Four decades of forest succession in the oak-dominated forest reserves in Slovakia
iForest - Biogeosciences and Forestry, Volume 7, Issue 5, Pages 324-332 (2014)
doi: https://doi.org/10.3832/ifor0996-007
Published: Apr 17, 2014 - Copyright © 2014 SISEF
Research Articles
Abstract
The study analyzes biodiversity, production and recovery processes in the natural oak-dominated forests in three protected areas in time span of four decades after the cessation of human impact. In each forest reserve, we established three permanent research plots of 0.5 ha, which were monitored regularly every 10 years. The obtained results confirmed an expected decrease of oak in all investigated areas, regardless of the level of past human intervention. Growth rates of oak stem density were negative in all reserves and ranged from -0.7% yr-1 (Kašivárová) to -2.1% yr-1 (Bujanov). A typical rotated-sigmoid diameter structure was confirmed during the whole observed period only in the reserve Boky. The moderate affected reserve (Kašivárová) showed a bimodal pattern and the most affected reserve (Bujanov) revealed the typical unimodal distribution. Considering spatial diversity, the recorded structure of investigated forests closely corresponds with the relative level of past intervention. In Boky, the most differentiated structure was reflected in the highest long-term mean stem density (890 ± 151 ha-1) and the lowest basal area and growing stock (36.5 ± 1.4 m2 ha-1 and 284 ± 22 m3 ha-1, respectively). The past-affected reserves reached higher values of basic stand parameters. In Kašivárová and Bujanov, the average basal area (36.5 and 44 m2 ha-1, respectively) and growing stock (284 and 518 m3 ha-1, respectively) correlated negatively with the level of structural heterogeneity, while the stem number revealed a positive correlation. We observed a continuous increase of deadwood volume and dead to live wood ratio. The recorded long-term volume of deadwood (43-128 m3 ha-1) and deadwood proportion (151-28%) tended to be higher in the past-affected reserves. The convergence towards the rotated-sigmoid distribution in both reserves with past human impact was recorded. Considering the vertical structure, the most dynamic changes were observed in the lower and middle layer. Moreover, the substitution of the light-demanding oak by shade-tolerant species (beech, hornbeam) was recorded. The intensity of substitution directly reflected the intensity of structure modification in the past. Our results suggest that the process of forest recovery and oak loss significantly depends on the level of past human intervention.
Keywords
Oak Decline, Past Human Impact, Recovery, Forest Reserve, Quercus petraea L
Introduction
Well preserved old-growth oak forests are quite rare in Europe and are mostly dominated by pedunculate oak (Quercus robur L. - [23], [24], [47], [33]). Natural mixed sessile oak (Quercus petraea L.) forests, as they are easily accessible and have been intensively used in the past ([22], [24], [47]), have remained untouched only in a very small area. In the western Carpathians, the remnants of old-growth mixed Q. petraea forests occur at intermediate and low altitudes ([3]).
Since the second half of the 20th century, many researchers from almost all European countries have investigated the symptoms and course of oak decline (e.g., [31], [15], [38], [45], [51]). The main goal of these studies was to identify crucial factors and the trigger mechanism of such phenomenon. In most cases, oak dieback was studied in large areas regardless of stand origin. However, in addition to the long-term monitoring, it is necessary to incorporate also the historical background, when the structure and development of recent natural forests are analyzed. Such approach may allow the identification of underlying factors that caused vegetation changes ([21]).
The origin and the future development of oak forests have been widely discussed, leading to several opposing theories. According to the classic high-forest theory ([56], [59], [26], [24], [2]), the oak-dominated climax (equilibrium) structure is the final step of a successional process and represents a stable adaptation to local environmental conditions ([4], [14]). Tree species composition could be temporarily modified by a small-scale canopy disturbance, but the ecosystem will immediately return to the equilibrium by its own self-regulation processes.
On the other hand, the disturbance theory ([11], [58], [32]) attributes the dominance of light-demanding oak to the impact of mid- to large-scale disturbances. It considers the oak-dominated forest to be the mid-stage of a continuous, but constantly ingressive process of forest succession leading to the final dominance of shade-tolerant species.
According to a third group of theories, the recent extent of oak-dominated forests in the temperate zone is most probably the result of the past human exploitation in favor of oak (for charcoal and fuelwood production, cattle pasture) or the impact of large herbivors ([54]).
Evidence from several recent studies support the last mentioned explanation. Based on 50-years monitoring of forest reserves, Brang et al. ([6]) and Rohner et al. ([41]) described a trend towards the reduction of species richness in Switzerland lowland forests. The light-demanding species promoted by former human intervention were increasingly shaded out after the cessation of forest management. Petritan et al. ([34]) provided a complex study of the stand structure of natural mixed Q. petraea forest in Runcu-Grosi (Romania). They conditioned the preservation of recent proportion of oak by the occurrence of either major canopy disturbance or silvicultural intervention. However, Cowell et al. ([12]) pointed at the minor role of canopy gaps in the development of oak-mixed forest remnants in Dobbs Natural Area (Indiana, USA) and outlined the parallel between the current species composition and historical shift in the light regime due to grazing. Similarly, Matuszkiewicz ([30]), Jakubowska-Gabara ([19]) and Hédl et al. ([18]) argue for the abandonment of forest exploitation and pasture as the main driving factor of the secondary succession of thermophilous oak communities towards the mesic forests.
This study focuses on the spatial analysis of natural succession and structural change of oak-dominated forests affected by different intensity of human impacts in the past. In particular, we tried to address the following questions:
- Does the nature and intensity of oak decline differ depending upon the intensity of former human impacts on the stand or is it a common phenomenon of recent times?
- Can the forest naturalness be inferred from the current distribution of tree diameters through its fitting to the rotated sigmoid function? How could a shift in diameter distribution in a time span of four decades reflect the intensity of past interventions?
- Can structural changes be traced in the vertical structure of the investigated forests?
Material and methods
Model protected areas under different exploitation regimes in the past were chosen in the western Carpathians of Slovakia according to the following criteria:
- a minimum area of the forest remnant (protected area) of 50 ha to secure the conditions for self-development (according to [9]);
- a proportion of oak by stand basal area of at least 90% in the first inventory;
- at least three decades covered by forest inventory campaigns;
- historical records describing the exploitation intensity in the past.
Three National Nature Reserves (NNR) met the above criteria:
- The NNR Boky, characterized by the lowest degree of hemeroby (sensu [16]); an old-growth (pristine) forest, with no-known human impact. The site is inaccessible, and no exploitation has been recorded on historical sources.
- The NNR Kašivárová, a natural forest (sensu [44]) located in an area where traces of past human impacts are still detectable (some scattered beech and oak individuals were harvested to produce charcoal).
- The NNR Bujanov, an area significantly affected by human activities, left for self-development. Before the NNR establishment, forest stands had undergone beech harvest for railway ties and potash until 1900 ([17]). The stands were under regular silvicultural management until the establishment of the protected area in 1964.
The three reserves have been strictly protected since 1930 (Kašivárová) or 1964 (Bujanov and Boky). The sites are located in the beech-oak altitudinal zone (Tab. 1) and belong to the alliance Carpinion betuli ([20]). Parent materials include andesite and granite, the prevailing soil type is cambisol. The mean annual temperature ranges from 7.0 to 8.2 °C and the annual precipitation from 675 mm (Bujanov) to 750 mm (Kašivárová).
Tab. 1 - Main environmental characteristics of the three localities selected for this study.
| Characteristics | Forest reserve | ||
|---|---|---|---|
| Kašivárová | Boky | Bujanov | |
| Latitude [°N] | 48°27’59” | 48°33’59” | 48°52’19” |
| Longitude [°E] | 18°46’28” | 19°01’22” | 21°03’38” |
| Years of measurement | 1966, 1976, 1986, 1996, 2011 | 1974, 1984, 1994, 2004 | 1966, 2009 |
| Altitude [m a.s.l] | 280-590 | 480-600 | 420-760 |
| Bedrock | andesite | andesite | granite |
| Soil type | eutric cambisol | eutric cambisol | dystric cambisol |
| Mean temperature [°C] | 8.0-8.2 | 7.5 | 7.0-7.2 |
| Mean precipitation [mm] | 750 | 720 | 675 |
| Area of forest reserve [ha] | 49.8 | 176.5 | 88.2 |
| Forest plant community | Carpinion betuli | Carpinion betuli | Carpinion betuli |
The dataset analyzed has originated from a 45-years (30-years in Boky) long-term research on permanent plots representing various developmental stages of selected oak-dominated forests ([24]). The outcomes of previous studies on the same data were published by Korpel ([24]), Halamová & Saniga ([14]), Saniga ([43]), Tomaštík & Saniga ([52]).
In each reserve, three 0.5 ha plots were established. On each plot, all living trees, standing deadwood (snags) and lying deadwood (logs) were recorded. The lower size limit for standing stems was a diameter at breast height (dbh) of 4 cm. As for downed deadwood, all logs or log parts with a large-end diameter ≥ 8 cm were recorded. For each standing tree, we recorded species, dbh, height and status (living or dead). Tree species, small- and large-end diameter and total length were measured for the logs.
Dominant height (h10%) was computed for each site as the mean height of the 10% tallest trees ([48]), and was used to assign each tree to one of three tree layers (lower, middle and upper layer) using the following thresholds: (i) lower layer (trees with height h < 1/3 h10%); (ii) middle layer (1/3 h10% ≤ h < 2/3 h10%); and (iii) upper layer h ≥ 2/3 h10%.
Volume of each tree was calculated according to the two-parameter (dbh and height) equations derived by Petráš & Pajtík ([35]). For the determination of the volume of broken standing deadwood Huber’s formula was used ([48] - eqn. 1):
where d1/2 is the mid-height diameter and hs is the height of the broken snag. The mid-height diameter was calculated by linear interpolation using the snag dbh and the respective tree height from the stand height curve.
The downed deadwood volume was computed by Smalian’s formula ([48] - eqn. 2):
where dS is the small-end diameter, dL the large-end diameter, and l is the log length.
The diameter structure was analysed by fitting either the negative exponential function, the single Weibull function (three-parameter form), or the finite mixture of two Weibull functions (seven-parameter form) to the empirical diameter distributions. More information on the functions used can be found in Westphal et al. ([57]) and Zhang et al. ([60]). The goodness-of-fit was examined using the likelihood-ratio χ2 test. All calculations were performed using the “mixdist” package of R software ([29], [37]).
Temporal changes in stem density and basal area were analyzed by assessing the population growth rates (GR) according to Taylor et al. ([50]). In each reserve, growth rates were calculated for oak and other tree species using the census data of the first and the last inventory. Moreover, the same calculation was performed for oak and other tree species in each stand layer (eqn. 3):
where Nt is the stem density (or basal area) at the time of the last inventory, N0 the stem density (or basal area) at the time of the first inventory, and t the census interval. Differences among the population growth rate values obtained were tested using the Kruskal-Wallis test (α = 0.05).
Results
Over the whole studied period, the most differentiated structure was observed in the reserve with no past human impact (Boky). Differentiated structure was reflected in the highest long-term mean stem density (890 ± 151 ha-1) and the lowest basal area and growing stock in this site (36.5 ± 1.4 m2 ha-1 and 284 ± 22 m3 ha-1, respectively - Tab. 2).
Tab. 2 - Basic stand characteristics (living trees of dbh >4 cm) in the investigated forest reserves.
| Parameter | Reserve/ | Boky | Kašivárová | Bujanov | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Decade | Together | Oak | % | Together | Oak | % | Together | Oak | % | |
| Stem density (ha-1) |
1960s | - | - | - | 284 ± 54 | 239 ± 33 | 84 ± 10 | 414 ± 77 | 381 ± 75 | 92 ± 1 |
| 1970s | 1068 ± 158 | 945 ± 42 | 89 ± 9 | 300 ± 50 | 239 ± 41 | 80 ± 10 | - | - | - | |
| 1980s | 921 ± 90 | 799 ± 27 | 87 ± 11 | 257 ± 42 | 227 ± 30 | 88 ± 8 | - | - | - | |
| 1990s | 867 ± 120 | 637 ± 70 | 74 ± 18 | 199 ± 23 | 183 ± 27 | 92 ± 7 | - | - | - | |
| 2000s | 703 ± 68 | 508 ± 36 | 72 ± 6 | 703 ± 229 | 163 ± 52 | 23 ± 18 | 482 ± 69 | 198 ± 8 | 41 ± 5 | |
| Mean ± SD | 890 ± 151 | 722 ± 190 | 80 ± 8 | 349 ± 202 | 210 ± 35 | 74 ± 26 | 448 ± 48 | 290 ± 129 | 67 ± 26 | |
| Basal area (m2 ha-1) |
1960s | - | - | - | 43.1 ± 2.1 | 42.3 ± 2.6 | 98 ± 1 | 42.5 ± 0.3 | 41.9 ± 0.5 | 93 ± 1 |
| 1970s | 35.1 ± 5.3 | 34.3 ± 5.9 | 98 ± 3 | 44.6 ± 2.3 | 43.3 ± 2.4 | 97 ± 1 | - | - | - | |
| 1980s | 38.1 ± 4.3 | 37.1 ± 5.1 | 97 ± 1 | 45.2 ± 2.2 | 44.5 ± 2.5 | 99 ± 1 | - | - | - | |
| 1990s | 37.2 ± 3.9 | 35.5 ± 5.1 | 95 ± 5 | 39.6 ± 1.7 | 39.2 ± 1.9 | 99 ± 1 | - | - | - | |
| 2000s | 35.5 ± 12.5 | 32.2 ± 13.4 | 90 ± 4 | 38.8 ± 3.3 | 32.0 ± 3.2 | 83 ± 9 | 45.4 ± 3.7 | 34.8 ± 1.8 | 77 ± 4 | |
| Mean ± SD | 36.5 ± 1.4 | 34.8 ± 2.1 | 95 ± 3 | 42.3 ± 2.9 | 40.3 ± 5.0 | 95 ± 6 | 44.0 ± 2.1 | 38.4 ± 5.0 | 88 ± 8 | |
| Growing stock (m3 ha-1) |
1960s | - | - | - | 467 ± 83 | 462 ± 84 | 99 ± 1 | 484 ± 28 | 479 ± 30 | 99 ± 1 |
| 1970s | 254 ± 58 | 252 ± 59 | 99 ± 1 | 483 ± 60 | 474 ± 60 | 98 ± 2 | - | - | - | |
| 1980s | 307 ± 56 | 303 ± 57 | 99 ± 2 | 508 ± 90 | 503 ± 92 | 99 ± 1 | - | - | - | |
| 1990s | 292 ± 47 | 286 ± 50 | 98 ± 3 | 457 ± 47 | 454 ± 46 | 99 ± 1 | - | - | - | |
| 2000s | 281 ± 132 | 266 ± 136 | 94 ± 3 | 422 ± 42 | 381 ± 44 | 90 ± 2 | 553 ± 37 | 440 ± 19 | 80 ± 2 | |
| Mean ± SD | 284 ± 22 | 276 ± 22 | 97 ± 2 | 467 ± 32 | 455 ± 45 | 97 ± 4 | 518 ± 49 | 460 ± 28 | 89 ± 10 | |
Natural reserves with various levels of past human impact showed a more homogenous stand structure, as revealed by a significantly lower stem density and higher basal area and growing stock. In the forest most strongly affected in the past (Bujanov) the long-term mean of basal area was higher by 20% (44.0 ± 2.1 m2 ha-1) and that of growing stock by 83% (518 ± 49 m3 ha-1) as compared with the Boky reserve. The long-term mean of deadwood volume (Tab. 3) in the site with past human impact (Kašivárová) was three times higher than in the old-growth forest in Boky. Similarly, the dead-to-live wood ratio in Kašivárová (28.1 ± 15.1%) was nearly twofold in comparison to the Boky reserve (15.0 ± 6.1%). In both sites a gradual increase of deadwood volume as well as its proportion from growing stock was observed over the whole period of investigation.
Tab. 3 - Deadwood volume and its proportion from the growing stock for the investigated forest reserves.
| Years |
Forest reserve | |||||
|---|---|---|---|---|---|---|
| Boky | Kašivárová | Bujanov | Boky | Kašivárová | Bujanov | |
| Deadwood volume (m3 ha-1) | Dead-to-live wood ratio (%) | |||||
| 1960s | - | 47 ± 18 | - | - | 10 ± 3 | - |
| 1970s | 17 ± 15 | 86 ± 28 | - | 7 ± 6 | 18 ± 3 | - |
| 1980s | 38 ± 19 | 107 ± 35 | - | 12 ± 4 | 21 ± 5 | - |
| 1990s | 52 ± 19 | 188 ± 36 | - | 18 ± 9 | 41 ± 7 | - |
| 2000s | 65 ± 25 | 212 ± 59 | 91 ± 60 | 23 ± 10 | 50 ± 16 | 17 ± 9 |
| Mean ± SD | 43 ± 21 | 128 ± 70 | - | 15 ± 6 | 28 ± 15 | - |
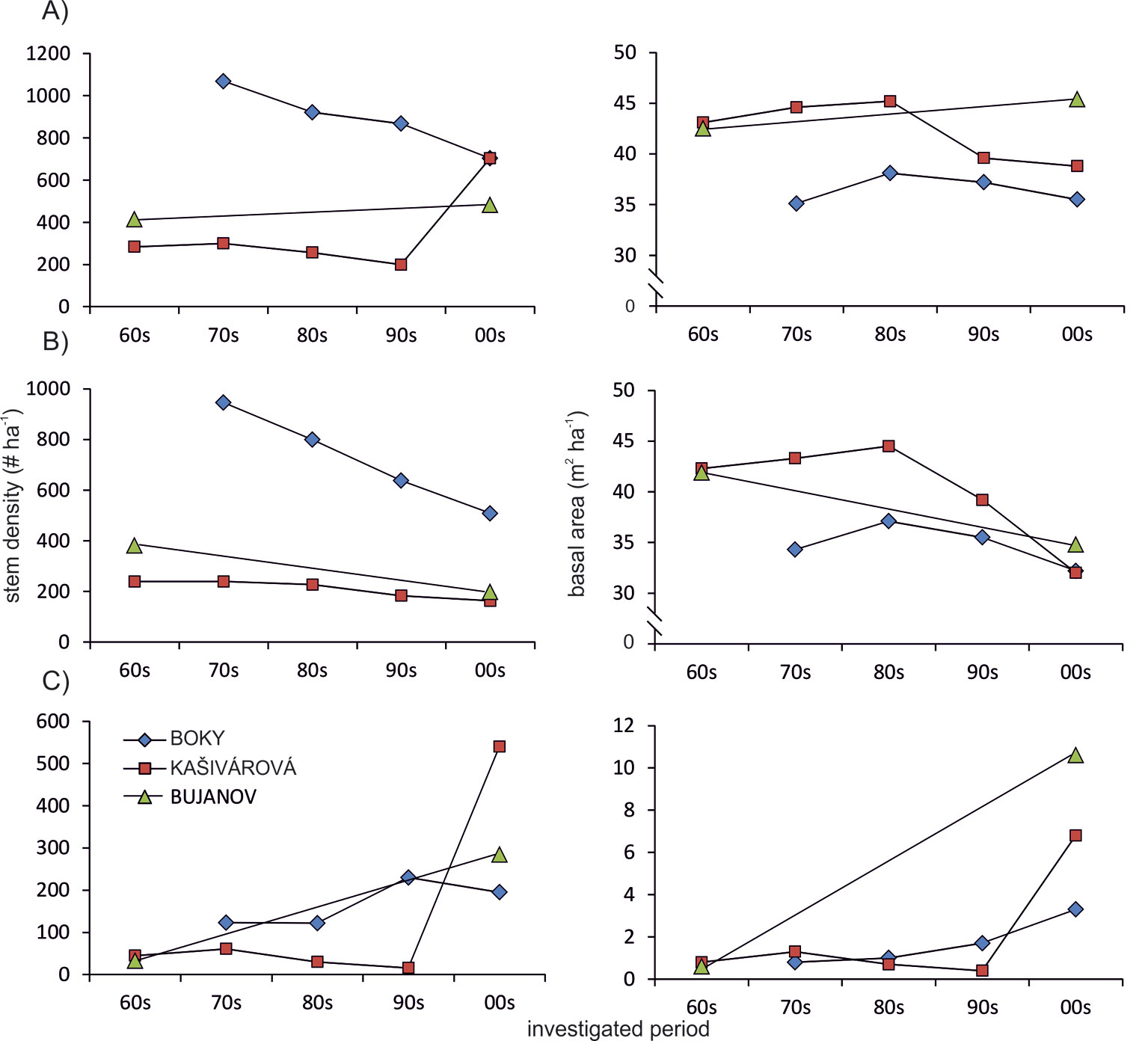
Regarding the temporal changes of basic stand characteristics, except for continuous increase of deadwood volume, no significant trends were observed over the entire study period. However, since the mid-1980s we recorded a gradual decrease of basal area and growing stock in the Boky and Kašivárová reserves (a detailed analysis was not possible in Bujanov). Such decrease was was moderate in Boky and stronger in Kašivárová, according to the level of past human impacts on the forest (Fig. 1).
Fig. 1 - Temporal development of stem density and basal area in the investigated forest reserves. (A): all tree species; (B): oaks; (C): other species.
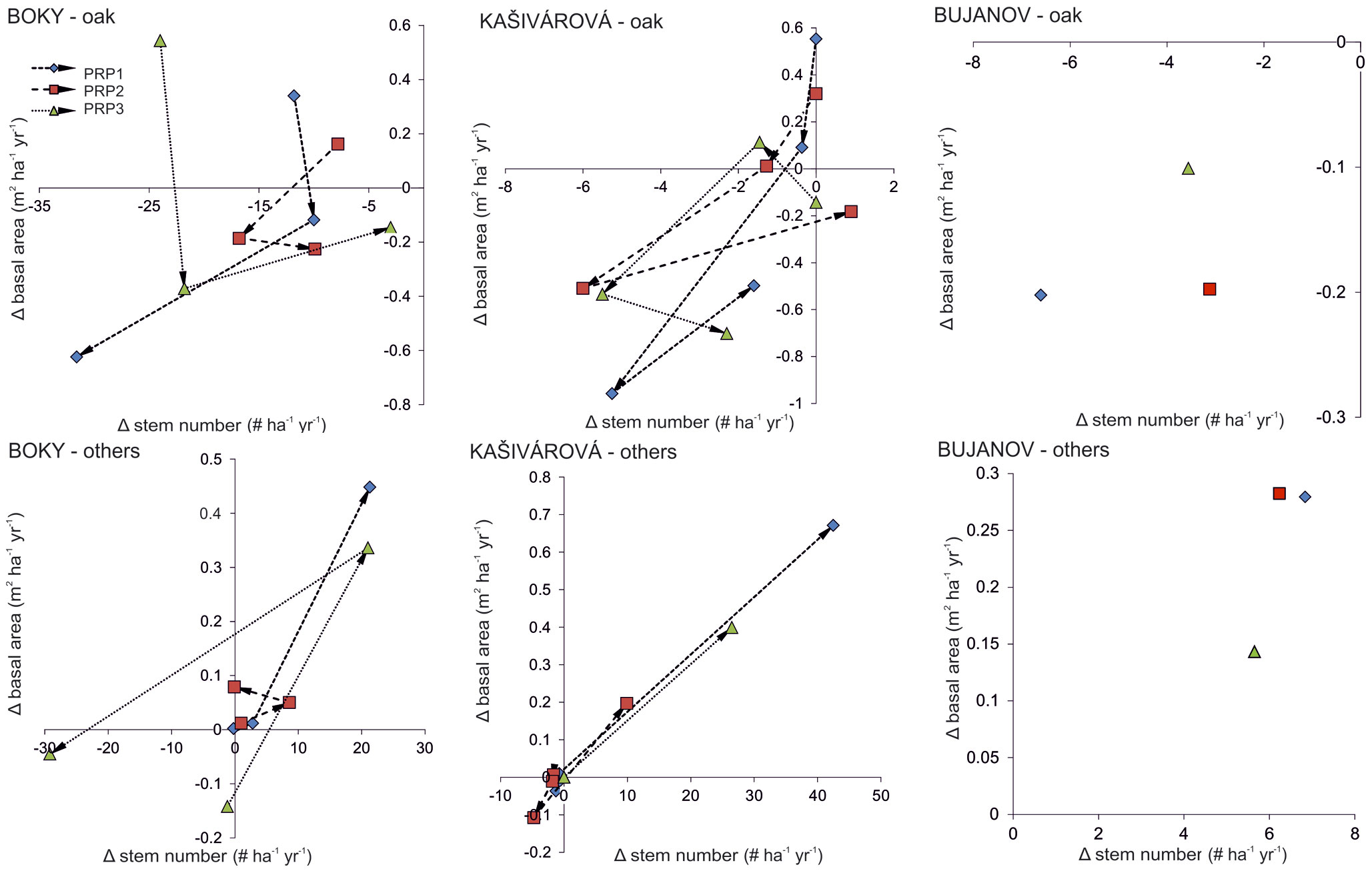
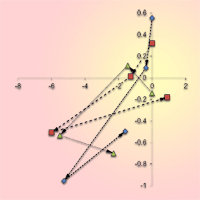
A continuous decline of oak stem density was recorded in all studied sites over the entire period of investigation. Moreover, since the 1980s a decrease of oak basal area and growing stock was also observed, yet much more intensive than that recorded for the whole stand (Fig. 1, Tab. 2). At the site with no past human interventions (Boky) the basal area of oak decreased from 37.1 to 32.2 m2 ha-1, whereas oak decline was more relevant (from 44.5 to 32.0 m2 ha-1) in the forest with past human impact (Kašivárová). At the same time, an increase of basal area and growing stock was recorded for the other tree species, especially over the last decade (Fig. 1). The above patterns were confirmed also at the research-plot level (see Fig. 2 for temporal changes in the stand parameters over the investigated period).
Fig. 2 - Annual changes (Δ ) in the stem density and the basal area according to separate permanent research plots (PRP) and inventory campaigns in the investigated forest reserves. Arrows indicate the temporal vector.
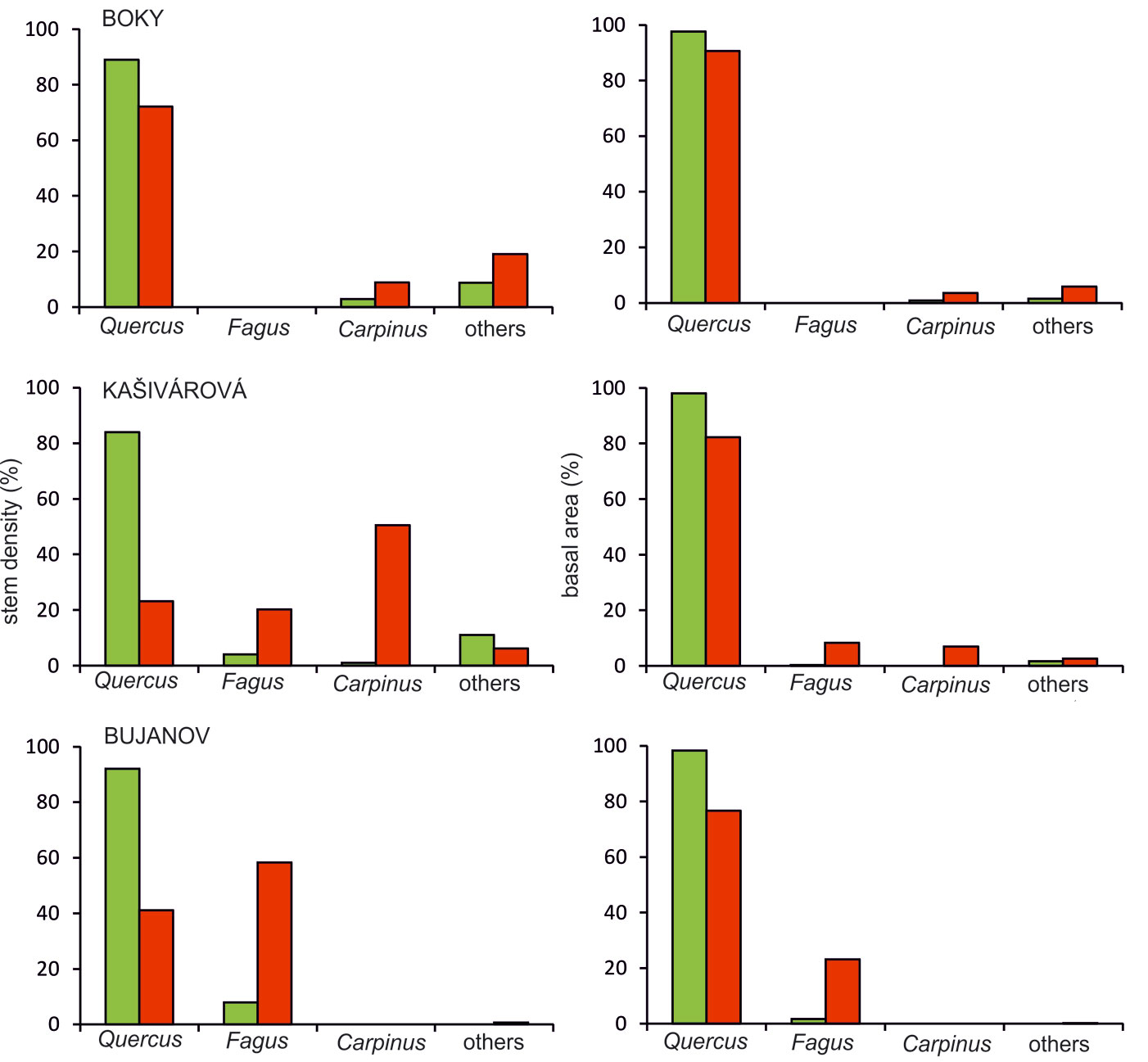
The starting species composition was very similar in all the investigated sites. At the time of the first inventory, oaks reached the highest proportion in terms of stem number or basal area (Fig. 3). The admixture of the other tree species varied among the studied sites. In Boky the admixture consisted of field maple (Acer campestre), hornbeam (Carpinus betulus) and wild service tree (Sorbus torminalis). In Kašivárová the most important additional tree species were beech (Fagus sylvatica) and hornbeam. In Bujanov the only additional tree species was beech.
Fig. 3 - Tree species structure calculated from the stem density (left) and the basal area (right) in the investigated forest reserves for the first (green columns) and the last inventory campaign (red columns).
The stand development over the census period led to a substantial shift in the tree species structure. Regardless of the interdecadal variation, the recorded structural changes resulted in a general decline of oak proportion in all studied reserves (Fig. 3). The decrease of oak proportion in stem number was smallest in Boky (from 89% to 72%). The largest decrease over the 45-years period was observed in Kašivárová, with a decline from 84% to 23%, while the proportion of hornbeam expanded from 1% to 51%. We also observed a significant increase of beech by 16% in this site. A similar change was recorded in Bujanov, where the share of oak decreased from 92% to 41% and the share of beech raised from 8% to 58%. Considering the proportions in basal area, the changes in species composition were not so apparent. The recruitment of other tree species reflected in stem numbers has not yet affected significantly the total basal area and oak has kept its dominant position in all investigated reserves. The recorded decrease of oak proportion in basal area was highest in Bujanov, however, not exceeding 22% (Fig. 3).
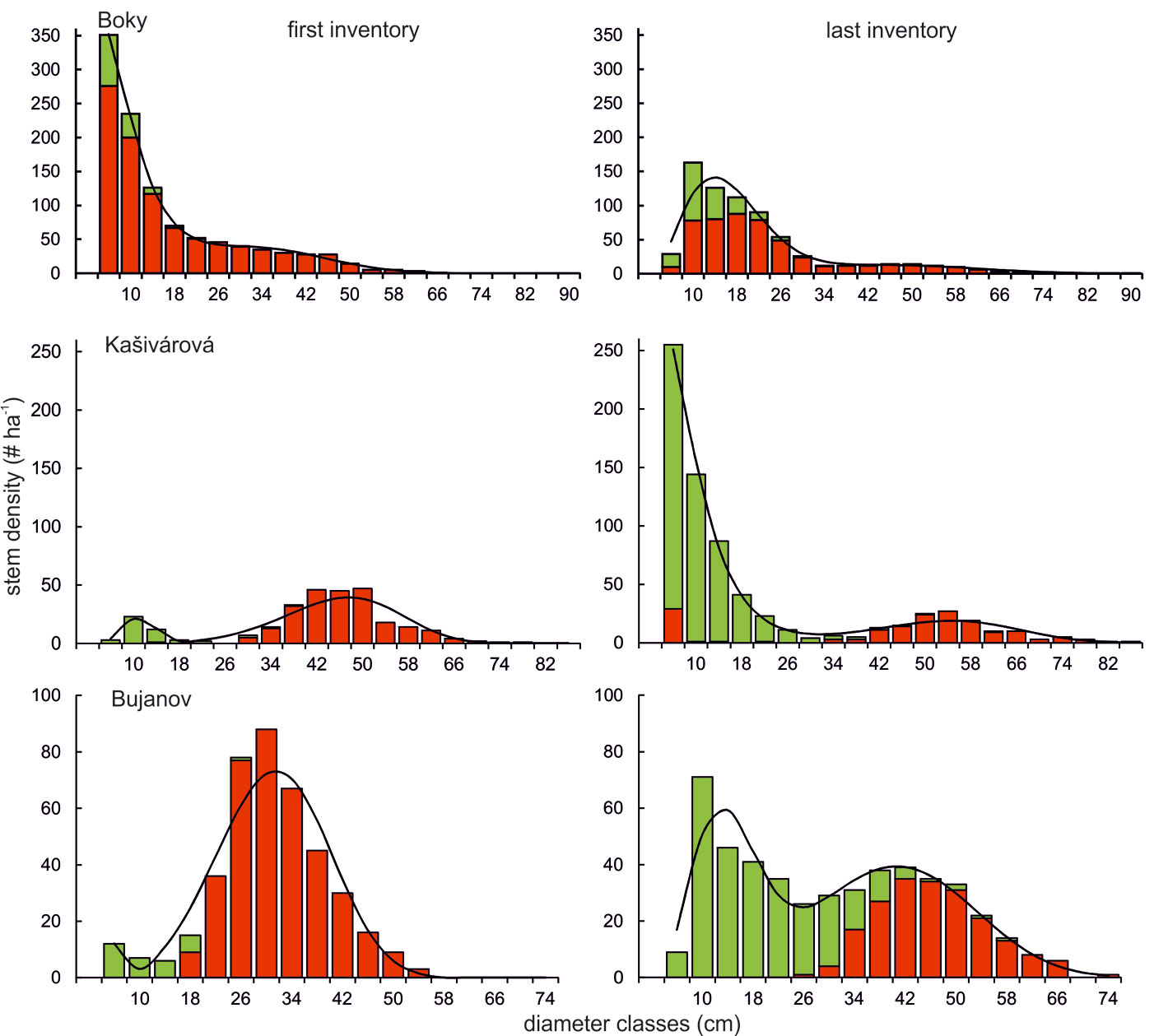
Diameter distributions observed at the beginning of the investigated period differed strongly among the studied reserves (Fig. 4). Unlike the old-growth forest Boky showing a typical rotated-sigmoid diameter distribution, diameter structures in Kašivárová and Bujanov showed bimodal patterns with a distinct dominance of the upper tree layer. However, over the studied period a convergence towards the rotated-sigmoid distribution was observed in both reserves. Contrastingly, the diameter structure kept the rotated-sigmoid form in the old-growth forest Boky over all the investigated period. Nevertheless, we observed a significant reduction of lower tree layer density, which can be mainly accounted for by the mortality of oaks and their shift into higher dbh classes. In the Kašivárová reserve, the diameter structure significantly changed over time. Although the diameter distribution maintained a bimodal shape, hornbeam and beech density in the lower tree layer largely increased, which resulted in a typical rotated-sigmoid form in 2011 (Fig. 4). The diameter structure of oak shifted from a unimodal shape with almost all oak stems in the upper tree layer to a bimodal distribution with some oak recruitment appearing in the lower tree layer. The diameter structure in Bujanov changed markedly (Fig. 4). The unimodally distributed oak layer with emerging beech in the lower tree layer in 1966 shifted towards a distinctively bimodal distribution in 2009. Currently, the stand is formed by oak in the upper tree layer and by beech in the middle and especially in the lower layer.
Fig. 4 - Empirical and fitted diameter distributions of the investigated oak-dominated forest reserves. Explanatory notes: red bars - oaks, green bars - other tree species, solid line - distribution predicted by the finite mixture of two Weibull functions. Please note that tree individuals were grouped into 4 cm diameter classes.
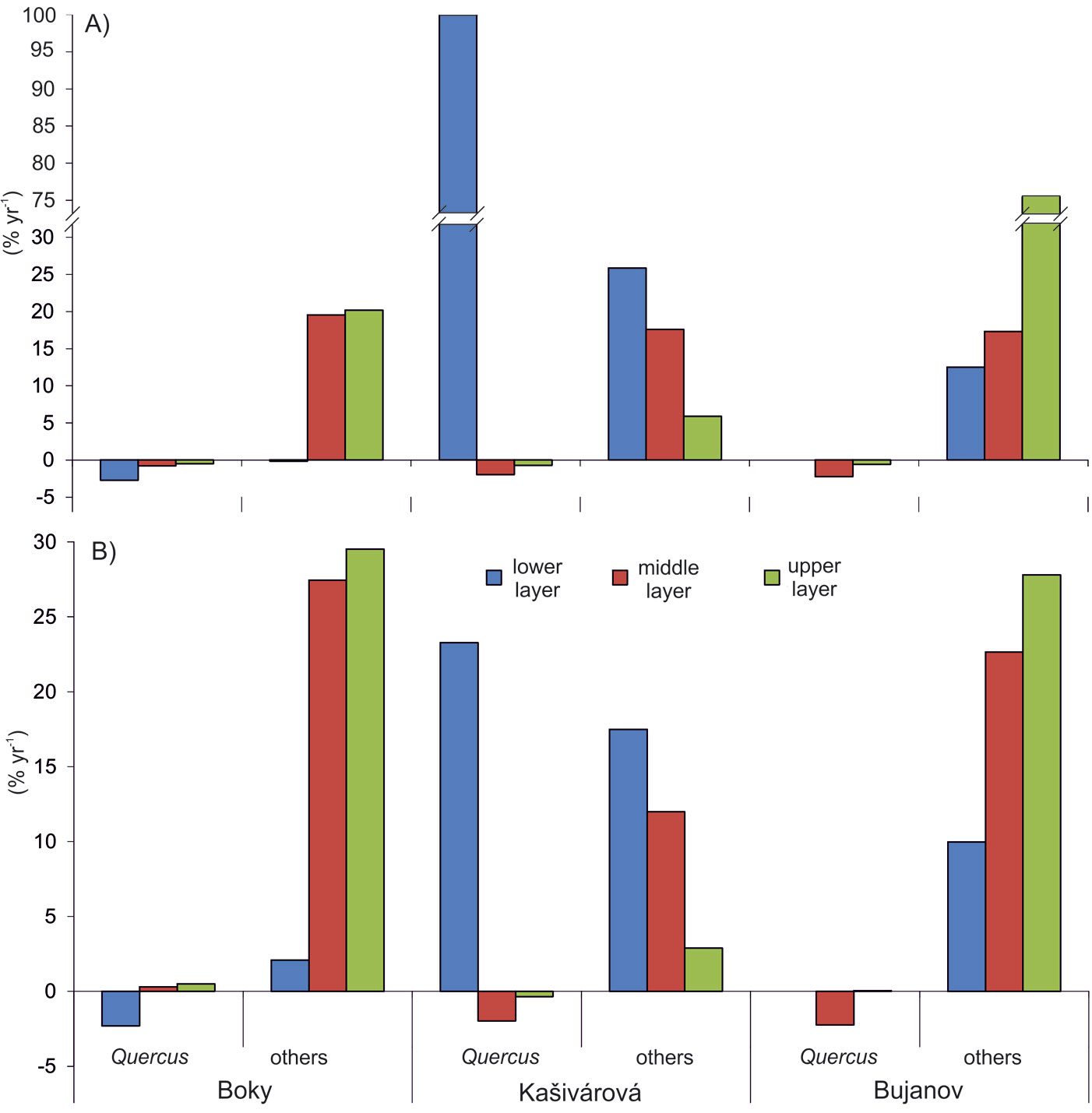
Structural shifts in species composition over the studied period disregarding the short-term (interdecadal) variation were calculated in the form of growth rates of stem density and basal area. Growth rates of oak were negative in all reserves, ranging from -0.7% yr-1 (Kašivárová) to -2.1% yr-1 (Bujanov) for stem density. The decrease of oak proportion in basal area was significantly less intensive and reached values between -0.2% yr-1 (Boky) and -0.7% yr-1 (Kašivárová). At the same time, in all investigated reserves we observed an increase of other tree species both in stem number (from 1.2% yr-1 in Boky to 2.1% yr-1 in Bujanov) and basal area (from 2.0% yr-1 in Kašivárová to 2.5% yr-1 in Boky). Growth rates varied significantly among tree layers (Fig. 5). The most dynamic changes in oak tree number and basal area were found in the lower layer (Boky), lower and middle layer (Kašivárová) and middle layer (Bujanov). In Bujanov, oak lower layer was virtually absent.
Fig. 5 - Growth rates of the stem density (A) and the basal area (B) according to respective tree layers and forest reserves, calculated using the eqn. 3.
The lower tree layer in Boky showed the highest oak mortality both in stem number (-2.7% yr-1) and basal area (-2.3% yr-1 - Fig. 5). Oak stem loss in the middle layer (-0.8% yr-1) did not affect basal area, which exhibited even an imperceptible growth. In the upper layer, oak basal area increased by 0.5% yr-1. Considering the dynamics of other species in the middle and the upper layer, we observed an increase of both stem number (19.5% yr-1 and 20.2% yr-1, respectively) and basal area (27.5% yr-1 and 29.5 % yr-1, respectively).
An opposite situation was observed in Kašivárová (Fig. 5). The increase of oak density in the lower layer by 100% yr-1 was reflected also by growth of oak basal area (23.3% yr-1). The overall decrease of oak was caused mainly by its reduction in the middle layer (-2% yr-1 both by stem number and basal area) and to a lesser extent in the upper layer. Other tree species (beech and hornbeam) invaded especially the lower layer (25.8% yr-1 and 17.5% yr-1 by stem number and basal area, respectively) and the middle layer (17.6% yr-1 and 12.0% yr-1, respectively). Their growth rates in the upper layer were significantly lower and did not exceed 6% yr-1 (stem density) and 3% yr-1 (basal area).
The development in Bujanov showed several peculiar characteristics (Fig. 5). In the lower layer, the absence of oak individuals was closely associated with an extensive expansion of beech density by 12.5% yr-1. In the middle layer, a massive increase of beech in stem number (17.3% yr-1) and basal area (22.6% yr-1) was accompanied by oak mortality (-2.2% yr-1 both by stem number and basal area). The upper tree layer was characterized by a slight loss of mature oak density (-0.6% yr-1); however, it was compensated by diameter growth of the remaining oaks (0.05% yr-1 by basal area). Regarding the growth rates of beech, their highest values were recorded in the upper layer (75.9% yr-1 and 27.8% yr-1 by stem number and basal area, respectively).
Discussion
The results obtained in this study confirmed the expected decrease of oaks in all investigated areas, regardless of the intensity of past human impacts. This is consistent with the general decline of oaks observed in many European forests during the last deca-des ([31], [38], [13], [46], [51], [41]). However, we discern some particularities regarding the oak loss according to the different levels of past human impact among the studied natural reserves.
Considering the spatial diversity, the recorded structures closely correspond with the relative intensity of past intervention on the investigated forests. This finding is in line with the conclusion of Clark & Covey ([10]) and Bauhus et al. ([1]) on a negative effect of size-class exploitation on the persistence of old-growth attributes and structural diversity.
The most differentiated initial structure, approximated by the rotated sigmoid function, was recorded in the reserve with no direct human intervention (Boky). The past-affected reserve in Kašivárová showed a bimodal pattern and the most affected Bujanov revealed even the typical unimodal distribution characteristic for even-aged forests ([36]). Accordingly, the more affected reserves reached higher values for the basic stand parameters, most likely due to a distinctive dominance of the upper layer. The average basal area and growing stock in Kašivárová and Bujanov (36.5-44 m2 ha-1 and 284-518 m3 ha-1, respectively) correlated negatively with their level of structural heterogeneity, while the stem number revealed a positive correlation.
The recorded amounts of deadwood are consistent with those reported for other oak-dominated natural forests in Europe ([39], [34]). Regardless of the historical background, we observed a continuous increase of deadwood volume and dead-to-live wood ratio in all the analyzed forests over 45 (or 30) years. Likewise, Vandekerkhove et al. ([53]) described the slow long-term process of deadwood accumulation in oak-dominated, man-made forests left to self-development 10 to 150 years ago. Considering the dead-to-live wood ratio, the obtained values in this study were slightly lower than those reported for other oak-dominated natural forests. Rahman et al. ([39]) presented dead-to-live wood ratios ranging from 30 to 40% depending on the forest associations, while Petritan et al. ([34]) found a deadwood proportion of 23% in sessile oak-dominated plots.
Regarding the interdecadal variation of stand parameters, we did not recognize a persistent trend over the analyzed period. The common feature of all investigated forests was a gradual decline of basal area and growing stock since the 1980s. This trend closely corresponds with the epidemic of oak wilt (Ceratocystis fagacearum) in Slovakia and Hungary ([8], [27]). However, the only considerable difference was the rate of such decrease. A more distinctive decrease in all stand parameters was confirmed for the reserves which experienced a stronger past human impact. This is probably due to the fact that forests with artificially modified structure are more susceptible to pathogens than natural ecosystems ([13], [49]). On the contrary, Brang et al. ([5]) reported for forest reserves in Switzerland almost opposite trends typical of the early- to mid-successional stages, namely an increase of basal area, growing stock and large trees density, as well as a decrease in stem number and species richness.
In this study several different patterns were revealed when the successional changes over the whole period of investigation were evaluated. First of all, a clear tendency was observed toward differentiated, close-to-nature structure throughout the structural shift from the bimodal diameter distribution in both past-affected reserves. The bimodal diameter distribution described at the beginning of the investigation period in Kašivárová reached the typical rotated sigmoid patterns at the time of the last inventory. Similarly, the diameter structure in the Bujanov reserve after 40 years of unhindered recovery showed a transformation from unimodal to bimodal distribution. It is very likely that the recovery process in this reserve follows the same direction as observed in Kašivárová. The rotated sigmoid diameter distribution in Boky persisted during the entire investigation period. A similar trend was described by Westphal et al. ([57]) and Kucbel et al. ([25]) in beech virgin forests in Europe. We have observed a tendency to the displacement of light-demanding oak by shade-tolerant species (beech, hornbeam). The intensity of such substitution directly reflected the intensity of the local structure modification in the past. Among the stand parameters considered, stem number showed the most distinctive change, while changes in tree species composition by basal area and growing stock were not so apparent yet.
In the Boky reserve, distinctive structural changes occurred within the lower and middle layer. A significant loss of oaks from the lower layer stems from a discontinuous oak recruitment in this site. The dieback of oaks resulted in an increase of stem number of hornbeam and other tree species. A continuous accumulation of deadwood in this site across all the investigated decades is directly linked to tree mortality. From the point of view of ecosystem stability, the loss of oak individuals is constantly counterbalanced by the ingrowth of accompanying tree species, e.g., hornbeam and field maple ([24]). In Kašivárová, oak abundance increased only in the lower layer. In spite of this fact, a decrease of oak proportion in the lower layer was observed as the result of the massive hornbeam expansion occurring periodically, and is a natural part of developmental cycle of natural oak forests ([24]). The real loss of oak individuals in this reserve was recorded in the middle and upper layers as a result of oak wilt, which occurred throughout Slovak forests in the 1980s ([8]). This is supported by a noticeable local increase of deadwood volume after the inventory in the 1990s ([42], [43]). Oak mortality favored a rapid natural regeneration of shade-tolerant beech in the lower layer and its growth into the middle layer. The most intensive change in tree species composition was observed in the Bujanov reserve. The absence of oaks in the lower tree layer and the dieback of oaks in the middle layer (outcompeted by beech) led towards a complex change of the spatial structure. A closed beech canopy in the lower layer hampers the natural regeneration of oaks. According to von Lüpke ([55]), successful regeneration of oaks occurs when the light availability does not fall below 15-20% of the full light, and at least 30-60% is required after two years. In the current situation, a successional transition from the dominance of oaks towards a closed canopy dominated by shade-tolerant beech is expected.
The vertical stand structure seems to drive the recovery process. In almost all cases, oak showed negative growth rates, while other tree species revealed rather positive growth rates in all tree layers of the investigated forests. The scarse initial abundance of the other tree species at the time of the first inventory may be the reason of the distinctive positive growth rates of such species in the Boky reserve. Moreover, a clear association between the intensity of past human impact and the outgrowing of the lower tree layer was recognized, suggesting that outgrowing of the natural regeneration is crucial for the recovery process. In addition to the competitive exclusion observed in the lower layer in Boky, we recognized a massive recruitment of other species to the lower layer in both past-affected reserves. Furthermore, successful regeneration of oak was recorded only in reserves with no or moderate level of past human impact (Boky, Kašivárová).
The successional shift toward the dominance of shade-tolerant species is expected based on the disturbance theory. However, our results are in agreement with the findings by Matuszkiewicz ([30]), Jakubowska-Gabara ([19]) or Hédl et al. ([18]), who report the replacement of oak-dominated thermophilous forest by mesic communities after the cessation of human intervention. Our study showed that the oak decline could be mostly a consequence of the recovery after the past third-party intervention rather than the effect of environmental changes. Regardless of the altered natural conditions, our results strongly support the hypothesis that the process of forest recovery and oak loss significantly depends on the intensity of past human impact. However, altered environmental conditions should be still considered an important factor lowering the resilience of past-affected ecosystems ([7], [28], [40]).
Acknowledgements
This study was supported by the Slovak Research and Development Agency, project APVV-0286-10. The authors would like to thank the anonymous reviewers for their helpful comments and suggestions on earlier version of the manuscript.
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Miroslav Balanda
Stanislav Kucbel
Ján Pittner
Technical University in Zvolen, T. G. Masaryka 24, SK 960-53 Zvolen (Slovak Republic)
Corresponding author
Paper Info
Citation
Saniga M, Balanda M, Kucbel S, Pittner J (2014). Four decades of forest succession in the oak-dominated forest reserves in Slovakia. iForest 7: 324-332. - doi: 10.3832/ifor0996-007
Academic Editor
Emanuele Lingua
Paper history
Received: Mar 22, 2013
Accepted: Feb 03, 2014
First online: Apr 17, 2014
Publication Date: Oct 01, 2014
Publication Time: 2.43 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2014
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 54852
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 45968
Abstract Page Views: 3453
PDF Downloads: 4130
Citation/Reference Downloads: 33
XML Downloads: 1268
Web Metrics
Days since publication: 4338
Overall contacts: 54852
Avg. contacts per week: 88.51
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2014): 23
Average cites per year: 1.92
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
The impact of seed predation and browsing on natural sessile oak regeneration under different light conditions in an over-aged coppice stand
vol. 9, pp. 569-576 (online: 04 April 2016)
Review Papers
Opportunities for coppice management at the landscape level: the Italian experience
vol. 9, pp. 775-782 (online: 04 August 2016)
Research Articles
Shrub facilitation of Quercus ilex and Quercus pubescens regeneration in a wooded pasture in central Sardinia (Italy)
vol. 3, pp. 16-22 (online: 22 January 2010)
Review Papers
Should the silviculture of Aleppo pine (Pinus halepensis Mill.) stands in northern Africa be oriented towards wood or seed and cone production? Diagnosis and current potentiality
vol. 12, pp. 297-305 (online: 27 May 2019)
Research Articles
Tree-oriented silviculture: a new approach for coppice stands
vol. 9, pp. 791-800 (online: 04 August 2016)
Research Articles
Equations for estimating belowground biomass of Silver Birch, Oak and Scots Pine in Germany
vol. 12, pp. 166-172 (online: 15 March 2019)
Research Articles
Geographic determinants of spatial patterns of Quercus robur forest stands in Latvia: biophysical conditions and past management
vol. 12, pp. 349-356 (online: 05 July 2019)
Research Articles
Species-specific climate response of oaks (Quercus spp.) under identical environmental conditions
vol. 7, pp. 61-69 (online: 18 November 2013)
Research Articles
Effect of origin and morphological characteristics of sessile oak (Quercus petraea) seedlings on the development of Cryphonectria parasitica
vol. 18, pp. 16-22 (online: 15 February 2025)
Research Articles
Optimizing silviculture in mixed uneven-aged forests to increase the recruitment of browse-sensitive tree species without intervening in ungulate population
vol. 11, pp. 227-236 (online: 12 March 2018)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword