinia pseudoacacia L. in Mediterranean ecosystems: a study in central Italy
vol. 9, pp. 822-828 (online: 25 May 2016)
Research Articles
Soil and forest productivity: a case study from Stone pine (Pinus pinea L.) stands in Calabria (southern Italy)
vol. 4, pp. 25-30 (online: 27 January 2011)
Research Articles
Are Mediterranean forest ecosystems under the threat of invasive species Solanum elaeagnifolium?
vol. 14, pp. 236-241 (online: 10 May 2021)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword