Calibration of a multi-species model for chlorophyll estimation in seedlings of Neotropical tree species using hand-held leaf absorbance meters and spectral reflectance
iForest - Biogeosciences and Forestry, Volume 9, Issue 5, Pages 829-834 (2016)
doi: https://doi.org/10.3832/ifor1785-009
Published: May 17, 2016 - Copyright © 2016 SISEF
Research Articles
Abstract
The aim of the present study was to calibrate a multi-species model for assessing leaf chlorophyll content in seedlings of six Neotropical rainforest tree species. Two hand-held chlorophyll absorbance meters (SPAD-502 and ClorofiLog) and the chlorophyll normalized difference leaf reflectance index (ND705) were tested. Measurements of leaf absorbance and reflectance, contents of chlorophyll a (Chl a), chlorophyll b (Chl b), and total chlorophyll (Chl t), leaf area (LA), and leaf mass per area (LMA) were performed on fully expanded leaves. A total of 200 leaves were used for the calibration of the multiple-species model. The relative root mean square calibration errors (RMSεc, %) were calculated based on estimated chlorophyll values for multiple-species models and on measured values for each of the six species. The average values of LA varied between 14.2 and 29.5 cm-2, LMA between 34.8 and 98.9 g m-2, and Chl t between 3 and 815 mg m-2. For all indices, the highest values of the coefficients of determination (R2) were observed for Chl a (R2 ≥ 0.91), followed by Chl t (R2 ≥ 0.89) and Chl b (R2 ≥ 0.82). The highest values of R2 were obtained for ND705 (R2 ≥ 0.86) followed by SPAD-502 (R2 ≥ 0.83) and ClorofiLog (R2 ≥ 0.82). The present study showed that ClorofiLog and SPAD-502 indices could be safely interconverted by a simple linear regression model (R2 = 0.98). RMSεc values were lower than 20%, which confirmed the feasibility of the multi-species model for estimating the chlorophyll content using hand-held chlorophyll absorbance meters and leaf reflectance.
Keywords
Chlorophyll Normalized Difference Index, Hand-held Chlorophyll Absorbance Meters, Leaf Reflectance, Neotropical Tree Species
Introduction
Chlorophylls are pigments responsible for capturing light for photosynthesis and are essential for the conversion of light radiation to chemical energy ([25]). Several studies showed that variations in chlorophyll content are related to plant photosynthetic capacity, development stage and environmental stresses ([17], [16], [23], [12], [3], [7]). Therefore, chlorophyll content is an important indicator of photosynthetic efficiency, carbon uptake, and fitness of plants and ecosystems ([26], [11]).
Spectrophotometry is the most conventional and precise technique for the determination of leaf chlorophyll content. It requires tissue homogenization with an organic extraction solvent, such as acetone or dimethyl sulfoxide (DMSO), and a spectrophotometer for the direct quantification of pigments ([19], [35]). Nonetheless, this technique is destructive and usually has to be performed under laboratory conditions. Other disadvantages of this technique are the low number of samples that can be processed per day and the necessity of transporting leaves from the field to the laboratory, which could interfere with the results, since more delicate leaves may suffer dehydration and pigment oxidation after harvesting.
With the development of optical instrumentation, the estimation of chlorophyll content has become easier, faster, nondestructive, and can be performed in the field. Currently, with the use of hand-held chlorophyll meters and portable reflectometers, it is possible to analyze a large number of samples in a single day and to measure the same leaf several times as it grows and develops. Hand-held chlorophyll meters are often calibrated to a single index and measure the absorption of electromagnetic radiation in the red and infrared regions ([21]). Red light is strongly absorbed by chlorophyll, whereas near-infrared light is used as an internal reference wavelength to adjust for differences in leaf structure. Conversely, portable reflectometers measure the radiation reflected by the leaf at a wide range of wavelengths ([28], [31]). Methods based on the reflection of electromagnetic radiation by leaves originated from remote sensing technology and are based on vegetation indices ([10], [9], [28], [31], [2]). Conceptually, vegetation indices are mathematical transformations of spectral bands used to estimate the contribution of vegetation in multispectral observations. The equations of most vegetation indices are mainly derived from bands of red and near infrared, such as the normalized difference vegetation index (NDVI) at an ecosystem scale ([26], [2]), or the chlorophyll normalized difference index (ND705) at a leaf scale ([10], [28]).
Hand-held chlorophyll meters and portable reflectometers are more convenient than extraction methods because they offer a more rapid, efficient, and nondestructive assessment of the leaf chlorophyll content. Nevertheless, optical methods also have limitations. First, they do not provide absolute chlorophyll content, but only relative indices that express the relative chlorophyll content ([28], [2]). Thus, the indices need to be previously calibrated with a more precise extraction method in the laboratory to provide absolute values of chlorophyll content. There are several mathematical models available in the literature for the calibration of hand-held absorbance chlorophyll meters. However, most of the available models are species specific and developed either for crop species ([32], [30], [4]) or temperate and sub-tropical forests species ([5], [34], [27], [13]). There are limited studies on the calibration of nondestructive methods for estimating leaf chlorophyll content in tropical tree species ([20], [8], [23]). Additionally, most of the available models are restricted to an estimation of total chlorophyll (Chl t) ([20], [8], [24]) and there are limited models for the estimation of chlorophyll a (Chl a) and b (Chl b - [28], [23]). Estimation of Chl a and b is important for the calculation of the ratio Chl a/b, which is an indicator of photosynthetic performance ([17]) and frequently used in studies on light acclimation capacity of seedlings when transferred from the nursery to the field in forest restoration programs ([12], [3]). Another limitation of optical methods refers to the physical properties of instruments that can result in non-linear relationships between chlorophyll indices and absolute chlorophyll content ([28], [31], [33], [32]). For instance, high leaf mass per area (LMA) may lead to the underestimation of chlorophyll content because high leaf thickness is usually associated with high mutual chloroplast shading and high overlapping of optical properties from distinct cell constituents that can affect the reflectance captured by the instrument ([18]). Heterogeneity in chlorophyll distribution may also create bias, from different leaf anatomical structures in distinct species, leaf developmental stages, and leaf vein networks. Finally, hand-held absorbance chlorophyll meters may lose sensitivity at high chlorophyll contents because of saturation of absorption in the red range ([32]). Therefore, the development of standard models that account for different leaf morphologies to be applied to different species and environmental conditions is necessary.
Estimation of leaf chlorophyll content of tree seedlings in areas of high biodiversity, such as tropical rainforests, that contain many species with different biochemical and anatomical structures, still represents a challenge for researchers. Coste et al. ([8]) developed a homographic model for the calibration of the SPAD-502 index to estimate chlorophyll content in a group of 13 Neotropical tree species with a wide range of LMA and chlorophyll contents. The developed homographic model was more accurate than the linear, polynomial, and exponential models and provided accurate estimates at high and low values of chlorophyll content. However, Coste et al. ([8]) did not calibrate their model for other chlorophyll indices, and did not test the model during initial tree development. Therefore, we conducted this study to test whether the multi-species model proposed by Coste et al. ([8]) is suitable for the calibration of the hand-held chlorophyll absorbance meters SPAD-502 and ClorofiLog, and of the leaf reflectance index ND705 ([10]) for the estimation of Chl a, b, and t in the leaves of seedlings at the nursery stage of six Neotropical rainforest tree species.
Materials and methods
Data were collected from seedlings of six Neotropical rainforest tree species representing six different botanical families (Tab. 1). The seedlings were grown under 50% artificial shading, in the nursery of Floresta Viva Institute (⇒ http://www.florestaviva.org.br/), Serra Grande, Uruçuca, Bahia, (Brazil). When seedlings were 2-4-months old, they were transported to the Plant Physiology Laboratory of the Universidade Estadual de Santa Cruz (UESC), Ilhéus, Bahia (Brazil), shortly before measurements. In the laboratory, we collected 30 to 37 fully expanded leaves from each species, with a total of 200 leaves. All the leaves collected had preserved edges and no sign of herbivory or disease symptoms. We measured the leaf absorbance, leaf reflectance, Chl a, b, and t contents, leaf area (LA), and leaf mass per area (LMA).
Tab. 1 - List of the six Neotropical tree species with their scientific names, family and type of leaves. (1): Only for seedlings. Adults of this species have compound leaves.
| Scientific name | Family | Leaves |
|---|---|---|
| Brosimum rubescens Taub. | Moraceae | Simple |
| Calophyllum brasiliense Cambess. | Calophyllaceae | Simple |
| Cytharexyllum myrianthum Chamiáo | Verbenaceae | Simple |
| Eriotheca macrophylla (K. Schum.) A. Robyns | Bombacaceae | Simple (1) |
| Inga capitata Desv. | Fabaceae | Compound |
| Tapirira guianensis Aublet. | Anacardiaceae | Compound |
Leaf absorbance and reflectance were measured immediately after their collection. Leaf absorbance was measured using the handheld chlorophyll absorbance meters ClorofiLog® (Falker, Porto Alegre, Brazil) and SPAD-502® (Minolta Inc., Osaka, Japan), for the determination of the Falker chlorophyll index (FCI) and the SPAD index (SPADi), respectively. Leaf reflectance of the adaxial leaf surface was measured with an USB4000-UV-VIS spectrometer using a LS-1 tungsten-halogen light source (Ocean Optics Inc., Dunedim, FL, USA). Based on the values of reflectance at 705 nm (R705) and 750 nm (R750), the normalized difference reflectance index was calculated as ND705 = (R750 - R705)/(R750 + R705) ([10]). We performed one measurement per leaf for each device. Measurements were always performed in the same place, at the middle of the leaf blade.
After absorbance and reflectance measurements, leaves were digitalized with an HP 2400 scanner. The digitalized images were saved in a TIFF format and the LAs were calculated using the ImageJ software (⇒ http://rsb.info.nih.gov/ij/). Then, three leaf-discs (6 mm diameter) were collected from the same area where the absorbance and reflectance measurements were taken. The leaf discs were placed in Eppendorf tubes with 5 mL dimethyl sulfoxide (DMSO) saturated with CaCO3 ([14]). The tubes were incubated at 65 °C for 24 h, for the total extraction of leaf pigments. An aliquot of 3 ml of the extract was used to measure the pigment absorbance at 649 nm and 665 nm, using a V-1100D® spectrophotometer (Mesu Lab Enterprise Co., Ltd, China). Chl a and b contents were calculated according to Wellburn ([35]). Finally, the leaves were dried at 65 °C until a constant mass. The dry mass and the leaf area were used for the calculation of LMA for each individual leaf.
Parameters of the homographic model Chl = (α · index)/(β - index) ([8]) were calibrated for chlorophyll contents (Chl a, b, and t) and chlorophyll indices (ND705, FCI and SPADi) using the entire data set (n = 200). The relative root mean square calibration errors (RMSεc, %) were calculated for each of the six rainforest tree species cited in Tab. 1 (n = 30-37), based on chlorophyll values estimated by the multi-species models and on the chlorophyll values measured by the indices. RMSεc was used as an indicator of the accuracy of predictive models and as a comparison with data from the literature ([28]).
Results
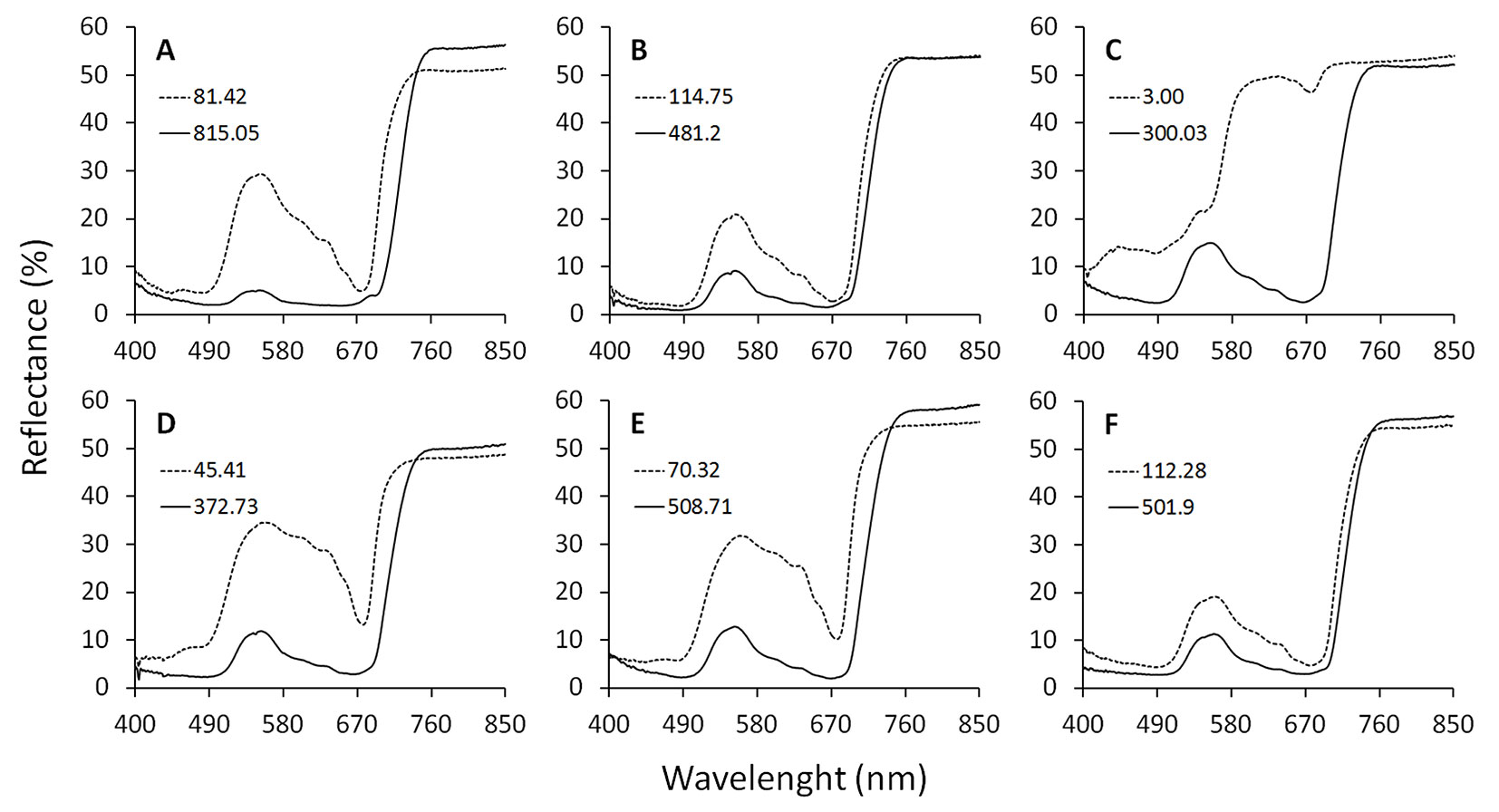
The average values for LA among the studied species ranged between 14.2 and 29.5 cm2, and for LMA, values ranged within 34.8 and 98.9 g m-2 (Tab. 2). The lowest and highest values for LA and LMA were found in T. guianensis and B. rubescens, and in C. myriantum and C. brasiliense, respectively. The average chlorophyll content obtained via traditional DMSO extraction methods ranged between 142.3-411.6 mg m-2 for Chl t, 98.1-270.1 mg m-2 for Chl a, and 44.2-141.5 mg m-2 for Chl b. The lowest and highest mean values for Chl t, b, and a were found in C. myrianthum and B. rubescens, respectively. The lower and higher individual values of Chl t contents for each of the six species used in this study are represented by the spectral properties of the leaves in Fig. 1.
Tab. 2 - Average leaf area (LA), leaf mass per area (LMA) and contents of chlorophyll a (Chl a), b (Chl b) and total (Chl t) of the seedlings of six Neotropical rainforest tree species.
| Species | n | LA (cm2) |
LMA (g m2) |
Chl a (mg m-2) |
Chl b (mg m-2) |
Chl t (mg m-2) |
|---|---|---|---|---|---|---|
| B. rubescens | 35 | 29.5 ± 9.5 | 69.0 ± 8.6 | 270.1 ± 126.4 | 141.5 ± 68.3 | 411.6 ± 194.4 |
| C. brasiliense | 32 | 19.1 ± 3.7 | 98.9 ± 21.4 | 162.6 ± 71.0 | 89.4 ± 32.8 | 252.0 ± 103.0 |
| C. myrianthum | 36 | 14.6 ± 3.9 | 34.8 ± 8.7 | 98.1 ± 57.9 | 44.2 ± 25.1 | 142.3 ± 82.8 |
| E. macrophylla | 37 | 22.8 ± 6.6 | 61.4 ± 6.0 | 102.4 ± 60.6 | 58.4 ± 33.9 | 160.8 ± 94.3 |
| I. capitata | 30 | 17.2 ± 5.2 | 90.8 ± 14.3 | 180.4 ± 90.8 | 90.3 ± 44.5 | 270.7 ± 134.7 |
| T. guianensis | 30 | 14.2 ± 3.8 | 55.5 ± 8.0 | 160.6 ± 72.1 | 106.6 ± 39.8 | 267.1 ± 111.4 |
Fig. 1 - Spectral properties of seedlings of six Neotropical rainforest tree species. The percentage of the irradiance reflected by the adaxial leaf surface was obtained with a spectrometer USB4000-UV-VIS using a LS-1 tungsten-halogen light source (Ocean Optics Inc. Dunedim, FL, USA). Dotted and continuous lines correspond to the lowest and the highest values of total chlorophyll content (mg m-2) for single-leaf measurements of each species, respectively, determined by dimethyl sulfoxide extraction and spectrophotometric measurements. (A): B. rubescens; (B): C. brasiliense; (C): C. myrianthum; (D): E. macrophylla; (E): I. capitata; (F): T. guianensis.
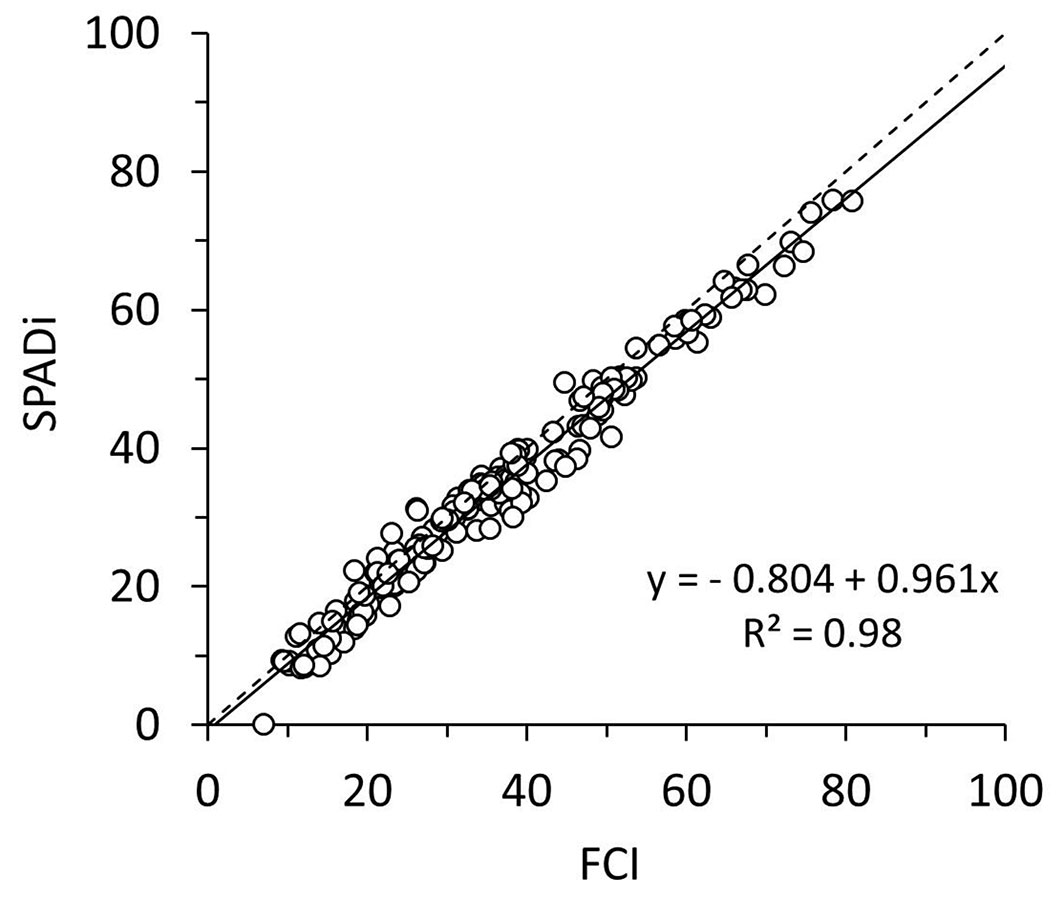
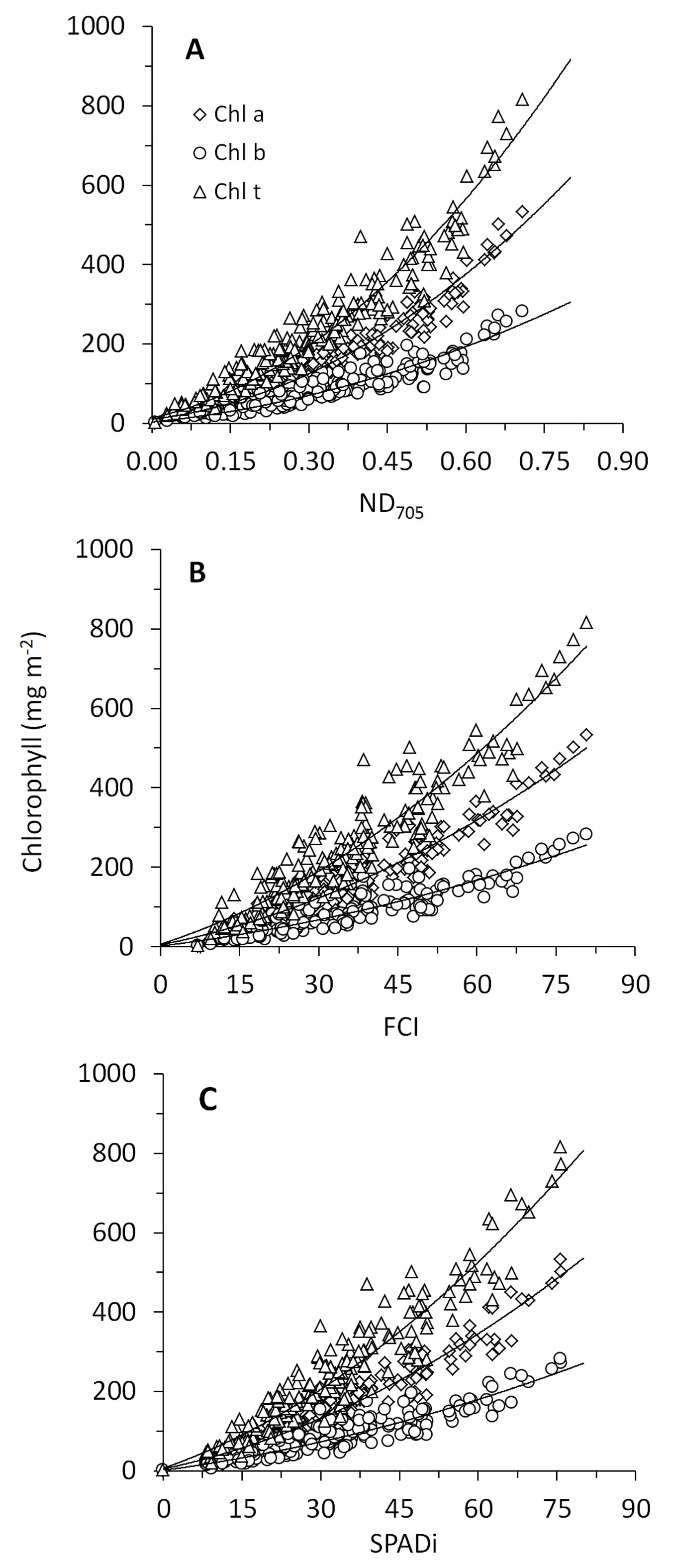
A strong linear relationship was observed between the two handheld chlorophyll meters, suggesting that data obtained from ClorofiLog (FCI) can be easily converted into SPADi (Fig. 2). Relationships between chlorophyll content and the nondestructive methods tested in this study were all curvilinear (Fig. 3) and the R2 values obtained for the calibrated homographic models were above 0.8 (Tab. 3). For all methods, the highest values of R2 were observed for Chl a, followed by Chl t and b. Although the R2 values were very similar among the three methods, the best fit was obtained for the data from ND705 (R2 ≥ 0.86) followed by SPAD-502 (R2 ≥ 0.83), and ClorofiLog (R2 ≥ 0.82).
Fig. 2 - Relationships between two non-invasive chlorophyll indexes based on leaves of seedlings of six Neotropical rainforest tree species (n = 200). Dotted line represent the 1:1 relationship between FCI and SPADi.
Fig. 3 - Relationships between chlorophyll contents and noninvasive chlorophyll indexes. Parameters of the homographic models based on leaves of seedlings of six Neotropical rainforest tree species (n = 200) are shown in Tab. 3.
Tab. 3 - Parameters of the homographic model Chl = (α · index)/(β - index) for relationships between non-invasive chlorophyll indexes and chlorophyll contents based on leaves of seedlings of six Neotropical rainforest tree species (n = 200).
| Index | Chl | α | β | R 2 |
|---|---|---|---|---|
| ND705 | Chl a | 515.04 | 1.45 | 0.95 |
| Chl b | 337.71 | 1.67 | 0.86 | |
| Chl t | 841.97 | 1.52 | 0.93 | |
| FCI | Chl a | 647.94 | 183.98 | 0.91 |
| Chl b | 417.25 | 211.20 | 0.82 | |
| Chl t | 1053.44 | 191.88 | 0.89 | |
| SPADi | Chl a | 690.35 | 181.89 | 0.92 |
| Chl b | 458.52 | 214.23 | 0.83 | |
| Chl t | 1132.38 | 191.07 | 0.90 |
For all species and indices, the calculated values of RMSεc were below 20% (Tab. 4). Among species, the lowest values of RMSεc were observed in C. myrianthum and E. macrophylla, whereas the highest value of RMSεc was found in T. guianensis. The ND705 index had the lowest average RMSεc values for Chl t, a, and b, followed by SPADi and FCI. The lowest and highest values of RMSεc for Chl a and b were found in C. myrianthum and E. macrophylla (ND705), and T. guianensis (FCI), respectively. The lowest and highest values of RMSεc for Chl t were found in E. macrophylla (ND705) and T. guianensis (FCI), respectively.
Tab. 4 - Relative root mean square calibration errors (RMSεc, %) based on the estimated chlorophyll values by the multi-species models (Tab. 3, n = 200) and measured values for seedlings of six Neotropical rainforest tree species.
| Species | Chl | ND705 | FCI | SPADi |
|---|---|---|---|---|
| B. rubescens (n = 35) | Chl a | 8.2 | 6.7 | 6.6 |
| Chl b | 10.0 | 7.7 | 8.1 | |
| Chl t | 8.6 | 6.7 | 6.8 | |
| C. brasiliense (n = 32) | Chl a | 4.8 | 6.2 | 4.2 |
| Chl b | 7.5 | 6.8 | 5.4 | |
| Chl t | 5.5 | 6.0 | 4.0 | |
| C. myrianthum (n = 36) | Chl a | 2.4 | 5.0 | 4.4 |
| Chl b | 6.4 | 5.9 | 5.4 | |
| Chl t | 3.0 | 4.5 | 3.8 | |
| E. macrophylla (n = 37) | Chl a | 3.1 | 4.9 | 3.7 |
| Chl b | 3.2 | 5.0 | 4.9 | |
| Chl t | 2.9 | 4.8 | 4.0 | |
| I. capitata (n = 30) | Chl a | 7.5 | 10.2 | 11.5 |
| Chl b | 10.9 | 12.9 | 14.0 | |
| Chl t | 8.3 | 11.0 | 12.2 | |
| T. guianensis (n = 30) | Chl a | 7.2 | 10.9 | 9.5 |
| Chl b | 14.8 | 18.7 | 17.2 | |
| Chl t | 9.7 | 13.5 | 12.0 |
Discussion
The results from our study show that the homographic model proposed by Coste et al. ([8]) is suitable for calibration of the relationship between absolute chlorophyll content and the chlorophyll indices ND705, SPADi, and FCI. As expected, there is a tendency towards a non-linear relationship between chlorophyll content and the chlorophyll indices ([28], [31], [33]). The nonlinear response may reflect the physical limitation of optical methods at higher chlorophyll concentrations ([21], [32], [30]), and the non-uniform distribution of chlorophyll in the leaf blade, particularly when there is high chlorophyll density in chloroplasts ([4]). Nonetheless, good fits can be obtained for Chl a (R2 ≥ 0.91), t (R2 ≥ 0.89), and b (R2 ≥ 0.82). A greater fit was observed for Chl a in all indices, which is particularly important because Chl a is always present in a greater amount than Chl b in the light harvesting complexes, thus having a significant contribution to Chl t ([25]).
Both handheld chlorophyll meters used in the present study operate in a similar way, with small differences. Although SPAD-502 measures absorption from leaves at two wavelengths, 660 nm (red) and 940 nm (near-infrared), ClorofiLog measures the absorption at three wavelengths, 635 nm and 660 nm (red) and 880 nm (near infrared - [1], [29]). We expected ClorofiLog, which measures a third wavelength near the second absorption peak of Chl b (642 nm), to provide greater accuracy for chlorophyll estimates. However, the values of R2 and RMSεc for both devices were similar, which means that SPAD-502 and ClorofiLog provide equally accurate chlorophyll estimates. Additionally, the indices provided by these devices (FCI and SPADi) showed a strong linear relationship. This result has an important application. Most regression models available in the current literature predicting chlorophyll content in tropical trees have been developed using SPAD-502 ([20], [8], [23]). ClorofiLog is newer and is currently being used more frequently in some countries. Based on the results from our study, the data from ClorofiLog can now be safely converted to the SPAD index and used for the estimation of chlorophyll content using regression with the models already available in literature ([20], [8], [23]).
The α parameter of the model described by Coste et al. ([8]) for Chl t (1.171 mg m-2) was similar to values found in our study for both chlorophyll meters, but the value of β (148.84) was lower than the values found in our study. Compared with the model reported by Coste et al. ([8]), our model would result in an underestimation of the chlorophyll content as the SPADi values increase. We attribute these discrepancies to differences in the maximum values of chlorophyll contents. In the present study, the maximum value of Chl t was 815 mg m-2 (B. rubescens), while Coste et al. ([8]) reported maximum values of Chl t around of 1500 mg m-2. The differences in chlorophyll content can be explained by differences in the ontogenetic stages of the plants, environmental conditions, and species used in the two studies. The plants used by Coste et al. ([8]) were around 2 years old, whereas the plants used in the present study were 2-4-months old. Different studies have shown that seedlings of tropical trees have lower nitrogen and chlorophyll contents per leaf area unit than adults ([16], [15]). Differences in chlorophyll content can also be explained by the distinct environmental conditions in which plants are grown. In the present study, seedlings were grown in nursery conditions, under 50% of full sun, whereas the plants studied by Coste et al. ([8]) were grown under three different light treatments: 5%, 10%, and 20% full sun. The reduction in light intensity induces an increase in chlorophyll content of leaves ([25]), which may explain the higher chlorophyll content reported in the study of Coste et al. ([8]). Although the maximum values of Chl t found in the present study were lower than the values reported by Coste et al. ([8]), they are in agreement with values reported for other tropical trees, for instance, Scleronema micranthum (600 mg m-2), Swietenia macrophylla, and Ceiba pentandra (900 mg m-2 - [20]). Additionally, the β values calculated individually for each of the 13 species ranged from 88.2 (Cecropia obtusa) to 299.2 (Eperua falcata), with a large dispersion of data in the SPADi range between 40 and 60, and chlorophyll content between 500 and 1000 mg m-2 ([8]). Based on this, the model proposed by Coste et al. ([8]) can be applied to different indices and equipment, but the range of chlorophyll contents should be taken into account.
The average RMSεc value calculated individually for each species is low compared with the values available in literature ([28], [8], [24]), indicating the high accuracy of our models. The average values of RMSεc for Chl a, b, and t in the present study were lower than 10%. Richardson et al. ([28]), for example, reported values of RMSεc for ND705 varying from 11.9% (Chl a) to 15.2% (Chl b), and values of RMSεc for SPADi from 15.6% (Chl b) to 20.3% (Chl a). The RMSεc values lower than 10% prove the feasibility of the multi-species model for estimating Chl a and b from hand-held absorbance chlorophyll meters and leaf reflectance. Our highest value of RMSεc was obtained for the estimation of Chl b in T. guianensis (18.7%). Yet, this value is similar to those reported by Richardson et al. ([28]) for estimates of Chl b in Betula papyrifera, using different hand-held chlorophyll meters and leaf reflectance indices (14.6% to 36.2%). It was also similar to values of RMSεc (14.6% to 29.2%) calculated by models based on reflectance indices in Eugenia uniflora by Mielke et al. ([24]).
Neotropical rainforests are recognized for their high biodiversity ([22]) and increasing human pressures. The growth of human populations and changes in land use are the main causes of forest degradation and losses of biodiversity. Sustainable use of forest remnants and restoration of degraded forest ecosystems are challenges for scientists and civil organizations in the tropics ([6]). Therefore, the suitability of scientific technology for the improvement the production and plantation of seedlings of native tree species is crucial to the success of commercial forest plantations and ecosystems restoration programs ([12], [3]). In the present study, we showed the feasibility of the multi-species model proposed by Coste et al. ([8]) to estimate leaf chlorophyll content with the use of different instruments, aiming the application at seedling production in forest nurseries and monitoring the physiological performance of seedlings after introduction to reforestation areas. All indices tested in the present study showed excellent fits for Chl t, b, and for Chl a in particular. We also demonstrated that the SPADi and FCI indices can be safely interconverted.
Acknowledgements
The authors thank Gerson J. Sales Neto, Nilson A. dos Santos, and Rones F. Souza of Floresta Viva Institute, and M.Sc. Murilo F. C. de Jesus for assistance with data collection. We thank Dr. Fábio P. Gomes of DCB/UESC for providing the SPAD-502 used in this study. Funding for Daniela V. Silva during this study was provided by a scholarship from Capes (Brazilian Higher Education Council). Marcelo S. Mielke and Ediófila Brito-Rocha gratefully acknowledge fellowships provided by CNPq (Brazilian National Council for Scientific and Technological Development). This study was supported by CNPq (Proc. 561933/2010).
References
CrossRef | Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Departamento de Ciências Agrárias e Ambientais, Universidade Estadual de Santa Cruz, Rodovia Jorge Amado km 16, Ilhéus, Bahia (Brazil)
Ediófila Brito-Rocha
Andrea Carla Dalmolin
Marcelo S Mielke
Departamento de Ciências Biológicas, Universidade Estadual de Santa Cruz, Ilhéus, Bahia (Brazil)
Corresponding author
Paper Info
Citation
Viera Silva D, Dos Anjos L, Brito-Rocha E, Dalmolin AC, Mielke MS (2016). Calibration of a multi-species model for chlorophyll estimation in seedlings of Neotropical tree species using hand-held leaf absorbance meters and spectral reflectance. iForest 9: 829-834. - doi: 10.3832/ifor1785-009
Academic Editor
Silvano Fares
Paper history
Received: Jul 30, 2015
Accepted: Jan 17, 2016
First online: May 17, 2016
Publication Date: Oct 13, 2016
Publication Time: 4.03 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2016
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 49127
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 39933
Abstract Page Views: 3079
PDF Downloads: 4520
Citation/Reference Downloads: 66
XML Downloads: 1529
Web Metrics
Days since publication: 3411
Overall contacts: 49127
Avg. contacts per week: 100.82
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2016): 9
Average cites per year: 0.90
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Relationships between leaf physiognomy and sensitivity of photosynthetic processes to freezing for subtropical evergreen woody plants
vol. 12, pp. 551-557 (online: 17 December 2019)
Research Articles
Spectral reflectance properties of healthy and stressed coniferous trees
vol. 6, pp. 30-36 (online: 14 January 2013)
Research Articles
Effect of Funneliformis mosseae on growth, mineral nutrition, biochemical indexes and chlorophyll content of Ziziphus spina-christi seedlings at different salinities
vol. 9, pp. 503-508 (online: 08 December 2015)
Research Articles
Wintertime photosynthesis and spring recovery of Ilex aquifolium L.
vol. 12, pp. 389-396 (online: 31 July 2019)
Research Articles
Magnolia grandiflora L. shows better responses to drought than Magnolia × soulangeana in urban environment
vol. 13, pp. 575-583 (online: 07 December 2020)
Research Articles
Sap flow, leaf-level gas exchange and spectral responses to drought in Pinus sylvestris, Pinus pinea and Pinus halepensis
vol. 10, pp. 204-214 (online: 01 November 2016)
Research Articles
Links between phenology and ecophysiology in a European beech forest
vol. 8, pp. 438-447 (online: 15 December 2014)
Research Articles
Leaf transpiration of drought tolerant plant can be captured by hyperspectral reflectance using PLSR analysis
vol. 9, pp. 30-37 (online: 05 October 2015)
Research Articles
Adaptive variation in physiological traits of beech provenances in Central Europe
vol. 11, pp. 24-31 (online: 09 January 2018)
Short Communications
Effect of four levels of shade on survival, morphology and chlorophyll fluorescence of Nothofagus alessandrii container-grown seedlings
vol. 8, pp. 638-641 (online: 08 January 2015)
iForest Database Search
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords