Trade-offs and spatial variation of functional traits of tree species in a subtropical forest in southern Brazil
iForest - Biogeosciences and Forestry, Volume 9, Issue 6, Pages 855-859 (2016)
doi: https://doi.org/10.3832/ifor1960-009
Published: Jul 07, 2016 - Copyright © 2016 SISEF
Research Articles
Abstract
Plant functional traits have been recognized as important factors related to the ecological strategies of species in forest ecosystems. We examined the relationships between functional traits and both tree species performance and environmental conditions in a subtropical forest in Brazil. Over four years (2008-2012), we investigated how demographic rates were related to functional traits (wood density, leaf area and tree height) of 20 species sampled within 50 plots of 10 × 20 m, which had previously evaluated as to environmental conditions. Non-metric multidimensional scaling was used to order the species by their functional traits. The demographic rates were fit a posteriori to the ordination, with significant rates (p < 0.05) plotted as vectors. The relationships between environmental conditions and the community-weighted means (CWMs) of trait values were verified using redundancy analysis. CWM wood density was positively correlated with soil pH. CWM leaf area and CWM maximum tree height were both negatively correlated with altitude and positively correlated with soil magnesium (Mg) content. The taller species with lower wood density, which occupied the forest canopy, had a greater diameter increment and lower recruitment than did the shortest species with higher wood density. The shorter species with higher wood density, which occupied the understory, had greater recruitment and a greater increase in abundance than did the taller/lower-wood-density species. Our study (i) revealed changes in the forest related to the light environment, with an increase in the relative participation of shade-tolerant species with higher wood densities, and (ii) detected small-scale spatial variation in community traits as a response to variations in soil chemical properties and topography.
Keywords
Araucaria Forest, Atlantic Forest, Environmental Heterogeneity, Multivariate Analysis
Introduction
Species coexistence and spatial turnover across environmental gradients have been reported as important mechanisms for promoting the high tree species diversity observed in forest ecosystems ([17], [27]). These processes can be partially explained by ecological niche partitioning by species as a consequence of biotic and abiotic filters. In turn, the spatial occurrences of species are mediated by the ecological and phenotypic traits of the tree species ([1]), which result in different species performances according to the environmental conditions ([21]). Thus, the fitness of different organisms in their environment reflects the adaptive values and life strategies of the species, and affects the demographic rates and spatial variation of traits in communities ([35], [32], [6]). In general, species with forms and functions that maximize net carbon balance and growth in a given environment will typically succeed in that environment ([35]).
Many studies have demonstrated the existence of a growth-mortality trade-off among species in closed canopy tropical forests ([40], [41], [30], [32]), which has mainly been associated with the responses of different life strategies of species to the environmental heterogeneity of light ([31], [41]). For shaded forest understory species, the investment in wood density and long-lived, well-protected leaves represents an important strategy for mechanical resistance to stem damage and the avoidance of biomass loss, thereby increasing survivability ([30]). In contrast, gaps and earlier successional forest patches are dominated by light-demanding species that invest in short-lived and physiologically active leaves, which results in a rapid growth and thus an increased access to light ([30], [32]).
Differences in species performance may represent a product of ecological filtering that reflects the species’ fitness to their existing environment. Therefore, linkages among community functional traits and environmental conditions are expected. These patterns have been observed at different spatial scales as a function of climatic variables, topography, altitude, soil and disturbances ([25], [18], [15]). Whereas acquisitive traits, such as rapid growth and soft wood density, are commonly found in environments providing greater resource availability (e.g., canopy gaps and nutrient-rich soils), conservative traits, such as slow growth and hard wood density, are usually observed in low-resource environments (e.g., shaded understories, low-fertility soils - [3], [2]). Thus, knowledge of these patterns contributes to the understanding of the breadth of tree species niches in forest ecosystems.
Studies that evaluate how variations in plant functional traits affect species performance and how functional traits are influenced by environmental conditions are important for understanding forest ecosystem ecology. In the present study, we aimed to investigate the relationships between functional traits and tree species performance, and between community traits and environmental gradients in a subtropical evergreen forest in Brazil. Our objective was to determine how the demographic rates of tree species are mediated by their functional traits and how community traits vary spatially in response to environmental heterogeneity. We tested the hypothesis that in the study forest, the ecological strategies of species differ under different light and edaphic environments, with species with acquisitive traits succeeding under high-resource conditions (e.g., canopies and nutrient-rich soils) and species with conservative traits thriving in low-resource environments (understorey and low-fertility sites).
Materials and methods
The present study was conducted in a fragment of subtropical evergreen forest, classified as Araucaria Forest, with an approximate area of 103.06 ha and an altitude ranging from 990 to 1000 m a.s.l. It is located at latitude 27° 51′ 19.20″ S and longitude 50° 10′ 33.39″ W in the municipality of Lages, Santa Catarina, Brazil. The mean precipitation and annual temperature for the region (1970-2010) are 1682.80 mm and 15.9 °C, respectively ([11]).
The 20 most abundant woody species, representing 69.9 and 83.1% of the community abundance and basal area, respectively, were sampled over 1 ha in 50 plots of 10×20 m. The environmental conditions of the plots (chemical and physical properties of the soil and topography) had been evaluated as part of the research conducted by Higuchi et al. ([11]). The richest botanical families in the study area are Myrtaceae (22 species), Lauraceae (7) Aquifoliaceae (5), Asteraceae (5), Fabaceae (5) and Salicaceae (5 - [11]). For the sampled species, the functional traits (wood density, leaf area and maximum height) were determined. The demographic rates (recruitment, mortality, basal area gain, basal area loss, basal area increment of survival, net change and turnover) were recorded for four years (2008-2012) by Salami et al. ([36]), based on Lieberman et al. ([22]), Korning & Balslev ([16]), Sheil & May ([37]) and Oliveira-Filho et al. ([28]) as follows (eqn. 1 to eqn. 8):
where Mort is the annual mortality rate, Recr is the annual recruitment rate, Loss is the annual basal area loss rate, Gain is the annual basal area gain rate, t is the time interval between inventories, No is the initial number of trees, Nt is the final number of surviving trees after t, m is the number of dead trees, r is the number of recruited trees, ABo is the initial basal area, ABt is the final basal area after t, ABm is the basal area of dead trees, ABd is the basal area loss (diametric reduction and partial loss of stems), ABr is the basal area of recruited trees, ABg is the basal area gain (tree growth), Nchg is the tree annual net change, Abchg is the basal area net change, Nturn is the tree turnover, and Abturn is the basal area turnover.
The determination of wood density data and leaf area was performed according to Pérez-Harguindeguy et al. ([29]). A total of 10 individuals per species were sampled (overall 200 samples) using an increment borer with a diameter of 5.15 mm. In the laboratory, these samples remained immersed in water to obtain a constant wet weight. The volumes were obtained by the water-displacement method (Archimedes’ principle). The samples were then dried in an oven for 72 hours until stabilization of the dry mass, and the dry weight was determined using an analytical scale. The wood density values were determined as the ratio between the dry weight and the volume (g cm-3). The leaf area, including petioles and rachis for compound leaves, was obtained by sampling 20 leaves from each individual of each tree species (10 individuals per species). Healthy leaves, without visible damage by herbivores, were collected. The leaf samples were stored in sealed plastic bags to prevent water loss during transport to the laboratory, where the leaves were photographed and the leaf area was determined using ImageJ® software ([34]). The maximum heights of the tree species was assessed through field observations and literature review ([23], [24]).
Non-metric multidimensional scaling (NMDS) was used to ordinate the species according to their functional traits. The demographic rates were fit a posteriori to the NMDS ordination, with the significant rates (p < 0.05) plotted as vectors. For assessing the community functional responses to the environmental gradient, the community-weight matrix and redundancy analysis (CWM-RDA) method was performed ([13]). For this method, the community-weighted means of trait values (CWMs) were determined for each plot. Then, a multivariate forward selection by permutation of residuals (n = 999), in which the data were centered and scaled, was performed to identify significant environmental variables in the CWM. Subsequently, the relationships among the environmental variables and the CWM were verified using a redundancy analysis (RDA). All of the analyses were performed using the R statistical programming environment ([33]) with the “vegan” ([26]) and “FD” ([19], [20]) packages.
Results
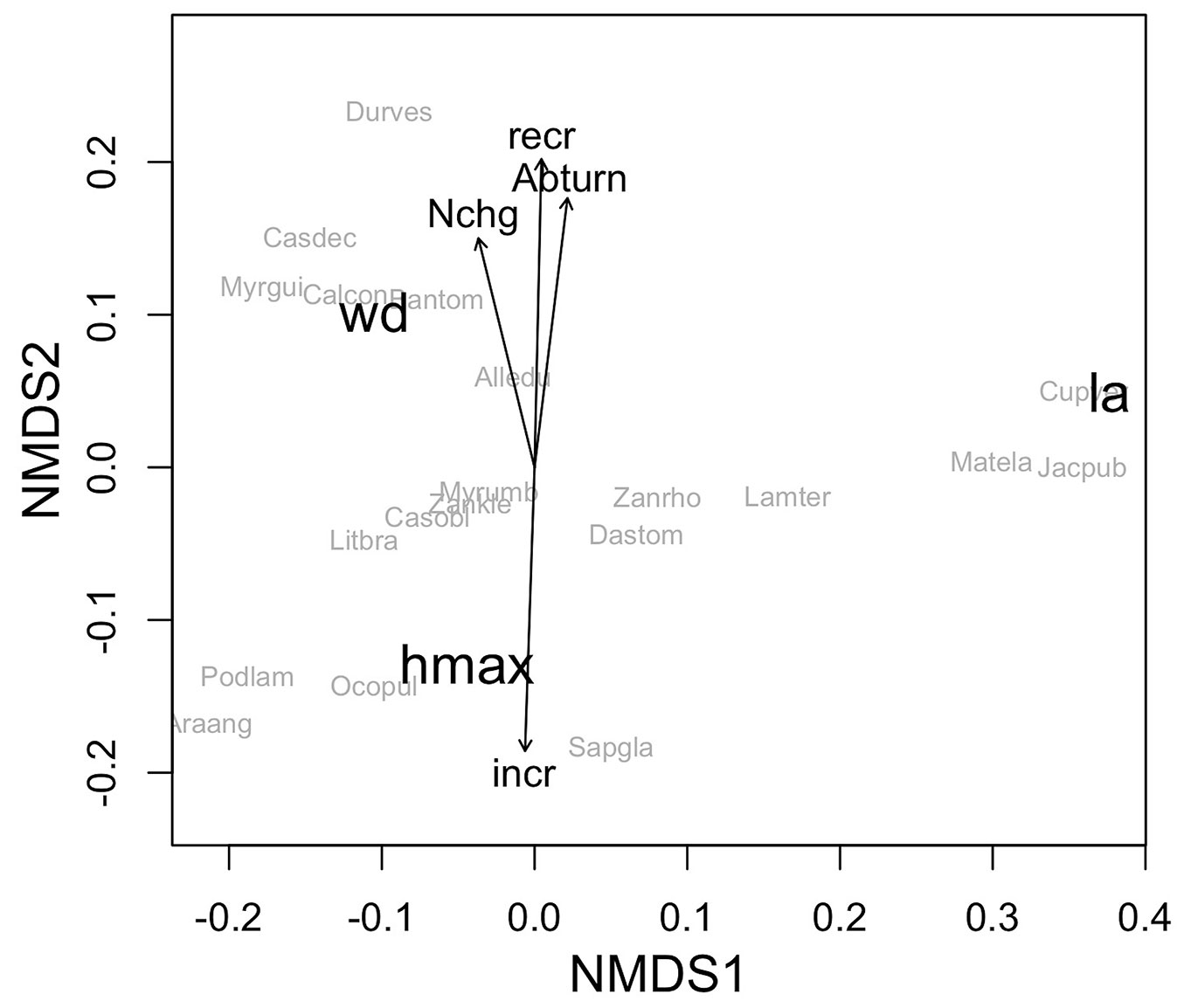
The mean values of maximum height, leaf size and wood density were 16.55 m, 33.86 cm2 and 0.44 g cm-3, respectively. The NMDS ordination indicated the existence of relationships between species functional traits and their demographic rates (p < 0.01 - Fig. 1). Whereas Axis 1 of the NMDS ordination reflected a gradient associated with leaf area, with small-leaved species on the left and larger-leaved species on the right, Axis 2 represented a gradient related to wood density and maximum height. The species that had greater recruitment and greater basal area turnover were predominantly those with lower maximum heights (< 14 m) and higher wood densities (> 0.60 g cm-3), which were located predominantly towards the top of the ordination plot. The species with the greatest increases in the diameter increment had the highest maximum heights (> 19 m), which occupied the upper forest canopy and were located towards the bottom of the ordination. No relationships were observed between functional traits and mortality rate, basal area loss, basal area gain or tree turnover. The size of the leaves also did not influence the demographic rates of the species.
Fig. 1 - Ordination produced by non-metric multidimensional scaling (NMDS) of tree species in a subtropical Araucaria Forest in southern Brazil as a function of their functional traits, with significant demographic rates (p < 0.05) plotted as vectors. (wd): wood density; (la): leaf area; (hmax): maximum height of trees: (Abturn): basal area turnover; (Nchg): tree annual net change; (recr): annual recruitment rate; (incr): survival diametric increment; (Alledu): Allophylus edulis; (Araang): Araucaria angustifolia; (Bantom): Banara tomentosa; (Calcon): Calyptranthes concinna; (Casdec): Casearia decandra; (Casobl): Casearia obliqua; (Cupver): Cupania vernalis; (Dastom): Dasyphyllum tomentosum; (Durves): Duranta vestita; (Jacpub): Jacaranda puberula; (Lamter): Lamanonia ternata; (Litbra): Lithraea brasiliensis; (Matela): Matayba elaeagnoides; (Myrgui): Myrcia guianensis; (Myrumb): Myrsine umbellata; (Ocopul): Ocotea pulchella; (Podlam): Podocarpus lambertii; (Sapgla): Sapium glandulosum; (Zankle): Zanthoxylum kleinii; (Zanrho): Zanthoxylum rhoifolium.
The forward selection identified three significant variables (elevation, soil pH and soil Mg - p < 0.05) that explained 17.72% of the variation in the community functional traits in the RDA (Tab. 1), with the first and second axis accounting for 73.15% and 24.53% of this total, respectively.
Tab. 1 - Environmental gradients and responses of the community traits of tree species represented by the correlation of each environmental variable with the first two axes of the redundancy analysis (RDA) for a subtropical Araucaria Forest in southern Brazil. The values presented between parentheses after the axis names indicate the percentages of explained variation associated with each axis.
| Redundancy Analysis | Statistics / Axis (var. explained) |
pH | Mg (cmol dm-3) |
Elevation (m) |
|---|---|---|---|---|
| Significant environmental gradient (p < 0.05) |
Minimum | 3.8 | 0.1 | 900.7 |
| Mean | 4.8 | 1.9 | 1000.5 | |
| Maximum | 6.3 | 4.5 | 1113.4 | |
| Response of community traits to gradients (Total variation explained = 17.72%) |
Axis 1 (73.15%) | 0.31 | 0.66 | -0.57 |
| Axis 2 (24.53%) | 0.95 | 0.47 | -0.38 |
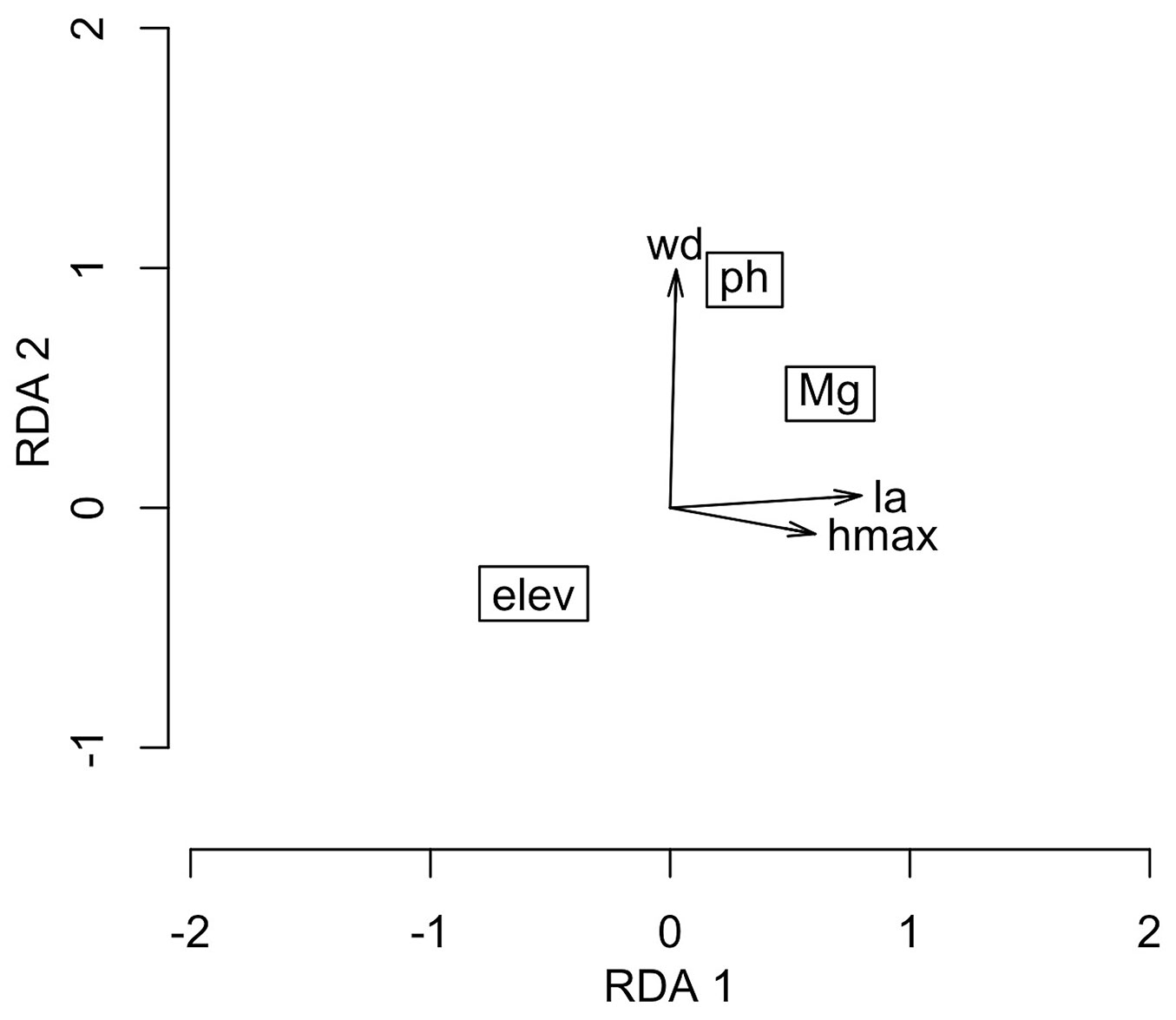
According to the RDA (Fig. 2), the sites with a higher wood density were associated with higher values of soil pH, and the plots with smaller leaves and shorter species were associated with lower values of soil Mg content and higher elevations. In general, the results (Fig. 1 and Fig. 2) demonstrate that the species with higher wood densities, higher rates of recruitment, and increases in both the numbers of individuals and basal area turnover rates occurred predominantly in sites with the highest pH values, whereas the larger species with the highest diameter increments occurred mainly in the lower elevation sites with soils with the highest Mg contents.
Fig. 2 - Ordination obtained by the Redundancy Analysis (RDA) of community traits as a function of environmental gradients in a subtropical Araucaria Forest in southern Brazil. (elev): elevation; (wd): wood density; (ph): soil pH; (Mg): soil Mg; (la): leaf area; (hmax): maximum height of trees.
Discussion
Considering the relationships between demographic rates and functional traits in tree species, a clear trade-off was evident between the allocation of resources for growth and the allocation for wood density. The species of larger size and lower wood density, which occupied the canopy of the forest, exhibited the highest diameter increments but lower recruitment, whereas the species of higher wood density and lower stature, which occurred in the understory, exhibited higher recruitment, greater increases in abundance and smaller diameter increments. This trade-off has been frequently cited for closed canopy forests ([40], [41], [32]), and it is a consequence of the classical model of forest dynamics ([7], [38]), in which the dynamics are determined by the divergent life strategies of species under different light environments. Whereas light-demanding, pioneer species, which colonize gaps and grow rapidly, represent one extreme of this gradient, shade-tolerant species represent the other extreme, growing slowly under the canopy and presenting elevated survival rates ([41]).
In a more stable, low-resource environment, such as the forest understory, the investment in wood density represents a strategy for mechanical resistance to stem damage, thereby increasing survivability, whereas the greater diameter increment of large trees can be explained by greater access to light ([32]). Furthermore, as noted by Chave et al. ([6]), the negative relationship between wood density and growth rate is expected because a higher wood density is the result of a greater investment in a smaller volume of wood per unit of biomass, and dense wood may result in a lower proportion of conduit, thus affecting transpiration, photosynthesis and growth.
Although some authors have demonstrated a strong association between mortality and the life strategies of species ([40], [41], [30], [32]), this was not the case in the present study. Our result might reflect the successional stage of the forest, where despite their lower recruitment, the large-sized species with lower wood densities and greater diametric increments had not yet reached the senescence phase.
Considering the spatial variation of community traits, the results indicate the influence of environmental conditions on these traits, which suggests the existence of ecological filters ([21]) that are crucial for the occurrence of functionally similar species, most likely reflecting the adaptation of the species across environmental gradients ([35]). The species with higher maximum heights and larger leaves were dominant in the lower-elevation plots with higher values of soil Mg content, whereas the species with higher wood densities were predominant in the more basic soils. These results indicate the existence of small-scale (1 ha) spatial variation of functional traits and life strategies in the community, which are promoted by the heterogeneity of soil chemical properties and topography. The different life strategies observed for different species are suggested to be an important mechanism of ecological niche partitioning, which may explain the coexistence of species over short spatial scales ([14]).
As low soil pH is an important factor limiting nutrient availability to plants, the pattern of higher community wood density in more basic soils observed in the present study was unexpected because a greater nutrient supply generally favors fast-growing species of lower wood density ([3]). However, the pattern observed here indicates that wood density may be also strongly affected by other ecological factors, such as disturbance history, that can influence the forest light environment and consequently, the spatial distribution of pioneer species, which usually have lower wood densities ([39], [3], [4]). Thus, in the present study the existence of lower wood density at sites with more acid soils could can be interpreted as the result of the occurrence of recent disturbances in this forest sector.
Despite the lack of relationship between leaf size and demographic rates, this functional trait was negatively correlated with terrain elevation and positively correlated with soil Mg content. Small leaves can increase the boundary-layer conductance ([10]), allowing better control of water loss ([8]). Thus, a small leaf size is usually recognized as a key adaptation for a greater water stress ([12]), typically in areas with high incidence of solar radiation and wind, such as the upper positions of topographic gradients ([5]). Similarly, as demonstrated by Fyllas et al. ([9]), leaf functional traits are also influenced by edaphic gradients, with species with greater leaf masses per area succeeding in high-fertility sites ([9]).
Conclusions
We conclude that a trade-off between the investment in growth and the investment in wood density led to an increase in the relative proportion of shade-tolerant species with higher wood densities in these forest fragments, thereby suggesting the predominance of stable, low-light environments. Furthermore, small-scale spatial variation in the community traits was observed as a response to the variations in soil chemical properties and topography.
Acknowledgements
The authors thank the Brazilian National Council for Scientific and Technological Development (CNPq) for the productivity research grant awarded to the second and third author, and Santa Catarina State University (UDESC) for the scholarship awarded to the first author.
References
CrossRef | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Online | Gscholar
CrossRef | Gscholar
Authors’ Info
Authors’ Affiliation
Pedro Higuchi
Ana Carolina da Silva
Bruna Salami
Angélica Dalla Rosa
Fernando Buzzi-Junior
Tiago de Souza Ferreira
Amanda Koche Marcon
Marco Antonio Bento
Forestry Department, Agroveterinary Center, Santa Catarina State University, Av Luiz de Camões, 2090, Conta Dinheiro, 88.520-000, Lages, SC (Brazil)
Forestry Department, Santa Maria Federal University, 97105-900 Santa Maria, RS (Brazil)
Corresponding author
Paper Info
Citation
Missio FF, Higuchi P, Silva AC, Longhi SJ, Salami B, Dalla Rosa A, Buzzi-Junior F, Ferreira TS, Koche Marcon A, Bento MA (2016). Trade-offs and spatial variation of functional traits of tree species in a subtropical forest in southern Brazil. iForest 9: 855-859. - doi: 10.3832/ifor1960-009
Academic Editor
Andrea Cutini
Paper history
Received: Dec 19, 2015
Accepted: Apr 19, 2016
First online: Jul 07, 2016
Publication Date: Dec 14, 2016
Publication Time: 2.63 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2016
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 51072
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 42965
Abstract Page Views: 2930
PDF Downloads: 3744
Citation/Reference Downloads: 85
XML Downloads: 1348
Web Metrics
Days since publication: 3475
Overall contacts: 51072
Avg. contacts per week: 102.88
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2016): 7
Average cites per year: 0.70
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Networking sampling of Araucaria araucana (Mol.) K. Koch in Chile and the bordering zone of Argentina: implications for the genetic resources and the sustainable management
vol. 2, pp. 207-212 (online: 22 December 2009)
Research Articles
Fluctuation of the ecological niche of Moringa peregrina (Forssk.) Fiori with topoclimatic heterogeneity in southern Iran
vol. 16, pp. 53-61 (online: 16 February 2023)
Research Articles
Fragmentation of Araucaria araucana forests in Chile: quantification and correlation with structural variables
vol. 9, pp. 244-252 (online: 28 August 2015)
Research Articles
Strong relationships between soil and vegetation in reference ecosystems of a riparian Atlantic rainforest in the upper Doce River watershed, southeastern Brazil
vol. 16, pp. 226-233 (online: 17 August 2023)
Research Articles
Do different indices of forest structural heterogeneity yield consistent results?
vol. 15, pp. 424-432 (online: 20 October 2022)
Research Articles
Effect of restoration methods on natural regeneration in the Brazilian Atlantic Forest
vol. 18, pp. 23-29 (online: 15 February 2025)
Research Articles
Soil chemical and physical status in semideciduous Atlantic Forest fragments affected by atmospheric deposition in central-eastern São Paulo State, Brazil
vol. 8, pp. 798-808 (online: 22 April 2015)
Research Articles
Environmental niche and distribution of six deciduous tree species in the Spanish Atlantic region
vol. 8, pp. 214-221 (online: 28 August 2014)
Research Articles
Leaf morphology of progenies in Q. suber, Q. ilex, and their hybrids using multivariate and geometric morphometric analysis
vol. 11, pp. 90-98 (online: 31 January 2018)
Research Articles
Aboveground tree biomass of Araucaria araucana in southern Chile: measurements and multi-objective optimization of biomass models
vol. 14, pp. 61-70 (online: 09 February 2021)
iForest Database Search
Search By Author
- FF Missio
- P Higuchi
- AC Silva
- SJ Longhi
- B Salami
- A Dalla Rosa
- F Buzzi-Junior
- TS Ferreira
- A Koche Marcon
- MA Bento
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
- FF Missio
- P Higuchi
- AC Silva
- SJ Longhi
- B Salami
- A Dalla Rosa
- F Buzzi-Junior
- TS Ferreira
- A Koche Marcon
- MA Bento
Search By Keywords
PubMed Search
Search By Author
- FF Missio
- P Higuchi
- AC Silva
- SJ Longhi
- B Salami
- A Dalla Rosa
- F Buzzi-Junior
- TS Ferreira
- A Koche Marcon
- MA Bento
Search By Keyword