The forest biodiversity artery: towards forest management for saproxylic conservation
iForest - Biogeosciences and Forestry, Volume 9, Issue 2, Pages 205-216 (2015)
doi: https://doi.org/10.3832/ifor1657-008
Published: Oct 26, 2015 - Copyright © 2015 SISEF
Review Papers
Abstract
One of the objectives of forest conservation is the set aside of unharvested areas. However, the fragmentation and lack of connectivity of protected areas make the integration of conservation measures in productive forests essential. Strategies to integrate conservation of saproxylic biodiversity in forest management have been developed, but often considering only specific aspects or remaining preliminary otherwise. As the impact of climate change and anthropogenic stresses increases, the development and the synthesis of this approach is crucial. We reviewed the key literature on forest management for biodiversity conservation, integrating forest science perspective to provide a practical management framework. Our goal is to present a management framework that could contribute to the effective preservation of forest insect biodiversity at the landscape scale, without high economic efforts, and addressing the conflicts that still jeopardize sustainable forest management. The results of our review support the creation of micro-reserves inside productive forests, to support large reserves in landscape conservation strategies. Micro-reserves increase the resilience of forest ecosystems to anthropogenic disturbances, through the development of a heterogeneous structure, maximizing microhabitat availability. Modeling forest management and harvest on local natural disturbance would extend the benefits of spatio-temporal heterogeneity in productive forests. Variable retention harvest systems, applied at the landscape scale, are a feasible and adaptable strategy to preserve and increase biodiversity, safeguarding structural legacies such as senescent trees and deadwood inside the productive matrix. The operational shift, from the stand to the forest landscape, is fundamental to extend the benefits of conservation measures. The Forest Biodiversity Artery, composed by several micro-reserves or îlots de senescence, connected by corridors of habitat trees and deadwood, constitutes a network that would deliver old-growth forests attributes to the productive matrix. This planning instrument would support forest connectivity, and socioeconomic constraints.
Keywords
Biodiversity, Deadwood, Gap, Habitat Tree, Integrative Conservation, Landscape, Microhabitat, Retention
Introduction
Changes in land-use confined old-growth forest to less than 0.5% of the forested area in Europe, United States and China ([173]). Structural and compositional changes that influenced forests in the last centuries brought a heavy depletion of deadwood ([68]). Old-growth and deadwood-associated species (i.e., saproxylic) are among the most threatened in temperate forest ecosystems ([55]). Species that require resources which have become rare in productive forests, such as large deadwood and open canopy areas ([148]), may face a high risk of extinction. The uncertainties associated with future climate and anthropogenic stresses make the development of new forest conservation strategies imperative ([105], [142]).
Traditionally conservation efforts brought the employment of natural forest as a model for restoration ([74]) and the set aside of unharvested areas rather than the restoration of harvested forests ([6]). Over the last 10 years the forest area allocated for biodiversity conservation in Europe increased of about half a million hectares every year ([43]); however, these reserves tend to be isolated and surrounded by intensively managed landscapes ([19]). Since state reserves cannot guarantee the survival of forest biodiversity ([104]), the integration of conservation measures in productive forests represents an urgent and vital intervention.
Since the establishment in 1811 of the Royal Saxon Academy of Forestry in Tharandt by Heinrich Cotta, the concept of sustainability, in its former sense of a sustained yield of wood, has been a leading principle for managing forests in Europe ([134]). The intensity of wood harvest depended on forest growth potential, to ensure a continuous supply of timber for various uses. Ecosystem approaches have been part of European forestry since its origin as a science in the 18th century, through the comprehensive study of forest ecology ([141]). Still, there is often a disparity between traditional silviculture and the complexity of forest ecosystems ([14]). Our aim was to derive a framework to preserve saproxylic diversity, relying on the last 20 years of literature on biodiversity conservation in productive forests. The proposed management strategies were derived answering the following questions: (1) which features of old-growth forests, fundamental for saproxylic conservation, could be feasibly applied to productive forests? (2) which harvest methods could be better reconciled with saproxylic preservation? (3) what are the current strategies to conserve saproxylic biodiversity in productive forests? and (4) what should be the scale of intervention?
Methods
For the selection of the reviewed studies, Oldeman ([115]), Kohm & Franklin ([71]) and Lindenmayer & Franklin ([86]) works were used as guiding principles. Their works provided the terms listed in Tab. 1, which were used to interrogate three databases: (i) ISI Web of Science; (ii) Scopus; and (iii) Google Scholar. The obtained literature was then screened: no restrictions related to the time since publication were applied, whereas the selection was based on the relevance of the studies, focusing on European forests. The term “old-growth” is used in this article for both managed and unmanaged forests in Europe and generally applies to stands with trees older than 200 years, therefore “old-growth” is not synonymous of “virgin” ([124]).
Tab. 1 - Used term combinations for the literature search. (*): wildcard character used for boolean search.
| Term 1 | - | Term 2: Management | - | Term 2: Conservation |
|---|---|---|---|---|
| Forest | - | Certification | - | Biodiversity |
| Saproxylic* | AND | Disturbance | OR | Biodiversity conservation |
| Dynamic | Deadwood | |||
| Eco unit | Habitat tree* | |||
| Gap harvest | Hollow tree* | |||
| Landscape approach | Îlot* de sénescence | |||
| Landscape management | Old-growth | |||
| Plantation | Structural legacies | |||
| Retention harvest | Tree retention | |||
| Sustainable management | Tree microhabitat* |
Forest management and conservation
Translating old-growth forest features into management principles
After the 1970s, the idea that forests were constant and stable systems was abandoned, recognizing the complexity associated to the different kinds of disturbance ([74]). Oldeman ([115]) seminal work highlighted how natural disturbances (e.g., wind storms, flooding, biotic-induced tree mortality) represent crucial processes and a permanent element of natural forest ecosystems. Far from being negative to the forest, these different types of disturbances are one of the main drivers of biological diversity. Stand heterogeneity is the result of gap dynamics, which is in turn influenced by small-scale (e.g., death of a single tree) and high-energy (e.g., wind throw) events ([12]). The interaction between climate, terrain and natural disturbance generates the spatial and structural heterogeneity of unmanaged forests ([86]). In shady and coarse woody debris (CWD) deprived managed forests, wind throw gaps act as biodiversity hotspots, providing deadwood and openings, which offer micro-habitats and trophic resources for a range of organisms ([17]). The early-seral stages post disturbance, with the occurrence of natural regeneration, have a positive influence on saproxylic composition ([61], [171]). Furthermore, the results presented by Beudert et al. ([11]) show that the “benign neglect” approach towards pest outbreaks not only increases the overall biodiversity of a site but also does not threat water quality in watersheds. Disturbance remnants, i.e., patches of surviving trees, may play a fundamental role in the recovery of large-scale and high-energy disturbances ([151]).
Oldeman’s view roots on the subdivision of forests in eco-units, forest areas whose development started at a unique moment in time, and “which architecture, eco-physiological functioning and species composition are ordained by one set of trees until the end”. Even if the pathways of forest development are countless and influenced by chance, succession models have been derived to group them and summarize the involved processes ([38]). Oldeman ([115]) identifies four phases forming the sylvatic mosaic: (i) innovation, (ii) aggradation, (iii) biostatic, and (iv) degradation. Likewise, Larrieu et al. ([80]) compared data from 32 unharvested European mountain mixed forests, and experimentally identified five forest development phases (FDPs): (i) regeneration, (ii) establishing, (iii) growing, (iv) culmination, and (v) disintegration. Hence, the forest is a mosaic of patches, characterized by their evolution and maturity ([144]): this dynamics in space and time contribute to the development of a heterogeneous environment, characterized by multiple functional cycles (i.e., silvigenic, humus, pedofauna - [4]). The sylvatic mosaic (sensu [115]) of a temperate natural old-growth forest is characterized by a kaleidoscope of different kinds of eco-unit in dynamic equilibrium: the structural difference between even-aged (managed, mixed or pure) and uneven-aged forests (natural forest) is related to the size and spatial distribution of the tree cohorts ([115]).
A silvicultural practice based on the occurrence of the different eco-units or FDPs, established considering reference conditions, would sustain structural biodiversity conservation ([172]). The explicit inclusion of natural eco-units or FDPs in forest management planning could favor the incorporation of the often missing culmination and disintegration phases, fundamental to guarantee high levels of microhabitats and deadwood ([82]). Furthermore, natural early-seral stages can present old-growth forest structural attributes, thus where forest management focuses on ecological complexity this phase should be valued as well ([38]).
The maintenance of natural forest regeneration, together with the protection of soil productivity, represent the heart of nature-based silviculture ([14]). From the early conceptualizations of a close-to-nature silviculture ([116]), it was clear that the application of a general panacea model was not appropriate: local, regional and temporal features of forests should act as guiding management principles. Models should favor the resilience of the forest, through the development of a heterogeneous structure, mimicking natural disturbances through gap harvest ([139], [32], [93], [94]). As it has been stressed by Shorohova et al. ([154]), the management and restoration of old growth-forests cannot be accomplished reproducing a static phase; conversely, it should focus on the emulation of natural disturbances and successional dynamics. Besides, silvicultural treatments that emulate natural disturbances, creating gaps in multi-aged forests, may increase resistance and resilience to future disturbance ([114]).
Senescent and dead trees, together with snags and logs left on the forest floor, constitute what Franklin et al. ([45]) defined “structural legacies”, i.e., elements that increase the post-disturbance complexity and provide habitat and food resources, promoting the survival and re-establishment of forest organisms ([5]). Regardless the considered geographic scale, the amount of available deadwood influences the functional composition of saproxylic organisms ([53]). Despite the inclusion of deadwood volume among the improved Pan-European indicators for sustainable forest management ([99]), “forest hygiene” still threatens its preservation. However, Stokland et al. ([157]) stressed that both the risk for visitors and that of fires could be reduced by management, and that pest outbreaks (e.g., Ips typographus) represent exceptional events related to lowland spruce introduction and introduced pest species.
Gossner et al. ([53]) argue that close-to-nature approaches may fail the attempt of preserving saproxylic beetle biodiversity, disregarding the relevance of large diameter and late stage of decay deadwood availability. The concurring effects of increased harvest, biomass extraction, and assumed pest management reduced the volume of available deadwood by 90% ([65]). Furthermore, the landscape scale removal of fuel wood endangers saproxylic organisms and other organisms affected by the physical and chemical changes of soil properties (see [18] for a review).
The productive forest matrix and its controversial role for biodiversity preservation
Even if the threat represented by the management of a single forest stand is rather limited, the cumulative effect of these activities across a landscape may be significant ([167]). Furthermore, the management intensity affects not only the present state of the stand but also its temporal sequence of states ([143]). The results obtained through the silvicultural management intensity indicator by Schall & Ammer ([143]) highlighted that modern continuous-cover systems, such as selective logging, may preserve productivity without an intense environmental alteration. Nevertheless, canopy closure can affect the distribution of heliophilous species, thus the effect of the amount of retained trees on microclimate should be tested ([162]).
The variable retention harvest
In retention forestry, a part of trees and snags are left after the harvest, to create in young successional forests structures similar to those created by natural disturbance ([60]).The variable retention harvest (VRH), retaining structural, functional and compositional elements after the harvest, ensures: (1) lifeboating species that would otherwise disappear from a logged site, (2) the increase of stand structural complexity, and (3) the enhancement of forest landscape connectivity ([44], [56]). The presence of a canopy closure gradient ensures the presence of a richer saproxylic fauna ([166], [52], [83]). Together with agro-forestry, retention forestry is considered an effective approach to reduce the conflict between biodiversity conservation and socio-economic needs at the landscape scale ([135]). The suitability of the VRH in multifunctional forestry is guaranteed by its adaptability: it can be adjusted to meet different objectives and include distinctive local features, ranging from forest types to regional policy ([56]). Gap-based approaches can be modeled on natural disturbance regimes: calibrating spatio-temporal components on local conditions in order to reach an effective and adaptive management ([139], [32], [100], [86], [94]). There are several applications of this method (few examples are listed in Tab. 2) showing their diversified extent, aims and adaptability. Partial cuts may also have the advantage of ensuring the continual availability of deadwood, retaining large trees and snags through rotations ([42]). The limits of retention forestry in preserving biodiversity include the lack of large-scale disturbances, as those generated by fire ([164], [60]) and other chronic natural disturbances as windthrow, drought, heavy snowfalls, flooding or biotic factors as diseases and defoliations ([124]).
Tab. 2 - Examples of gap based harvest with variable retention systems, applied to different forest types and various objectives.
| Forest type |
Aim | Harvest method |
Unit size |
Source |
|---|---|---|---|---|
| Boreal forest | Study the effect of green tree retention on forest biota |
Retain 0, 10, 50 m3 ha-1 of the standing volume |
3-5 tree groups | Hyvärinen et al. ([62]) |
| Boreal mixed forests | Increase the density and vigour of spruce in aspen-dominated mixed woods |
Alternate harvested and unharvested corridors |
10 m wide | Man et al. ([91]) |
| Boreal mixed forests | Evaluate regeneration after partial cutting and natural disturbance |
Remove 47.9-63.2% of pre-harvest basal area |
400 m2 | Gendreau-Berthiaume et al. ([49]) |
| Boreal mixed forests | Emulate natural disturbance, maintain beetle diversity | Remove 10-20% of the canopy in harvest gaps |
0.1-0.2 ha | Thomas et al. ([161]) |
| Boreal riparian forest | Emulate natural disturbance, increase habitat complexity through early succession forest regeneration | Partial harvest, up to 50% of basal area |
10 - 400 m2 | Mallik et al. ([90]) |
| Floodplain forest | Natural reserve management emulating disturbances, saproxylic species conservation | Randomized small-gaps cutting with retention elements, waterlogging | 250-300 m 2 | Mason ([94]) |
| Mixed hardwood forest | Restore and maintain ground-layer vascular plant diversity in a second-growth forest | Dormant season timber harvesting in the gap and thinning outside |
0 to 46 m of diameter | Kern et al. ([69]) |
| Mixed temperate forests and plantations | Limit environmental impact, emulate disturbance, re-establish multi-layer structure and re-naturalization of conifer plantations | Eco-units with retention of deadwood | 300-1000 m 2 | Mercurio et al. ([101]) |
| Spruce-fir (Picea abies - Abies alba) | Strengthen forest resilience to climate change increasing structural irregularity | Group selection with <50% of harvested trees (minimum diameter 52.5 cm) |
500 m2 | Lafond et al. ([78]) |
| Lenga beech (Nothofagus pumilio) | Assess regeneration under different microenvironmental conditions | Retain evenly distributed dominant trees between aggregates | 30 m radius aggregates | Pastur et al. ([119]) |
The plantation opportunity
The general view on plantations is that they represent a sort of green desert. However, they can actually represent a potential habitat for biodiversity, since they resemble the structural complexity of natural forests more than other more intense land uses ([48]). Their ecological value depends on the previous land use, alternative land uses, species involved and purpose ([21]). Where reforestation entails the replacement of natural forests, plantations may be detrimental for conservation, whereas if established on anthropogenic grasslands that were once forested, they could confer environmental benefits to the landscape ([121]). Plantations should be designed to deliver socio-economic benefits while providing ecological services, and should be included in a broad scale land-use planning ([118]).The extent and productivity of forest plantations are increasing worldwide, and the application of ecosystem approaches represents the vital and feasible strategy to support social and ecological outcomes ([141]). Koch Widerberg et al. ([70]) suggest that the retention of oaks and deadwood in spruce plantations, along with the creation of small clearings, could represent a cost-effective intervention to preserve saproxylic beetle diversity. Plantations could contribute to the land sparing approach, segregating production in these areas and focusing conservation efforts in high quality natural habitats ([125], [165]). Land-sparing logging could be an effective strategy to preserve forest biodiversity in areas of large-scale interventions ([96], [40]).
Current strategies: forest micro-reserves
Conservation efforts in productive forests include the protection of “tree islands”, where no harvest takes place ([2]). The îlot de senescence (IdS), described by Lachat & Bütler ([76]), is a small and permanently unmanaged patch, distributed throughout managed forests. The IdS are a stepping stone that provide suitable habitat for saproxylic organisms. The retention of these groups of trees within a harvested forest ensures the availability of present and future deadwood, favoring both species with reduced dispersal and meta-community dynamics ([107]). Lachat & Bütler ([76], [77]) presented a practical design of forest conservation network, arguing that the conservation of saproxylic organisms in Switzerland could be achieved through the integration of large protected areas, IdSs and habitat trees. Further conceptualizations were presented by Cateau et al. ([27]) for the Mont Ventoux (France), based on the balance between ecological, economic and social issues. For Fennoscandian countries, a similar setting aside of small habitat patches of productive forest to preserve landscape-level biodiversity was carried out from 1992 ([159]), naming these areas “Woodland Key Habitats” ([163]). The small area of the IdS (ranging from one ha to a maximum of 20 ha) should not discourage on their effectiveness: small reserves can target high-quality remnants ([54]), and their contribution to biodiversity conservation is increased by the fact that they are in non-wilderness areas ([86]). Where the establishment of protected areas is economically infeasible, biodiversity preservation could be achieved integrating reserves and managed matrix ([8], [154]). A further contribution could be represented by large marginal forests where management has been abandoned for socio-economic reasons ([156]). Nevertheless, the role of large reserves for the preservation of undisturbed ecosystems in optimum conditions is irreplaceable and cannot be granted by the matrix management alone ([86]): micro-reserves and natural reserves fulfill complementary functions and should be considered together in conservation strategies ([159]).
Scale of intervention: forest landscape conservation
Saproxylic species assemblages that characterize forest environments live in association with their surroundings, depending on heterogeneous conditions in time and space ([160]), and responding to substrate availability at various spatial scales ([10]). The effectiveness of a micro-reserve depends on the intensity of the edge effect and the consequent disturbance (e.g., micro-climate alteration), as well as on the availability of “ecological memory” ([8]), which ensures the resilience of a landscape through buffering and renewal. Planning and management need to be implemented at multiple spatial scales, from single trees to key habitats and finally to large reserves ([88]).
The general definition of stand, as an area characterized by its internal uniformity in terms of “composition, age, arrangement or condition” ([111]) diverges deeply from Oldeman’s eco-unit. Forest ecosystem management at the stand level is a laudable objective, but alone it cannot ensure landscape biodiversity conservation ([9]). Hence, overcoming the concept of stand is fundamental to increase forest landscape permeability, favoring habitat diversity and reducing intensive land uses ([142]), as well as being flexible enough to accommodate wood market modifications ([150]). The coordinated management of the components of a forest landscape ensures a more cost-effective and long-term use of conservation resources ([22]). Forest management should take into account that the spatial configuration of forest patches affects natural dynamics, ecosystem services and the sustainability of the extracted products ([67]). The extension of conservation measures in commercial and plantation forests needs a major operational shift: from stand-focused silviculture to a forest management that recognizes the landscape as the working unit ([121]).
A synthesis: the forest biodiversity artery and its incorporation in productive forests
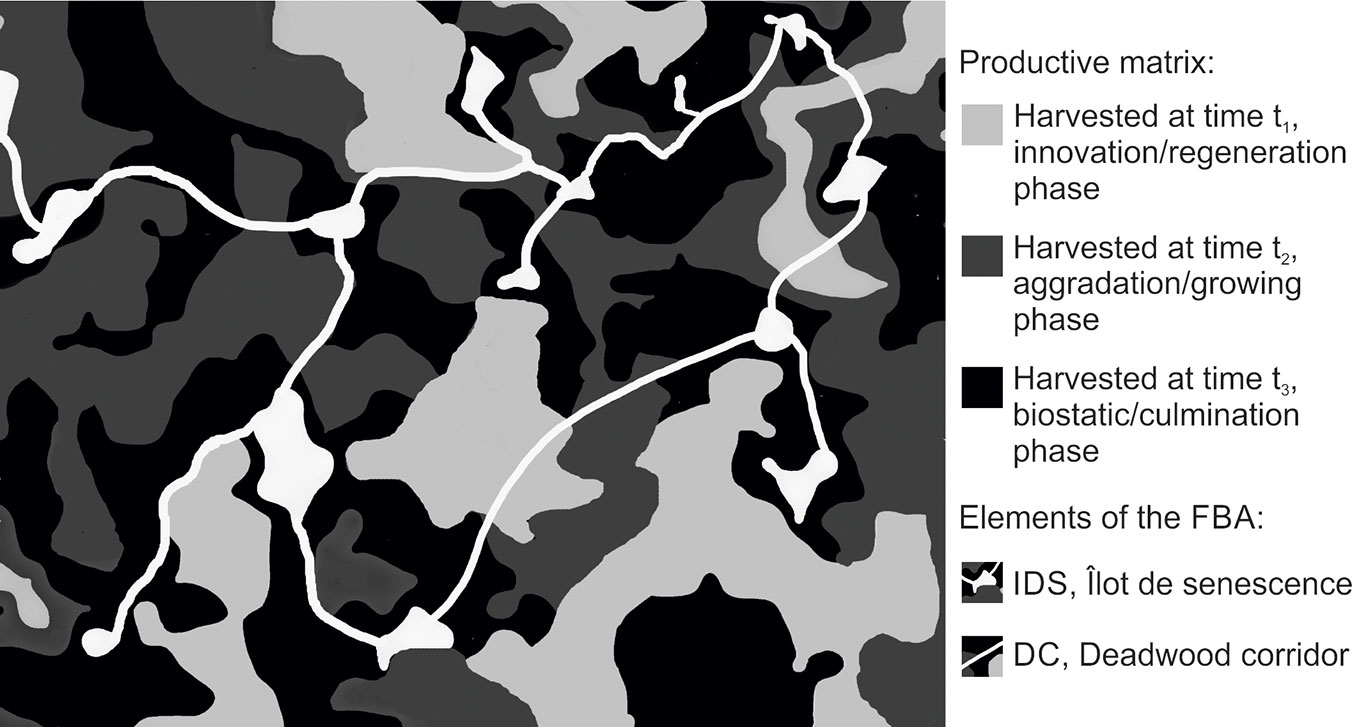
For the effective preservation of forest biodiversity, an extensive strategy integrating reserves and matrix management must be developed ([86]). The framework presented here aims at reaching an operational integration of biodiversity conservation in multi-functional forestry, and consists of two elements: a forest matrix managed with VRH in which the Forest Biodiversity Artery (FBA) is embedded. It represents an integrative conservation approach (sensu [13]), which seeks to deliver old-growth forest attributes to the entire forest matrix. Structural legacies would be retained indefinitely inside the productive matrix, calibrating their number on the natural disturbance regimes and eco-unit/development phase. The FBA should be composed by several IdSs, connected each other by strips of similar habitats (habitat trees and deadwood corridors - DC). In the long term, the FBA system would constitute a network of old-growth forest patches integrated within productive forests (Fig. 1). The practical application of the proposed framework has been built up on the review presented in the previous chapters and is based on five main points, described below, which would ensure the simultaneous presence of the elements listed in Tab. 3.
Fig. 1 - Representation of the proposed management system: the Forest Biodiversity Artery (FBA). The FBA is embedded in the productive matrix, showing the Deadwood Corridors (DC) and the îlots de senescence (IdSs). The productive forest matrix would be managed with gap harvest, with different eco-units or forest development phases. Landscape planning would facilitate the simultaneous presence of innovation/regeneration, aggradation/growing and biostatic/culmination phases (sensu [115] and [82], respectively). Deadwood thresholds and number of veteran trees in the productive matrix would be calibrated on the eco-unit/forest development phase, considering natural disturbance. The FBA would sustain saproxylic biodiversity providing high amounts of habitat trees and deadwood, diversified for decay and type. The landscape distribution of IdSs and DC would be established in order to ensure connectivity without affecting harvest operations (e.g., taking advantage of rides and glades, favoring sheerest areas).
Tab. 3 - Key elements favoring the preservation of forest biodiversity at the landscape scale.
| Element | Function | Characteristics | Source |
|---|---|---|---|
| Îlot de senescence (IdS) | Conserving biodiversity in commercial forests | Part of the sylvatic mosaic, size established considering local disturbance dynamics. High availability of deadwood and veteran trees, never harvested |
Lachat & Bütler ([76]) |
| Deadwood corridor (DC) |
Favoring the connections and meta-community dynamics | Approximate interior forest conditions | Warren & Fuller ([169]) |
| Variable retention harvest (VRH) | Sustaining productive values without compromising ecosystem services | Gap harvest, with the retention of living structures and deadwood | See Tab. 2 |
| Structural legacies |
Lowering critical trophic and raising microhabitat resources | Coarse woody debris: chablis or uprooted trees, volis or snags, high and low stumps | Hedgren ([59]), Abrahamsson & Lindbladh ([1]) |
| Habitat tree |
Life boating for species and ecosystem processes | Green-tree retention in the harvested gaps, preserving over-mature trees (DBH>90 cm) in the matrix and, indefinitely in the FBA |
Franklin et al. ([44]), Lachat & Bütler ([76]) |
Incorporating natural disturbance
Learning and inferring from natural models are key instruments to reach both economic production and biodiversity conservation ([86]). The amount and severity of disturbances associated with climate and land use changes has raised over the last decades, and understanding how these drivers interact is a prerequisite of their mitigation ([149]). The frequency and severity of natural disturbance (both at regional and local level) can constitute the preliminary data to establish retention approaches ([137]) and small gap cutting treatments (Tab. 4). Disturbance regimes could be classified considering how probabilistic (e.g., fire intervals for a forest type) and random (e.g., where the fire happens) events interact ([3]). The integration of these processes through spatio-temporal scales offers the means for the development of a more adaptive management ([112], [149]), strengthening ecosystem resilience ([31], [105]). Seidl et al. ([152]) applied future scenario simulations and statistical modeling to show the upcoming intensification of disturbance regimes (due to fire, bark beetles and wind) under climate change, if management strategies remain unchanged. The deadwood created by these natural disturbances represents a valuable resource, which tends to host a higher biodiversity compared to deadwood artificially created ([72]). Hence, policy measures should consider natural disturbance as a free conservation tool, allowing natural recovery and limiting salvage logging ([11]). For example, the model LANDIS-II (⇒ http://www.landis-ii.org/) may simulate forest succession and natural/anthropogenic disturbances at the lansdcape level, and could be a useful tool for conservation planning ([84]). Managing a forest in terms of eco-units could represent a useful approximation that could allow the inclusion of the diversity of compositional and structural phases. Among the selective cutting techniques, the most suitable treatments to restore the natural dynamics of the eco-units, characterizing the main part of the natural European forests ([124]), is represented by variable retention harvest (VRH).
Tab. 4 - Types of the small patch cutting as reviewed by Mercurio ([100]). (D): larger diameter of the eco-unit; (H): predominant height of the trees in the eco-unit treatment; (NA): not available.
| Eco-units | Source | |
|---|---|---|
| Size (m2) | Shape | |
| 500-1000 | D = ½ H | Cappelli ([26]) |
| < 400-500 | Circular | De Philippis ([36]) |
| 600-1500 | 1-1.5 H | Del Favero et al. ([35]) |
| NA | NA | Pavari ([120]) |
| 500-1500 | NA | Perrin ([123]) |
| 1000-1500 | D =1-1.5 H | Piussi ([126]) |
| NA | D = 2 H | Roussel ([138]), Rojo Saiz ([136]), Boudru ([16]), Mattews ([102]) |
Criteria for the selection of retained structures
Tree microhabitats are diverse (e.g., wounds, sap exudations, fungi fruiting bodies, cavities, cankers, bark pockets, etc.) and host a rich fauna ([155]). Biodiversity could be preserved safeguarding valuable structures such as snags and veteran trees ([132], [168]), which are particularly prone to develop such microhabitats. The same method adopted for selective logging ([51]) should be applied to identify the retained structures, focusing on the phenotypes showing age-related features, such as those identified by Lindenmayer & Franklin ([86]): large-diameter trees (ensuring the development of deadwood cavities per se), complex canopies, rough bark. The criteria that could be applied for the selection of future habitat and hollow trees are listed in Tab. 5. Trees selected according to these characteristics would ensure a life-boating function (increasing microhabitat availability) and a structural enrichment (enhancing canopy and vegetation structure complexity - [45]). The high value of a tree for biodiversity can also be determined focusing on its age, size and crown shape, or on the rarity of the species and its low mortality ([137], [168]). Allowing a sub-population of trees to complete their natural cycle would ensure both the presence of microhabitats and the volume and diversity of deadwood ([81]). Large senescent trees could be excluded from the harvest applying DBH thresholds, thus the scarce high ecological value elements still present in productive forests would never be logged ([2]). These elements have the key role of promoting the colonization of harvested sites, thus they should be as many as to sustain the species in this process ([6]). Management recommendations for the preservation of these structures are listed in Tab. 6.
Tab. 5 - Characteristics that increase the probability of microhabitat and hollow formation.
| Tree species | Characteristics | Focus | Source |
|---|---|---|---|
| Abies alba, Fagus sylvatica | Minimum DBH: beech>90 cm, fir>100 cm | Montane forests | Larrieu & Cabanettes ([79]) |
| Eucaliyptus sp. | Stage of senescence, tree form and DBH determine the occurrence of hollows | Model probability of hollow occurrence | Rayner et al. ([131]) |
| Eucalyptus marginata | Tree heights> 19 m, tree DBH>45 cm | Reptiles | Craig et al. ([33]) |
| Eucalyptus sp., Corymba sp. | DBH>110 cm | Reptiles | Croak et al. ([34]) |
| Fagus sylvatica, Picea abies | Minimum DBH: beech>72 cm, spruce>43 cm; the number of microhabitats increases for DBH>68 cm |
Saproxylic beetles | Larrieu et al. ([81]) |
| Fagus sylvatica, Picea abies | Minimum DBH: beech>50 cm, spruce>65 cm | Saproxylic beetles | Larrieu et al. ([80]) |
| Fagus sylvativa, Quercus sp., Abies alba, Picea abies | Snags host more microhabitats than living trees. More microhabitats on oaks than on the other species | Biodiversity | Vuidot et al. ([168]) |
| Nothofagus pumilio | DBH 60.6 ± 16.4 cm | Birds | Diaz & Kitzberger ([37]) |
| Pinus sylvestris | Minimum DBH for snags created from Scots pine to provide suitable cavity nest for birds and wildlife >40 cm | Birds and wildlife | Summers ([158]) |
| Pseudotsuga menziesii | DBH≥70 cm | Birds and mammals | Michel & Winter ([103]) |
| Quercus ilex, Q. pubescens | Good predictors: time since last cutting, diameter class. Non-coniferous species have more microhabitats |
Birds, mammals and saproxylic beetles | Regnery et al. ([133]) |
| Quercus robur | 50% chance of hollow presence for trees 258 years old | Invertebrates, birds and mammals |
Ranius et al. ([130]) |
| Various | High DBH; balanced and pointed crown; straight trunk; dominant trees; local patterns related to altitude and slope | Wildlife conservation | Gibbons & Lindenmayer ([50]) |
Tab. 6 - Management recommendations for the preservation and increase of habitat and hollow trees.
| Tree species | Recommendations | Focus | Source |
|---|---|---|---|
| Abies alba, Fagus sylvatica | Favor mixed forests:differentiate microhabitats. Allow that a part of the trees complete their natural cycle |
Montane forests | Larrieu & Cabanettes ([79]) |
| Eucalyptus sp. | Time lag in the use of hollow-bearing trees in harvested areas: ensure landscape availability | Mammals | Cawthen & Munks ([29]) |
| Eucalyptus sp. | Lag effects on the availability of hollows and conservation scenarios: model number of potential trees of high DBH/age | Birds | Manning et al. ([92]) |
| Fagus sylvatica | Take into account spatial distribution, avoiding safety conflicts | Saproxylic beetles | Müller et al. ([109]) |
| Fagus sylvatica, Picea abies | Include a sub-population (10-20% of the surface area) that will complete the natural silvigenetic cycle | Saproxylic beetles | Larrieu et al. ([81]) |
| Fagus sylvatica | Preserve and create pollard trees | Saproxylics | Cantero et al. ([25]) |
| Pseudotsuga menziesii | Microhabitat abundance is higher with low treatment history. Increase structural complexity: group harvest, small gap creation, retention of biological legacies |
Birds and mammals | Michel & Winter ([103]) |
| Quercus ilex, Q. pubescens | High stem density has a negative effect | Birds, mammals and saproxylic beetles | Regnery et al. ([133]) |
| Quercus robur | Hollows generated early in fast-growing trees | Invertebrates, birds and mammals |
Ranius et al. ([130]) |
| Quercus rubra, Platanus hybrida, Juglans nigra | Guidelines for the artificial creation of microhabitats | Saproxylics | Mason et al. ([95]) |
| Salix alba | Pollarding increases the probability of hollows formation | Saproxylic beetles | Sebek et al. ([147]) |
| Broadleaf species | Hollow tree spatial distribution less than 100 m | Saproxylic beetles | Dubois et al. ([39]) |
| Maturing hardwood forests | Uneven-aged harvest with group selection had a lower impact on hollow availability |
Harvest regimes | Fan et al. ([41]) |
| Various | Retain species with higher probability of developing hollows of survival |
Wildlife | Gibbons & Lindenmayer ([50]) |
| Various | Management guidelines in coppice | Insects | Fry & Lonsdale ([46]) |
Deadwood thresholds
The deadwood amount required to preserve biodiversity should be calibrated considering several taxonomical groups, taking into account the community composition rather than relying on the misleading species richness ([108]). Organisms that live in association with deadwood, a dynamic compound of living decaying trees and CWD ([15]) depend on a diverse and ephemeral substrate, and this inner stochasticity should be taken into account for its proper management ([64]). Müller & Bütler ([108]) reviewed the deadwood threshold values recorded in the main forest types in Central Europe, suggesting that an amount of 20-50 m3 ha-1 should be guaranteed in several forest stands as part of a landscape network. This benchmark range could be used to calibrate the intervention according to productivity targets, higher wherever possible and lower to meet specific economic needs.
Forest restoration methods generally include tree girdling and felling to increase the availability of deadwood ([75]) and tree microhabitats ([174]). “Morticulture”, the production of woody detritus to ensure ecosystem function ([58]), can be applied extending stand developmental models to deadwood dynamics in the considered forest management system ([65]). The amount of deadwood could be artificially increased using the techniques presented by Cavalli & Mason ([28]) and the retention of legacies (sensu [45]). Among these legacies we can mention high stumps ([113], [1], [20]), and chablis and volis, two French words full of forestry meaning, that indicate an uprooted tree and a broken tree-trunk, respectively ([115]). The availability of snags has been increased topping trees with dynamite, chainsaw, girdling the canopy and with fungal inoculation ([23], [85], [24]). Models allow the simulation of CWD volumes per decay class that should be found in a stand as a function of its age ([129]). Mortality and decomposition should be taken into account as well, since spatio-temporal dynamics are fundamental to understand the functionality and sustainability of deadwood compartment management ([107]). Additional factors to be considered include the stability and longevity of the structures, and how to optimize their spatial arrangement in order to reduce safety risks and do not affect forestry operations ([7]). The development of deadwood thresholds at the landscape scale represents a future challenge in the preservation of meta-population dynamics ([108]).
Landscape approach
Patch-based conservation networks have been designed specifying a minimum number of required patches, having certain habitat conditions (e.g., size, age distribution, structural complexity, composition, deadwood availability and decay rate - [87], [146]). For Fennoscandian and Baltic countries, identified criteria fell in three categories: (i) stand features (e.g., soil, slope, age, structure, and deadwood); (ii) habitat elements (e.g., habitat trees, boulders, streams, high stumps, pollard trees); and (iii) indicator species (i.e., specialized saproxylics - [163]). The amount of retained habitat within a productive forest should be above 5-10%, and possibly significantly higher, to achieve an ecological enrichment ([56]). Managers should be aware that the benefits of these permanent small reserves tend to be tangible in the long-term (i.e., there could be a substantial lag time), and that these areas are sensitive to edge-effects and their effectiveness can be threatened by the surrounding harvest ([106]). The performance of the potential IdS can be evaluated maximizing the ecological value and minimizing their cost ([27]). Concerning the area effect, Lindenmayer et al. ([89]) performed the first experimental evaluation of the SLOSS (single large or several small areas for conservation) debate to VRH systems, and their results showed no significant area effect on bird species richness: thus IdS area could be adapted to local socio-economic requirements without compromising their value. Moreover, considering the extinction risk of saproxylic species, Ranius & Kindvall ([128]) suggested that small reserves in landscapes characterized by commercial forestry have the advantage of containing higher quality fragments, which do not suffer the time-lag in deadwood restoration, as much as larger and younger areas.
The application of predictive forest models to establish the IdS minimum size in beech (Fagus sylvatica L.) forests proved that as little as 0.5 ha could ensure a high probability of continuous deadwood availability ([63]). The ecological connectivity of the IdS should be ensured by the presence of DC, made of veteran trees prone to develop microhabitats and to produce deadwood in its whole range of forms (e.g., position and decay stage). These two elements together constitute a network, continuous in both space and time, ensuring the preservation and dissemination of saproxylic species. The species’ life-history traits, and in particular their mobility, could be considered to best define the balance between number and size of the IdS, thus between the life-boating function (smaller and more numerous IdSs) and habitat quality (larger, fewer and less affected by edge effect IdSs - [6]).
The management system offered by the FBA ensures habitat continuity and the presence of a more complete set of eco-units, to preserve the saproxylic species that occur either as habitat-tracking meta-populations ([145]) or as classical meta-population ([127]), according to their phylogenetic adaptation ([157]). Further benefits of managing forests at the landscape scale include the combination of retention forestry with other land uses ([56]) and vice versa, the integration of conservation actions in the existing land use practices ([57], [175]). The combination of statistical and remote sensing methods, such as LIDAR, could allow the calibration of forest harvesting on local stochastic natural dy-namics, overcoming artificial stand constraints. In addition, tools that allow the study of spatial patterns, like FRAGSTATS® ([97]), can increase the sustainability of forest products including spatio-temporal land cover changes ([67]). Lastly, the experimental application of landscape retention forestry would allow the establishment of operative thresholds that could be used to reach specific conservation goals, according to disturbance regimes and site heterogeneity and composition ([137]). Combining different measures optimized according to site features and cost-effectiveness of interventions, can increase the feasibility of conservation strategies ([66]). Conservation planning software like MARXAN ([170]) offers further instruments to effectively allocate conservation efforts.
Bottom-up conservation strategies and certification
Nature-based silviculture relies on a thorough understanding of socio-economic and ecological components ([30]), hence the collaboration and support of different stakeholders is strongly desirable ([14]). The effectiveness of landscape approaches, in both addressing the effect of growing anthropogenic pressures and finding ways to support the development of desirable conditions, has gained an extensive support ([140]). Silvicultural practices integrating natural disturbances are especially suitable for public lands, where the management is generally not focused on timber production and/or traditional practices ([114]). On the other hand, since the conservation approaches associated with it (e.g., retention forestry) tend to be bottom-up, owners and license holders are those who generally bear the economic costs ([56]). New policy frameworks should be developed to allow forest landscape planning through the incorporation of opportunity costs, to support landholders in the establishment of an “eco-eco” (economically and ecologically) viable productive forests. Examples of certifications for marketable products include labels that target species (e.g., bird-friendly shade-grown coffee), and their success depends on the consumers awareness of the biodiversity benefits and conversely on their willingness to pay for the additional value of the products ([117]). To achieve the non-static landscape management, incentives and structures that coordinate the different stakeholders are essential ([8]).
Conclusions
A heterogeneous range of substrate quality and management practices are essential to preserve saproxylic beetle diversity in productive forests ([98]). Preserving the functionality of forest ecosystems at the landscape-scale mitigates the detrimental effects of climate change ([153]), and contributes to the connectivity, critical for protected area networks ([105]). Landscape approaches offer a widely accepted framework to deal with sustainable resource management and the growing pressures that threaten water, land and forests ([140]). The implementation of a holistic system requires the combined effort of forestry technicians, conservation biologists and spatial statisticians, to understand disturbance regimes and design biodiversity networks. Quantitative approaches have long been applied in silviculture, providing fundamental instruments, unavailable in other environments, to establish conservation measures ([110]). New standards for sustainable forest management are spreading, such as the certifications of the Forest Stewardship Council ([47]) and the Program for the Endorsement of Forest Certification ([122]), that could feasibly take into account the key features of FBA. These planning instruments, considered together, would feasibly meet the critical targets identified by Lindenmayer & Franklin ([86]): supporting species populations, allowing the movement of organisms, maintaining the reciprocal buffering between productive matrix and protected areas and, at the same time, ensuring production, commodities and services.
Since the expansion of protected areas is limited by competing socio-economic goals, the shift towards the integration of reserves and structural legacies in production and protection forests ([73]) represents a promising approach. Recognizing that for biodiversity preservation in productive forests the benefits offered by the micro-reserves can exceed those of larger isolated reserves ([56]), we believe that the FBA and similar approaches represent a cost-effective tool to work towards a truly multi-functional forestry.
Acknowledgements
Franco Mason was supported by the MiPAAF, Ministry of Agricultural Policy and Forestry, Italian National Forest Service, Central Biodiversity Office, Rome, in the framework of the LIFE Project ManFor C.BD. (LIFE09 ENV/IT/000078); Livia Zapponi was supported by the LIFE Project ManFor C.BD. We would like to thank our colleagues Serena Corezzola and Davide Badano, who kindly read an earlier version of the manuscript. We would like to thank Randy Rollins for language revision, and Zarina Dalla Santa Brown for the final reviewing of grammar and style. We thank Giorgio Matteucci for his constant support in the framework of the Life Project ManFor C.BD., Alessandro Bottacci, Marco Panella and Gianni Zanoni (Central Biodiversity Office of the Italian National Forest Service), Paola Favero (local Biodiversity Office of Vittorio Veneto, Italy) for the keen interest in applying for the first time in Italy the îlot de senescence concept in the Natural Biogenetic Reserve “Campo di Mezzo-Pian Parrocchia”, as an innovative action in the framework of the LIFE Project ManFor C.BD. Lastly, we thank Paolo Cantiani, Fabrizio Ferretti and Umberto Di Salvatore (CREA, Council for Agricultural Research and Economics - Arezzo, Italy) for the constructive discussions on the proposed method.
References
Gscholar
Online | Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Online | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Online | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
CrossRef | Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
National Center for Study and Conservation of Forest Biodiversity “Bosco Fontana”, Laboratory for the Taxonomy of Invertebrates (CNBFVR-LANABIT). Via Tomaso Da Vico 1, 37123 Verona (Italy)
National Center for the Study and Conservation of Forest Biodiversity “Bosco Fontana”, Strada Mantova 29, 46045 Marmirolo, MN (Italy)
Corresponding author
Paper Info
Citation
Mason F, Zapponi L (2015). The forest biodiversity artery: towards forest management for saproxylic conservation. iForest 9: 205-216. - doi: 10.3832/ifor1657-008
Academic Editor
Massimo Faccoli
Paper history
Received: Apr 01, 2015
Accepted: Aug 18, 2015
First online: Oct 26, 2015
Publication Date: Apr 26, 2016
Publication Time: 2.30 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2015
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 44656
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 35980
Abstract Page Views: 3021
PDF Downloads: 4382
Citation/Reference Downloads: 110
XML Downloads: 1163
Web Metrics
Days since publication: 3107
Overall contacts: 44656
Avg. contacts per week: 100.61
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Feb 2023)
Total number of cites (since 2016): 28
Average cites per year: 3.50
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Biodiversity conservation and wood production in a Natura 2000 Mediterranean forest. A trade-off evaluation focused on the occurrence of microhabitats
vol. 12, pp. 76-84 (online: 24 January 2019)
Research Articles
The effects of forest management on biodiversity in the Czech Republic: an overview of biologists’ opinions
vol. 15, pp. 187-196 (online: 19 May 2022)
Research Articles
Stand structure and deadwood amount influences saproxylic fungal biodiversity in Mediterranean mountain unmanaged forests
vol. 9, pp. 115-124 (online: 08 September 2015)
Research Articles
Early responses of biodiversity indicators to various thinning treatments in mountain beech forests
vol. 11, pp. 609-618 (online: 25 September 2018)
Review Papers
Biodiversity assessment in forests - from genetic diversity to landscape diversity
vol. 2, pp. 1-3 (online: 21 January 2009)
Review Papers
Linking deadwood traits with saproxylic invertebrates and fungi in European forests - a review
vol. 11, pp. 423-436 (online: 18 June 2018)
Book Reviews
National forest inventories: contributions to forest biodiversity assessments (2010)
vol. 4, pp. 250-251 (online: 05 November 2011)
Research Articles
Investigating the effect of selective logging on tree biodiversity and structure of the tropical forests of Papua New Guinea
vol. 9, pp. 475-482 (online: 25 January 2016)
Short Communications
An approach to measuring biodiversity and its use in analysing the effect of nitrogen deposition on woodland butterfly populations in the Netherlands
vol. 2, pp. 46-48 (online: 21 January 2009)
Research Articles
Exposure elevation and forest structure predict the abundance of saproxylic beetles’ communities in mountain managed beech forests
vol. 16, pp. 155-164 (online: 08 June 2023)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword