Assessing escapes from short rotation plantations of the invasive tree species Robinia pseudoacacia L. in Mediterranean ecosystems: a study in central Italy
iForest - Biogeosciences and Forestry, Volume 9, Issue 5, Pages 822-828 (2016)
doi: https://doi.org/10.3832/ifor1526-009
Published: May 25, 2016 - Copyright © 2016 SISEF
Research Articles
Abstract
Black locust (Robinia pseudoacacia L.) is a fast growing tree species native to temperate North America, and widely diffused and naturalized in Europe. It is one of the candidate species for establishing bioenergy plantations on marginal lands in temperate and sub-Mediterranean regions. This potential is in contrast to its well-known invasive habit, leading to a potential damage to plant biodiversity in many European countries. Advise against black locust plantation in regions where it is already invasive has been issued by several international reports, as well as the adoption of mitigation measures (e.g., “containment” buffer zones) to prevent the spread of the species into natural and semi-natural habitats. In the Mediterranean basin, however, no studies have been carried out aimed at quantifying the escape rate of black locust saplings from plantation stands and its recruitment into natural habitats, together with the effectiveness of a buffer zone in reducing the spread. In this study we investigated the spread of black locust along 35 transects surrounding three 20-year- old plantations and including three different land cover types: abandoned arable land, semi-natural woodland and a buffer zone (orchards) with a low degree of farming input. In addition, the effect of soil disturbance on seed propagation was investigated. Our results demonstrate that the density of black locust regeneration is strongly affected by the land cover, abandoned agricultural land being the most prone to black locust colonization. Contrastingly, the spread was minimal in the buffer zone and negligible in semi-natural woodland. During the investigated year, seed generative propagation was also negligible. The semi-natural woodland seems to resist well to black locust invasion, though further observations are needed to assess the consequences of stand harvesting disturbance as well, according to local standard forest management. Buffer zones seem to be very effective in controlling black locust invasion. Best management practices, with active farming inputs, are also discussed.
Keywords
False Acacia, Mediterranean Region, Risk Assessment, Containment, EU Regulation, Invasive Species
Introduction
Anthropogenic alterations of natural resources through logging and land use change have often resulted in forest fragmentation and the degradation of wildlife habitats, biodiversity and other ecosystem services ([32], [30]). This is particularly true for the Quercus pubescens and Q. cerris woodlands. ([21]). On the other hand, wood bioenergy is now widely recognized as a valid alternative to fossil fuels, and its implementation in energy production systems is strongly recommended for mitigating global changes ([24]). Wood biomass for energy conversion is mostly produced by managed natural forest ecosystems, as well as by silvicultural systems specifically designed for maximizing biomass production such as short rotation forestry (SRF). However, many of the trees currently used in (or proposed for) SRF on marginal lands, such as Acacia saligna, Ailanthus altissima and Robinia pseudoacacia, are often recognized as being invasive in the Mediterranean region after weed risk assessment, due to their capacity to escape from the plantation site ([12]). However, no specific study in the field has been undertaken to directly assess the escape rate of these species from tree plantations in Mediterranean Europe.
Escape from plantation sites of SRF species carrying aggressive genotypes (regardless their wild types are indigenous or not - [8]) could harm the surrounding vegetation and the semi-natural woodlands. This includes alterations in their natural recruitment or expansion, thereby strongly affecting ecosystem services and biodiversity ([7]).
This research focuses on Robinia pseudoacacia (commonly named black locust, robinia or false acacia), a tree native to the north-eastern United States. It is widely planted and naturalized in many temperate areas of the world, but is also considered an invasive alien in many regions ([26]). In Europe, black locust is common in areas with continental sub-Mediterranean climates with warm summers, and is still expanding and sprouting up near its plantations ([6]) due to its strong ability to spread beyond its original place of cultivation ([13]). At present, at least twelve varieties and several hybrids and cultivars are known ([6]). In addition, engineering through inoculation has been undertaken to improve growth ([23]).
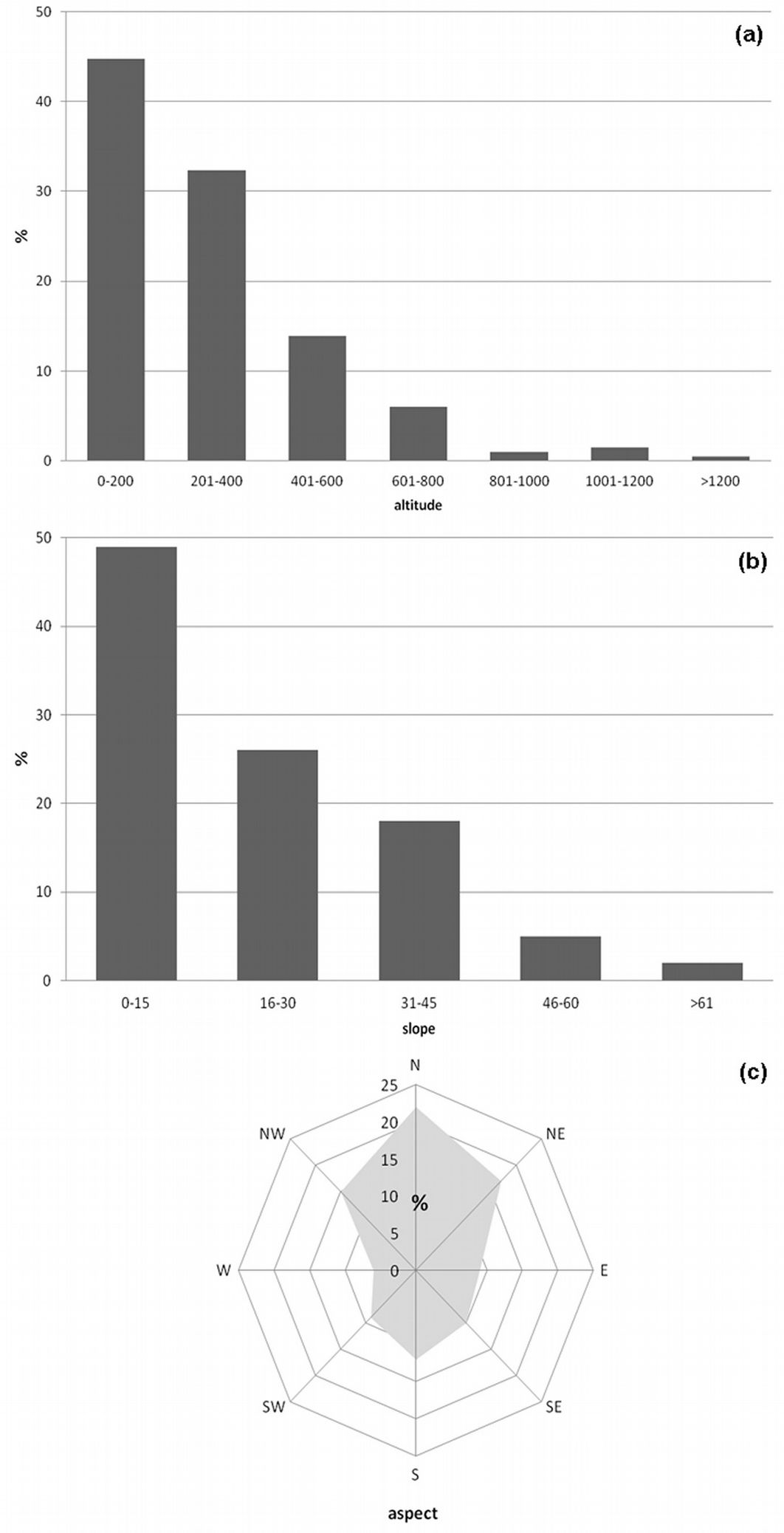
Black locust was introduced in Europe in the early 1600s and reached a wide diffusion as early as the beginning of the XIX century. In Italy, the first record in the botanical gardens of Padua dates back to 1660. Rather curiously, soon after the first European introductions, the species also reached northern Italy from France without spreading widely, so that it was reintroduced at the end of the 18th century ([18]). Black locust, together with Ailanthus altissima, covers an area of approximately 233.500 ha in Italy, representing 2.22% of the total forest area in Italy ([48]). It is a nitrogen-fixing tree, and its use for bioenergy production (mainly fuelwood) is expected to increase the phytonutrient content of the soil, unlike poplar SFR plantations ([39]). Since the early 1990s, research programs have implanted black locust SRF plantations in Italy (mostly coppices) in order to produce biomass for industrial energy production ([20], [35]). Based on more than 200 plots recorded in the Georeferenced Vegetation Database of “La Sapienza” University of Rome ([1]), the most suitable site conditions for the species in central Italy are north facing and gentle slopes between 0 and 400 m. a.s.l. (Fig. 1a, Fig. 1b, Fig. 1c). Black locust commonly occurs in stands of the thermo and meso-Mediterranean belts, together with Quercus cerris, Quercus pubescens, Carpinus betulus, Castanea sativa, Fraxinus ornus, Ostrya carpinifolia on the hills, and with Alnus glutinosa, Populus alba, Populus nigra, Quercus robur, Salix alba, Salix purpurea, Sambucus nigra along the river margins in riparian woodlands.
Fig. 1 - Black locust frequency distribution (in percentage, %) in central Italy, by altitude, aspect and slope (a, b, c, respectively) based on more than 200 plots examined from the Georeferenced Vegetation Database of “La Sapienza” University of Rome, Italy ([1]).
Black locust is an aggressive pioneer species showing several traits typical of invasive species: it can adapt to drought, easily propagates by seed and quickly resprouts from root suckers after coppicing ([33]). Moreover, in the Mediterranean region it has colonized a wide variety of disturbed sites, such as abandoned arable fields, dry sites, roadsides and other areas where the natural vegetation has been severely disturbed. Furthermore, black locust is regularly planted along roadsides and public open spaces. According to Boring & Swank ([4]), the abundance of black locust decreases along the natural forest succession, due to its limited competitive ability that is also supported by planting experiments ([15]).
In the introduced regions, black locust trees live longer than in the original habitat, likely due to lower herbivore pressure and fewer pathogens from the soil biota ([5]). In addition, its wood is particularly resistant to insect damage ([45], [6]). Fruiting begins at the age of 6 years and vegetative dispersal over short distance prevails over seed dispersal. However, seeds inside the seed pod are dispersed by wind and can travel for long distances. Seeds exhibit an impermeable seed coat that allow their persistence in the seed bank; seed shedding occurs between May and September. Most of the physiological attributes of the species are undoubtedly typical of an aggressive invasive species, as described by Rédei et al. ([44]). In Hungary, the species is widely used and provides significant socio-economic benefits; thus Hungary’s position regarding black locust, within the framework of the recent European Union Regulation (EU) No 1143/2014 on the “prevention and management of the introduction and spread of invasive alien species”, is not to include the species in the list of invasive alien species and its management should be maintained under the scope of national legislation ([17]).
In 2009, the EU issued a Directive (2009/ 28/EC) aimed to promote the use of renewable energy, calling on member states to monitor the impact of biomass cultivation, including the displacement and introduction of invasive alien species and consequent effects on biodiversity. The Standing Committee of the Bern Convention ([47]) also adopted a Recommendation (Rec. No. 141/2009) on “Potentially invasive alien plants being used as biofuel crops”. According to the recommendation, contracting parties should: “avoid the use of species that are already considered invasive in the region, screen for the invasiveness of new species and genotypes, monitor the possible spread of biofuel crops into natural habitats and introduce appropriate mitigation measures to limit crop escapes”. Similarly, several international organization for environment conservation (e.g., [25]) promoted guidelines to reduce the spread of biofuel species into the natural environment. Finally, according to the recent Regulation (EU) No 1143/2014, “invasive alien species of European Union concern, such as species causing significant damage in a Member State, should not be intentionally reproduced, grown or cultivated”.
Although invasive, black locust has many valuable characteristics for the establishment of bioenergy plantations on marginal lands of Mediterranean countries ([38], [19]). For example in Sicily (southern Italy) the species is largely planted under the regional reforestation scheme ([29], [41]).
The main aim of this research is to assess black locust spread outside plantation sites by comparing its colonization of different land cover types. To this purpose, we analyzed previously established experimental plantations of black locust in an experimental area of central Italy. In addition, we investigated the effectiveness of a buffer zone (such as a tree orchard with a low degree of farming input) as a barrier against black locus spread in surrounding habitats.
Materials and methods
Study area
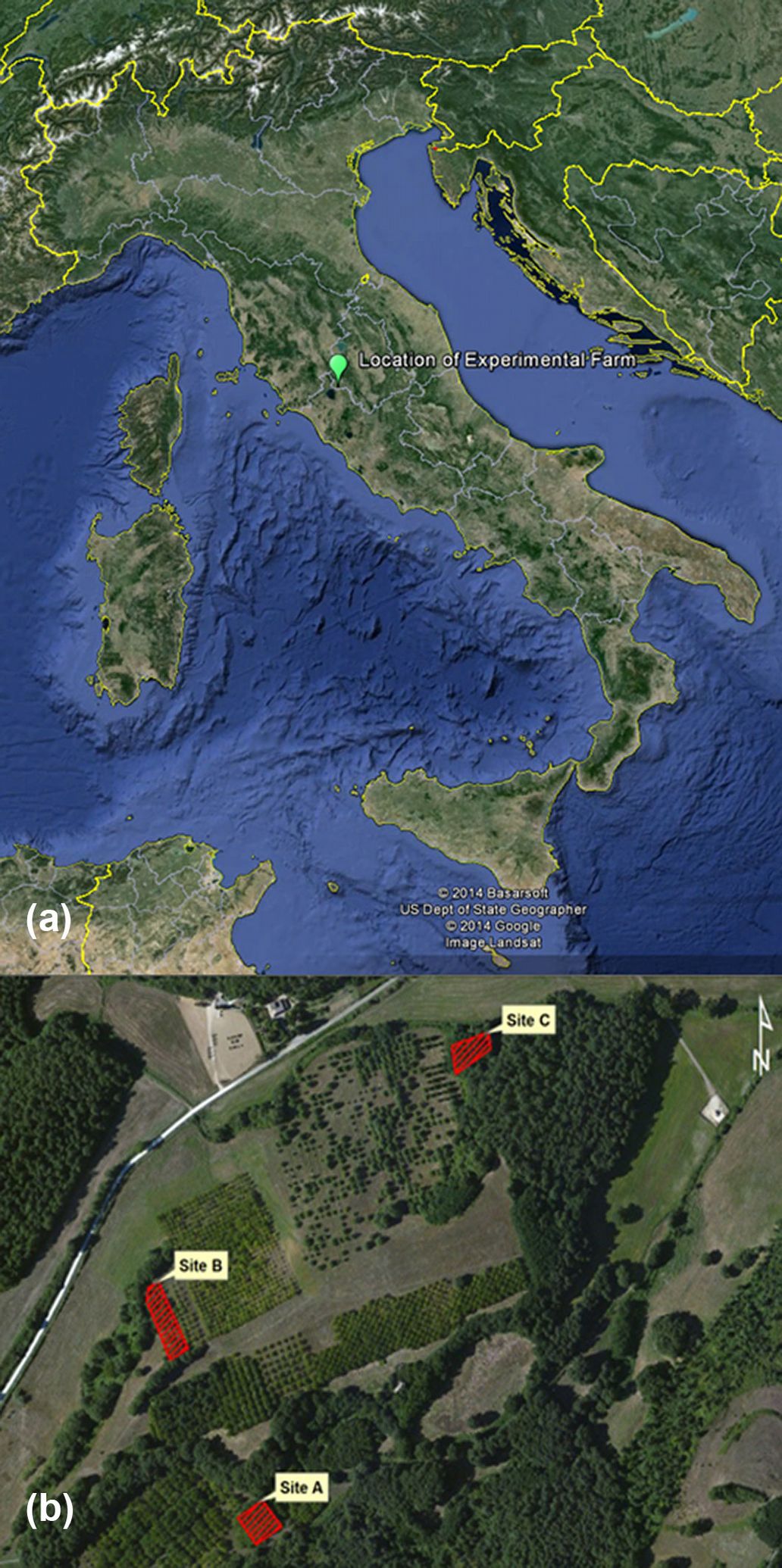
The research was conducted in the Biagio experimental farm managed by the Institute of Agro-environmental and Forest Biology of the National Research Council of Italy (CNR-IBAF) near the town of Orvieto (central Italy). The farm is located in the hilly area of the Monti Volsini (42° 40′ 23.61″ N, 12° 02′ 40.12″ E, 550 m a.s.l.), approximately 50 km from the Tyrrhenian Sea coast on soils of volcanic origin. The bioclimate is meso-Mediterranean ([42]) with higher temperatures in July-August and low precipitation in summer.
The farm has a total area of 22.8 ha and is surrounded by a wire mesh fence, about 2 m high, which completely secludes the area from large herbivores (though the scattered presence of wild boar has been noticed). Grazing was prevented until the year before the present study, when sporadically a herd of sheep was allowed to graze inside.
Black locust parental plantations
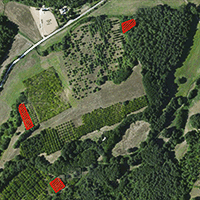
Fig. 2 shows the location of the three black locust plantations analyzed (A, B, C) within the farm. The size of each stand is between 0.1 and 0.2 ha. The black locust trees have different genetic origins.
Fig. 2 - Study area (a) and black locust study plantations (b) in the CNR-IBAF Experimental Farm, municipality of Orvieto, Italy (Source: Google Earth®).
The area surrounding the studied plantations is a semi-natural warm-temperate woodland dominated by native broadleaved oaks such as Quercus pubescens and Q. cerris. In addition, there is an altered grassland vegetation growing on abandoned arable land and fruit orchard/ex situ conifers. These were the land cover types at the time of the plantations.
The main dominant native species characterizing most of the biomass cover were: (i) Quercus pubescens, Q. cerris, Crataegus monogyna, Euonymus europaeus, Acer campestre, Corylus avellana, Fraxinus ornus, Spartium junceum, and Rosa sp. pl., Rubia peregrina in the woodlands; (ii) Arisarum vulgare, Geranium rotundifolium, G. molle, Sonchus spp., Dorycnium spp., Crepis spp., Lonicera implexa, Daucus carota, Dactylis glomerata and Plantago media in the abandoned arable land.
Sampling
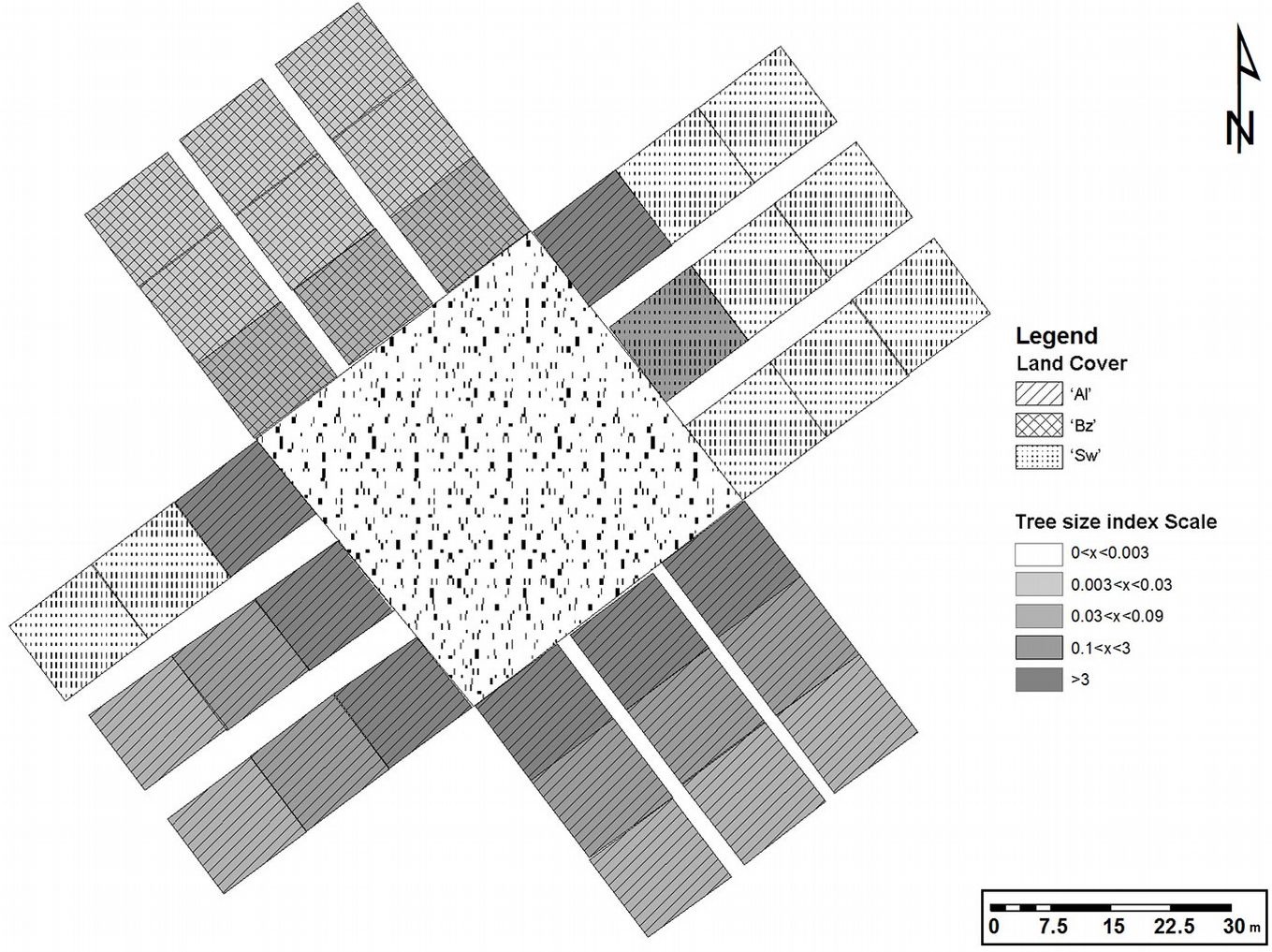
Following Langdon et al. ([31]), three perpendicular strip transects were established from the edge of each of the four sides of each plantation (Fig. 3), which totaled 35 transects (only two transects were established on the short side of stand B). Each transect was 10 m wide with a maximum length of 30 m: in some cases transects were shorter due to the presence of large roads/tracks for transit. Within each transect, starting from the edge of the black locust plantation, the presence of black locust individuals was assessed inside ninety-seven 10×10-m squared plots, which were considered as the experimental units (Fig. 3).
Fig. 3 - Black locust tree size for plantation A in relation to land cover category and distance from the plantation edges. (Sw): Semi-natural woodland; (Al): Abandoned land; (Bz): Buffer zone.
In each plot, the land cover type was classified in only one of the following categories: (i) semi-natural woodland (“Sw”); (ii) abandoned arable land (“Al”); (iii) tree orchard/buffer zone(“Bz”). These land covers types correspond to the following CORINE Land Cover classes: (i) “Sw”, 3.1.1.2 Broad-leaved forest with predominance of deciduous oaks; (ii) “Al”, 2.4.3.2 Agricultural areas with significant share of natural vegetation and with prevalence of grasslands; (iii) “Bz”, 2.2.2.1 Orchards areas of fruit orchards and ligneous crops.
In each plot, the following parameters were recorded for black locust: number of trees (adults: individuals with height > 1 m; saplings: individuals with height ≤ 1 m), diameter at breast height (DBH) and total height. Most individuals were smaller than 5 m, therefore the use of equations for predicting the aboveground volume based on DBH and height ([49]) was not possible. Given that the estimation of tree volume was beyond the scope of this study, we simply calculated the tree size by multiplying DBH by the height (in m) as a proxy of recruitment capacity ([10], [28]). Collected data were then grouped together based on the distance from the plantation edge (0-10 m, 11-20 m, 21-30 m) and the land cover category (“Sw”, “Al”, “Bz”).
To evaluate the presence of black locust individuals within the ecotone between the plantation and the native vegetation, the presence of the species in the first two meters bordering the plantation was also recorded, thus enabling to assess the implication of the “edge effect” on black locust spread.
Tab. 1 summarizes the number of plots grouped by land cover type and distance from the plantation edge.
Tab. 1 - Number of plots (10 × 10 m) grouped by land cover category and distance from the plantation edges.
| Distance (m) |
Semi-natural woodland | Abandoned land |
Buffer zone |
Total |
|---|---|---|---|---|
| 10 | 6 | 20 | 9 | 35 |
| 20 | 18 | 6 | 9 | 33 |
| 30 | 14 | 6 | 9 | 29 |
| Total | 38 | 32 | 27 | 97 |
Statistical analysis
Tree size was compared among groups (quadrates 10×10 m grouped by land cover type, distance and site) using the General Linear Model (GLM) for unbalanced data. Tree size was considered as a function of: type of land cover, distance from the site/plantation and plantation variables. Relationships between tree size for each type of land cover and distance were also tested by calculating the Pearson’s correlation coefficients. All data were checked for matching test assumptions, and no or mild violations were observed.
Assessment of germination and frontal dispersal capacity
To assess the germination capacity of black locust on disturbed soil with no vegetation, 12 germination plots of size 1 × 1 m were established near the border of plantations. Germination plots were cleared and ploughed just before the natural seed shed, and the presence of emerging seedlings was recorded in the following spring.
To quantify the frontal dispersal capacity of the species, the distance of the furthest black locust from the plantation edge was recorded by lengthening the strip transects. Such measurements were taken only on one side of plantation A, where there is a large area of abandoned arable land and potential dispersal was more likely to occur.
To assess the reproductive capacity of the black locust individuals under investigation, an indirect test of viability (“cutting test”) was applied ([43], [50], [11]). Seeds from the three study plantations were collected during shedding, pooled together and then divided into four lots of 25 seeds. Each seed was opened by cutting, and seeds showing intact and healthy embryos, as well as clean and firm endosperm, were considered viable. Previous studies on black locust reported that the percentage germination of apparently viable seeds based on cutting tests was 66% ([16]). In our study, the cutting test results revealed that most seeds (84.3% ± 12.6) could be considered as viable and consequently capable of germination.
Results
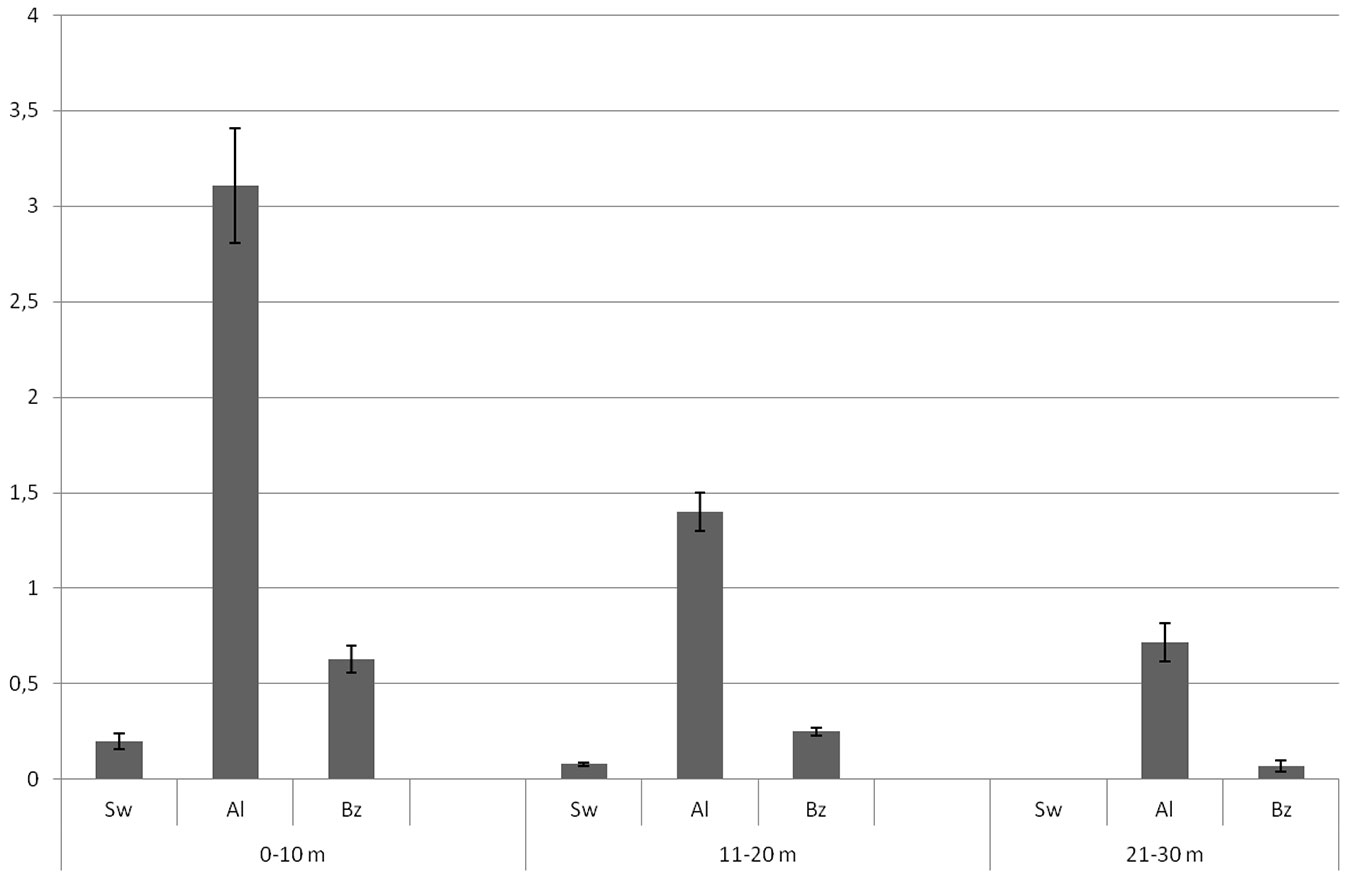
In the land cover category “Al” (abandoned land), black locust occurred copiously in the first 10 m from the plantation edge, both as saplings (<1 m) and trees (>1 m - Tab. 2, Tab. 3), and had a high tree size value (Fig. 4). At a distance of 20 m from the plantation edge, tree size value decreased by approximately five times as compared with the first distance class, while the number of individuals was four times lower. At 30 m from the plantation tree size was about 20 times lower and the number of individuals was more than 15 times less than in the first distance class. Likewise, in the first 20 m from the plantation edge of the category “Bz” (buffer zone) both the number of saplings and tree size values were similar to the 10 m plots.
Tab. 2 - Mean number (± SE) of black locust saplings (height <1 m) in the 10 × 10 m plots.
| Distance (m) | Semi-natural woodland | Abandoned land | Buffer zone |
|---|---|---|---|
| 10 | 0.50 ± 0.03 | 5.20 ± 0.64 | 0.80 ± 0.07 |
| 20 | 0.05 ± 0.003 | 1.60 ± 0.01 | 0.30 ± 0.02 |
| 30 | 0 | 0.50 ± 0.03 | 0.02 ± 0.01 |
Tab. 3 - Tab. 3 - Mean number (± SE) of black locust trees (height >1 m) in the 10 × 10 m plots.
| Distance (m) | Semi-natural woodland | Abandoned land | Buffer zone |
|---|---|---|---|
| 10 | 0.30 ± 0.02 | 7.30 ± 0.86 | 0.20 ± 0.014 |
| 20 | 0.01 ± 0.001 | 2.50 ± 0.03 | 0.01 ± 0.001 |
| 30 | 0 | 0.20 ± 0.018 | 0 |
Fig. 4 - Black locust tree size values (after square root transformation) in relation to distance from the study plantation edges and land cover category. (Sw): Semi-natural woodland; (Al): abandoned land; (Bz): buffer zone.
Contrastingly, in the land cover category “Sw” (semi-natural woodlands), very few black locust trees and saplings were found in all distance classes, and were totally absent at 30m from the plantation edge (Tab. 2 - see also the tree size results from plantation A in Fig. 3). Indeed, the maximum abundance of the species in this category was in the first two-meter ecotone.
GLM analysis revealed that land cover and distance from the plantation significantly affect the black locust tree size (P < 0.0001 and P < 0.001, respectively - Tab. 4). On the other hand, the site/plantation showed no significant relationship with tree size (P = 0.424 - Tab. 4).
Tab. 4 - GLM results for black locust tree size in relation to distance from the study plantation edges and micro-site land cover category.
| Source | df | F | P value |
|---|---|---|---|
| Land cover type | 2 | 32.38 | 0.000 |
| Plantation | 2 | 0.87 | 0.424 |
| Distance | 2 | 13.17 | 0.000 |
| Error | 90 | - | - |
Pearson’s correlation analysis revealed a significant negative association of tree size with “Al” (r = -0.73, df = 2, P < 0.001), and a weak negative association with “Sw” (r = -0.43, df = 2, P < 0.01) and “Bz” (r = -0.51, df = 2, P < 0.001).
In the twelve 1×1 m germination plots that were cleared and ploughed, only one black locust seedling was recorded during the survey, compared to five oak seedlings found in four plots.
Spontaneous frontal dispersal of black locust from plantation A was up to 44 ± 4.6 m, which is equal to 2.2 m per year.
Discussion
In more than 20 years from the establishment of plantations, black locust had spread outside of the original stands. The colonization rate varied depending on habitat disturbance (i.e., land cover types), and was highest in the abandoned arable land area. In the semi-natural woodland, black locust occurred sporadically and does not seem to represent a threat to the natural (native) vegetation. However, these results should be regarded with caution, because since the establishment of the black locust plantations the surrounding stands (coppice-with-standards) have never been harvested, according to the local forest management records, which provide a rotation length of 30-40 years. Coppice harvesting creates large canopy gaps and determines a high disturbance of the soil. All these factors can favor the expansion of black locust, both via root suckers and seed germination, as demonstrated in coppice stands in northern Italy ([40]).
Evidence of invasions by black locust in many forest types have also been reported by Maltoni et al. ([34]) in areas close to our study sites. Therefore, extreme caution should be taken in establishing new black locust bioenergy plantations in the proximity of existing forests stands, because their future harvesting may create favorable conditions to black locust expansion.
It is important to consider that disturbances can also occur naturally in native vegetation, due to, e.g., wildfire, landslides, severe storms, or just tree senescence. The high dispersal of invasive species (such as that originating from a black locust plantation) could hamper (or even nullify) the natural recruitment process of native species. As confirmed in our study, black locust can easily establish in the new ecotone, thus expanding the invasion front. Our study also showed that the presence of a buffer zone (in our case an orchard), with only a low degree of farming input, prevented black locust from spreading. This was likely due to the periodic cultivation practices carried out in the orchard ground floor. Indeed, farming practices are extremely efficient in suppressing both vegetative and generative reproduction of the species. This is confirmed by the fact that black locust is also an agroforestry tree species in Europe, forming tree hedgerows along field margins in agricultural landscapes, but it did not spread to nearby agro-ecosystems. This is further confirmed by recent experiences in Germany, where the impact of innovative alley cropping systems has been studied on arable lands ([36]). In this agroforestry system, 10-m wide strips of trees managed as bioenergy SRF are alternated with wide alleys of arable crops and soil cultivation. Crop herbicide spraying and crop harvest are carried out in close proximity to the black locust SRF strips. However, the impact of Robinia pseudoacacia on plant biodiversity (in particular on the understorey) is still under discussion. According to the literature, in the sub-Mediterranean and temperate ecosystems of central Europe, particularly in deep alluvial soils, the impact of black locust on natural vegetation seems remarkable, and in some cases it may become the dominant tree species, to the extent that a distinct phytosociological class has been recognized (Robinietea Jurko ex [22]).
In Italy, the impact of black locust on the understory is still debated. Benesperi et al. ([3]) found that plant communities of native tree species (Ostrya carpinifolia and Quercus cerris) are more diverse than pure black locust stands, while Sitzia et al. ([46]) reported that in stands where native woody species, such as oaks and manna ash, were replaced by black locust, the latter species has only a small effect on the understory composition, as compared with secondary native stands.
By monitoring the surroundings of an 80-year old black locust plantation in Japan, Morimoto et al. ([37]) found only few isolated individuals of black locust along a river up to a distance of more than 2500 m from the plantation. In the same study, seed traps captured seed shed approximately 70 m away from parent trees. Del Favero ([14]) in a recent secondary woodlands including black locust trees of Colli Euganei (northern Italy) recorded a spontaneous frontal expansion in abandoned fields of approximately 2 m per year, which is similar to the findings of our study.
In this study, the buffer zone with active farming was very effective in blocking black locust from spreading from its plantation site. Indeed, we recorded a maximum frontal expansion of about 50 m after 20 years since the establishment of the plantations. However, other authors reported maximum distances of frontal expansion up to 100 m ([27], [37]). The optimal width of the buffer zone to prevent black locus escape from plantations is difficult to estimate. Nevertheless, the probability of invasive crop species to establish in a natural habitat increases with the number of propagules and their dispersal, and decreases according to the distance between the SRF and the natural habitat ([9]).
Conclusions
Several tree species proposed for bioenergy production in SRF share traits that are considered as successful in invasive species ([2]). To this purpose, precautions are necessary to avoid newly-introduced species to escape from plantations, since in some cases it may take long time before their establishment in native ecosystems. Thus, species/genotypes used in SRF should be subject to pre-entry weed risk assessments before cultivation and to a post-entry monitoring programme.
The plot-based transect design ([31]) applied in this study provided satisfactory results. The extension of similar investigation to different geographical and environmental context could improve our knowledge on the impact of blck locust on natural vegetation. Based on our results, we recommend black locust not to be planted next to sites acting as source systems, stepping stones, or ecological corridors, such as abandoned land, disturbed areas, or river banks (SRF plantations are often established close to rivers, which can act as a preferential pathway of propagule dispersal. Moreover, a managed buffer zone surrounding black locust plantations should be established to act as a biological barrier for the invasive species. Buffer zones should be planted with other (non-invasive) cultivations, although periodic ploughing or harrowing may not be sufficient to prevent resprouting of high-density root suckers. Furthermore, the width of the buffer zone could vary depending on the dispersal distance of the invasive species. Finally, the management of woody semi-natural vegetation surrounding black locust plantations needs extreme caution in order to understand the long-term vulnerability of forest habitats to black locust invasions ([40]).
Acknowledgements
This research was supported by the project PROFORBIOMED (ref. 1S-MED10-009) co-financed by the European Regional Development Funds (ERDF) through the MED program. The financial and scientific assistance by Forum Plinianum (ref. 087/12/ CF-addendum Prot. 023399/2013 ISPRA) is also gratefully acknowledged. Support for the preparation of the manuscript was provided through the AGFORWARD project. We are also grateful to Dr. Carmela Cascone and Dr. Salvatore Cipollaro for their collaboration in the early stages of this project. The authors in the manuscript authorship are listed in alphabetical order, except the first author.
References
Gscholar
Online | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Online | Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
ISPRA-IV Dipartimento, STS Palermo (Italy)
IUCN-CEM- Ecosystems and Invasive Species (Italy)
Anna Testi
Dip. Biologia Ambientale, La Sapienza Università degli Studi di Roma (Italy)
ISPRA, Dipartimento Difesa della Natura; Roma (Italy)
Dip. STEBICEF, Università degli Studi di Palermo (Italy)
CNR-IBAF Porano (Italy)
Corresponding author
Paper Info
Citation
Crosti R, Agrillo E, Ciccarese L, Guarino R, Paris P, Testi A (2016). Assessing escapes from short rotation plantations of the invasive tree species Robinia pseudoacacia L. in Mediterranean ecosystems: a study in central Italy. iForest 9: 822-828. - doi: 10.3832/ifor1526-009
Academic Editor
Andrea Cutini
Paper history
Received: Dec 11, 2014
Accepted: Jan 28, 2016
First online: May 25, 2016
Publication Date: Oct 13, 2016
Publication Time: 3.93 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2016
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 51367
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 42225
Abstract Page Views: 3449
PDF Downloads: 4199
Citation/Reference Downloads: 52
XML Downloads: 1442
Web Metrics
Days since publication: 3463
Overall contacts: 51367
Avg. contacts per week: 103.83
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2016): 20
Average cites per year: 2.00
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Review Papers
Risk assessment of GM trees in the EU: current regulatory framework and guidance
vol. 6, pp. 127-131 (online: 08 April 2013)
Research Articles
Climatic fluctuations trigger false ring occurrence and radial-growth variation in teak (Tectona grandis L.f.)
vol. 9, pp. 286-293 (online: 28 September 2015)
Research Articles
Examining the evolution and convergence of wood modification and environmental impact assessment in research
vol. 10, pp. 879-885 (online: 06 November 2017)
Research Articles
Essential environmental variables to include in a stratified sampling design for a national-level invasive alien tree survey
vol. 12, pp. 418-426 (online: 01 September 2019)
Research Articles
Are Mediterranean forest ecosystems under the threat of invasive species Solanum elaeagnifolium?
vol. 14, pp. 236-241 (online: 10 May 2021)
Research Articles
Early flowering and genetic containment studies in transgenic poplar
vol. 5, pp. 138-146 (online: 13 June 2012)
Short Communications
Ozone flux modelling for risk assessment: status and research needs
vol. 2, pp. 34-37 (online: 21 January 2009)
Research Articles
Variation of wood and bark density and production in coppiced Eucalyptus globulus trees in a second rotation
vol. 9, pp. 270-275 (online: 08 September 2015)
Research Articles
Windstorm disturbance triggers multiple species invasion in an urban Mediterranean forest
vol. 11, pp. 64-71 (online: 25 January 2018)
Review Papers
Should the silviculture of Aleppo pine (Pinus halepensis Mill.) stands in northern Africa be oriented towards wood or seed and cone production? Diagnosis and current potentiality
vol. 12, pp. 297-305 (online: 27 May 2019)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword