Stand dynamics and natural regeneration in silver fir (Abies alba Mill.) plantations after traditional rotation age
iForest - Biogeosciences and Forestry, Volume 7, Issue 5, Pages 313-323 (2014)
doi: https://doi.org/10.3832/ifor0985-007
Published: Apr 08, 2014 - Copyright © 2014 SISEF
Research Articles
Abstract
In the Apennine mountains most pure silver fir stands originated from planting and were managed according to the traditional model, with clear cutting and a 100 year rotation. In the last decades in most of these stands there has been a change towards nature conservation and active management has stopped. The aim of this work was (1) to analyse stand dynamics and spontaneous regeneration processes that are taking place with increasing age in even aged fir plantations, and (2) to discuss if these processes can be useful for defining management approaches answering biodiversity conservation aims. The study was carried out in the Vallombrosa Forest (Central Italy). The relationship between fir stand age and structural parameters was analyzed using inventory data. Gap dynamics were monitored between 1983 and 2007 on the basis of remote sensing data. Based on a field survey of a sample of gaps, species composition and density of seedlings and saplings were analysed in relation to gap size and within-gap position. From 1983 to 2007 the number of gaps and their total area increased, following increasing stand age. Significant relationships (p < 0.01) were found between fir stand age and number of trees per hectare (r = -0.30), quadratic mean diameter (r = 0.65) and volume per hectare (r = 0.50). In the fir stands with lower stand density, a layer of trees with DBH < 15 cm had filled in the structure: fifteen different broadleaf species were recorded in this layer, usually mixed with fir. Gap size had an influence on presence of young firs (seedlings and fir < 0.5 m), which were significantly more numerous in gaps < 200 m2, but it had relatively limited influence on species diversity. Within-gap position did not influence regeneration density. Results indicate that a possible management option for gradually transforming even aged fir plantations in the Apennines into mixed, naturally regenerating systems, could be based on the creation of small gaps (< 200 m2) in the canopy cover, simulating the natural dynamics that are taking place in ageing fir plantations.
Keywords
Abies alba Mill., Plantations, Gap Dynamics, Natural Regeneration, Forest Biodiversity
Introduction
In the last decades forest management has shifted the focus from wood production to the provision of multiple environmental services and biodiversity conservation. Following this trend the conversion of forest plantations into naturally regenerating and more diverse and complex systems has become one of the aims of the sustainable forest management, often trying to revert a process which had instead transformed vast areas of natural forests into plantations across Europe since the XIX century ([45], [11]).
Silver fir (Abies alba Mill.) has frequently received attention from foresters and researchers, partly because there has been a long tradition of forest management in central and southeast Europe in areas where the natural share of fir is significant (e.g., [51], [16]), but mainly because of fir’s economic, environmental and social significance. Signs of decline in fir presence have also been investigated (e.g., [35], [38]). Recently, Volarik & Hedl ([94]) suggested that periods of both decline and expansion have underpinned silver fir dynamics. While natural mixed beech-fir forests were largely converted to spruce plantations in central European mountains and in many Alpine areas ([46], [94]), in the Apennine Mountains (Italy) fir - naturally present in beech dominated mixed hardwood forests - was generally disfavoured by forest exploitation and has become relatively rare ([69]). Today silver fir is an important species of five Natura 2000 habitats in the Apennines, three being priority habitats (*): 9130: Asperulo-Fagetum beech forests; 9210*: Apennine beech forests with Taxus and Ilex; 9220*: Apennine beech forests with Abies alba and beech forests with Abies nebrodensis; 9410: Acidophilous Picea forests of the montane to alpine levels (Vaccinio-Piceetea); 9510*: Southern Apennine Abies alba.
Fir had often been preserved in small areas around monasteries, such as Vallombrosa and Camaldoli (Tuscan Apennines) where fir cultivation has been documented at least from the XVI century ([85], [40]). Since late XVIII century fir planting started in various forests in the Tuscan Apennines, and this trend continued until the middle of the XX century. Most fir plantations are on State property and until the 1970s they were managed following the traditional model based on area regulation methods with clear cutting and 100 year rotation age ([92], [13]).
In the 1970s and 1980s pure fir stands in the Apennines started showing symptoms which recalled fir decline and dieback observed in central Europe ([54], [47]), raising some concern about the future of this species. Together with these symptoms, Heterobasidion annosum (later identified as H. abietinum - [67]) also caused some damage, consisting mainly in the death of individual or small groups of trees ([37], [8], [20]).
At the end of the 1970s the State forests were declared Nature Reserves and management aims were reoriented towards multifunctionality and biodiversity conservation. Furthermore, in the last decades fir stands have gained increasing importance for their role in shaping a “cultural forest landscape” in these mountain areas ([24]).
Shifting from artificial to natural regeneration in pure even-aged fir stands has long been a matter of concern among silviculturists in Italy ([9]). In a field study in pure even-aged stands in the Vallombrosa State Forest, Magini ([58]) showed that fir regeneration developed spontaneously along the edges of fir stands, while under dense stands or in large openings no fir regeneration was found. Nevertheless, results of this research were not transferred to the management level and the fir stands have evolved “naturally” for over 35 years. Only dead or dying trees were felled along forest roads for visitor safety ([25], [13]).
Understanding how forest structure develops in response to natural disturbances is essential to evaluate current forest conditions in relation to management practices and conservation status ([4], [76], [32], [78], [83]). The “gap phase” is considered important in forest regeneration by providing opportunities for tree recruitment, establishment and development ([97], [19], [81], [74], [17], [62], [65], [5]). Gaps are also important in maintaining plant species diversity ([77], [18], [17], [28]). The size, shape, age and temporal changes of gaps influence the regeneration patterns of tree species, due to the different ecological traits of the particular tree species and to the effects on the herbaceous layer (e.g., [27], [19], [77], [98], [29], [74], [30], [41], [31]).
Many authors have examined the regeneration dynamics of conifer plantations as a basis for conversion towards mixed stands (e.g., [50], [30], [101], [59], [1]), but fewer studies have investigated these processes in relation to natural gap formation in this type of stands (e.g., [61], [49]).
The aim of this work was: (1) to analyse stand dynamics and spontaneous regeneration processes that are taking place with increasing age in even-aged fir plantations, in particular following natural gap formation; and (2) to discuss if these processes can be useful for defining management approaches answering biodiversity conservation aims (e.g., Natura 2000).
Material and methods
Study area
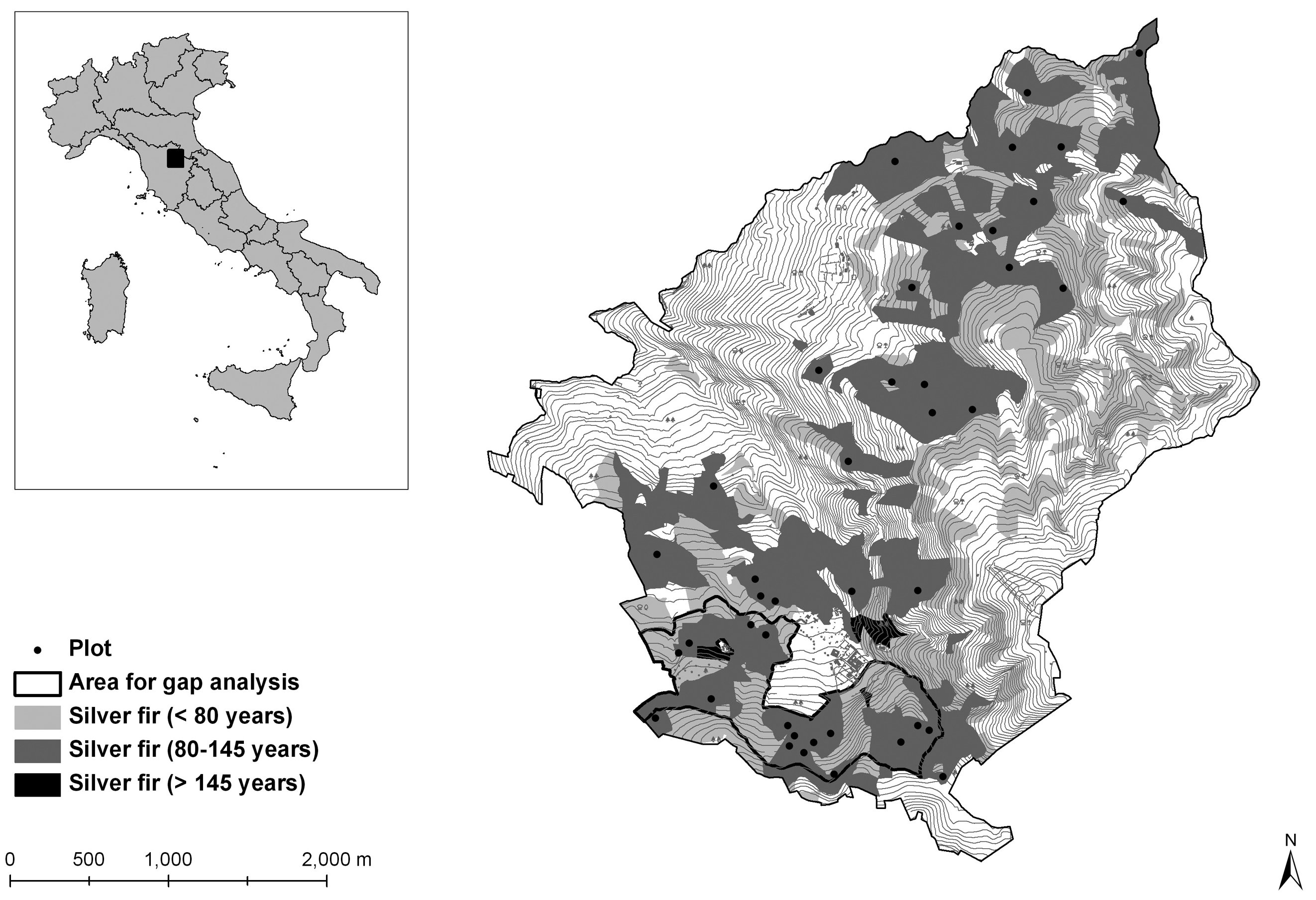
Our research was carried out in fir stands growing in the Vallombrosa State Forest on the Apennine Mountains, 40 km east of the city of Florence (43°43’ - 43°46’ N, 11°31’ - 11°35’ E - Fig. 1).
Fig. 1 - The Vallombrosa Forest. The area where gap analysis was carried out is outlined in black; black points show the 42 circular plots for stand structure analysis.
The forest covers a total area of 1273 hectares between 470 and 1440 m a.s.l. on the western slope of Monte Secchieta (1447 m a.s.l.); over 600 ha are pure, even-aged fir stands.
The climate is temperate-humid with Mediterranean type rainfall with a summer minimum. Mean annual temperature is 9.7 °C and total annual rainfall is 1337 mm, with an average of 71.2 mm in the summer months (June-August). Parent material is Oligocene sandstone of the “Macigno del Chianti” formation. The forest grows on sandy-loamy or loamy soils, rich in humus on the surface horizons. Soil depth varies. The slopes are generally steep or very steep.
In the past forest cover in the area was mainly chestnut and mesophilous oaks at intermediate altitudes and beech forests at higher elevation. Fir probably grew in small groups or mixed stands scattered throughout the forest, mostly within the beech area. The Vallombrosan (Benedictine) monks started fir cultivation in the XIV century ([39]). In 1866 the Vallombrosa Forest was transferred to the Italian State Forestry administration. The first management plan for the Vallombrosa fir forest was drafted in 1876, silviculture was based on clear cutting and artificial regeneration, with a 80 year rotation, and managed according to the age class area regulation method. This management model remained practically unchanged in the 7 following plans (1886, 1896, 1923, 1936, 1949, 1960, 1970), the only change being rotation age which was increased to 100 years in 1896. Total area covered by fir plantations in the forest increased from 217 ha in 1876 to 680 ha in 1960. Most fir plantations replaced beech forests ([14]).
In 1977 the Vallombrosa Forest was classified as a National Nature Reserve and is now included in the Site of Community Importance (SCI) “Vallombrosa and S. Antonio Forest” (IT5140012) under the Natura 2000 Network (Habitats Directive 92/43/EC). Two priority habitats (9210* and 9220*) are included within the SCI. As a Nature Reserve, hunting is banned in the Vallombrosa Forest, and in the last decades wild ungulates are increasing in number and distribution ([3]), similarly to other areas in the northern Apennines. In the Vallombrosa Forest estimated densities of roe deer (Capreolus capreolus L.), fallow deer (Dama dama L.) and wild boar (Sus scrofa L.) are very high: 13 ha-1 have been estimated for fallow deer, which is at least 3 times greater than the estimated carrying capacity, and 27 ha-1 for wild boar, i.e., 6 times higher than the carrying capacity ([22]).
Data collection and analysis
For stand structure analysis we selected all compartments which hosted pure silver fir stands with current age ranging from 80 to 145 years, according to the preceding management plans (Fig. 1). Forty-two 20 m radius circular plots (1256 m2) were randomly distributed in these compartments. In each plot we recorded species and diameter at 1.30 m (DBH) of all living trees with DBH > 2.5 cm; we also measured total height on a sample of trees (35 % of trees in each 5 cm DBH class).
Fir trees belonging to the original planted stands (aged 80 to 145 years in the different plots) were identified during the survey and recorded separately, so as to obtain a picture of the fir plantation development as compared to other species spreading naturally into the stands. Volume was estimated using a one entry volume table ([26]). Large trees with DBH > 50 cm and > 70 cm were analysed separately for their importance for biodiversity ([68], [87]). Linear regression models were used to determine the relationship between stand age, number of trees per hectare (N), volume per hectare (V) and quadratic mean diameter (QMD, diameter of the tree with average basal area). For each model the correlation coefficient (r) was computed. Additionally, the relationship between density of the original fir stand (quantified by basal area per hectare, BA) and density of the lower stratum was investigated.
We monitored canopy gap dynamics over 103 hectares in the southern portion of the forest (Fig. 1). Since both VHR satellite images - an effective alternative to field surveys for canopy gap detection ([42], [79]) - and Airborne Laser Scanning data - useful for automatic gap detection over large areas ([102], [6]) - were not available in the study area, canopy gaps were monitored using multitemporal aerial remote sensing data. We used 1983 aerial photographs (flying height 750 m), and 1997 and 2007 digital orthophotos with 1 m and 0.5 m pixel size, respectively. The 1983 images were scanned and orthorectified with 1 m pixel size (RMSE = 1 pixel) using the OSUIPEOHJOF software package (PCI Geomatics, Richmond Hill, Ontario, Canada). Because no information about the camera was available, the Rational Functions Model was used ([72]). To this purpose, a digital terrain model with a 5 m spatial resolution was created using contours extracted from topographic maps (1:10000) and ground control points were collected using the orthophotos as reference images.
Canopy openings ≥ 50 m2 were considered gaps. Such threshold was chosen since 50 m2 is the average crown projection area for fir trees in stands aged 100+ years in similar areas of the Apennines ([90]). Gap margins were photointerpreted and digitized on-screen into a polygon layer on a GIS database. The following parameters were then calculated: number of gaps; total gap area; percent of total surveyed area covered by gaps (gap fraction); mean, standard deviation and maximum size of gaps for each survey year. We also examined the fate of the individual gaps, following the method proposed by Kenderes et al. ([48]), i.e., creation of new gaps, gap closure, dissection of gaps and merging of neighbouring gaps. Because stereopairs were available only for the 1983 aerial photographs, we could not use the height of the vegetation filling the gap as an indication of gap closure ([82], [73]). Therefore, a gap was classified as closed when its area was smaller than 50 m2 in the following orthophoto interpretation.
A field survey of the tree regeneration in all gaps ≥ 100 m2 located in stands 100+ years and detected both in 1997 and 2007 was carried out, revealing 30 gaps matching the above characteristics. Number, height, species and impact by wild ungulates was recorded for all seedlings and saplings in 3 circular, 2m-radius plots (12.56 m2), placed along the major axis of the gap, one in the center, and the other two in the midpoint between the center and the gap margin. Each sampled individual was recorded in one of the following height classes: (i) one year-old seedlings; (ii) < 0.5 m; (iii) 0.5-1 m; (iv) 1-2 m; and (v) > 2 m. Individuals were considered as impacted by wildlife when the leading stem and the branches showed clear signs of grazing.
We calculated the average number of individuals in each height class for the following gap size classes: (i) < 200 m2; (ii) 200-400 m2; and (iii) > 400 m2. The non-parametric Mann-Whitney U-test (α=0.05) was applied to test for differences in the average number of individuals in the different height and gap size classes, using the statistical software SPSS v. 20. Additionally, the Mann-Whitney U-test was used to compare the density of the regeneration layer in the center of the gaps in relation to density occurring closer to gap edges. The percentage of individuals impacted by wild ungulates was also computed.
Results
Stand structure
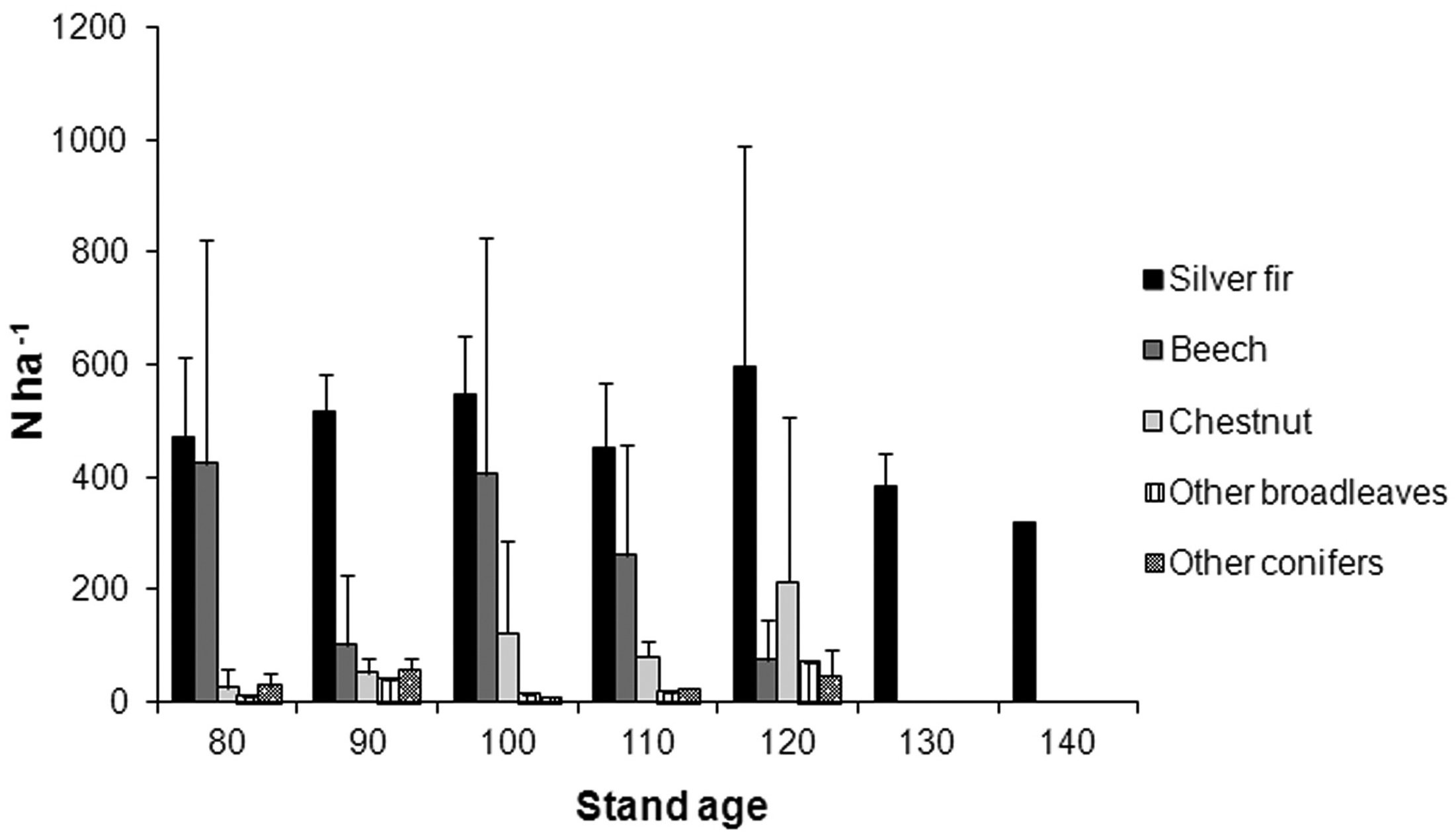
In all examined plots fir still occupied the dominant layer, sometimes with few other conifers (Picea abies (L.) Karst., Pinus nigra Arn., Pinus sylvestris L. and Pseudotsuga menziesii Mirb. Franco). In most plots there was a lower stratum (DBH < 15 cm) of fir and many different broadleaves: Fagus sylvatica L., Castanea sativa Mill., Acer pseudoplatanus L., Acer opalus Mill., Acer platanoides L., Carpinus betulus L., Ostrya carpinifolia Scop., Quercus cerris L., Prunus avium L., Fraxinus excelsior L., Fraxinus ornus L., Ulmus glabra Huds., Populus tremula L., Sorbus aucuparia L., Tilia cordata Mill., Corylus avellana L., Ilex aquifolium L. Beech and chestnut were by far the most frequent species (Fig. 2).
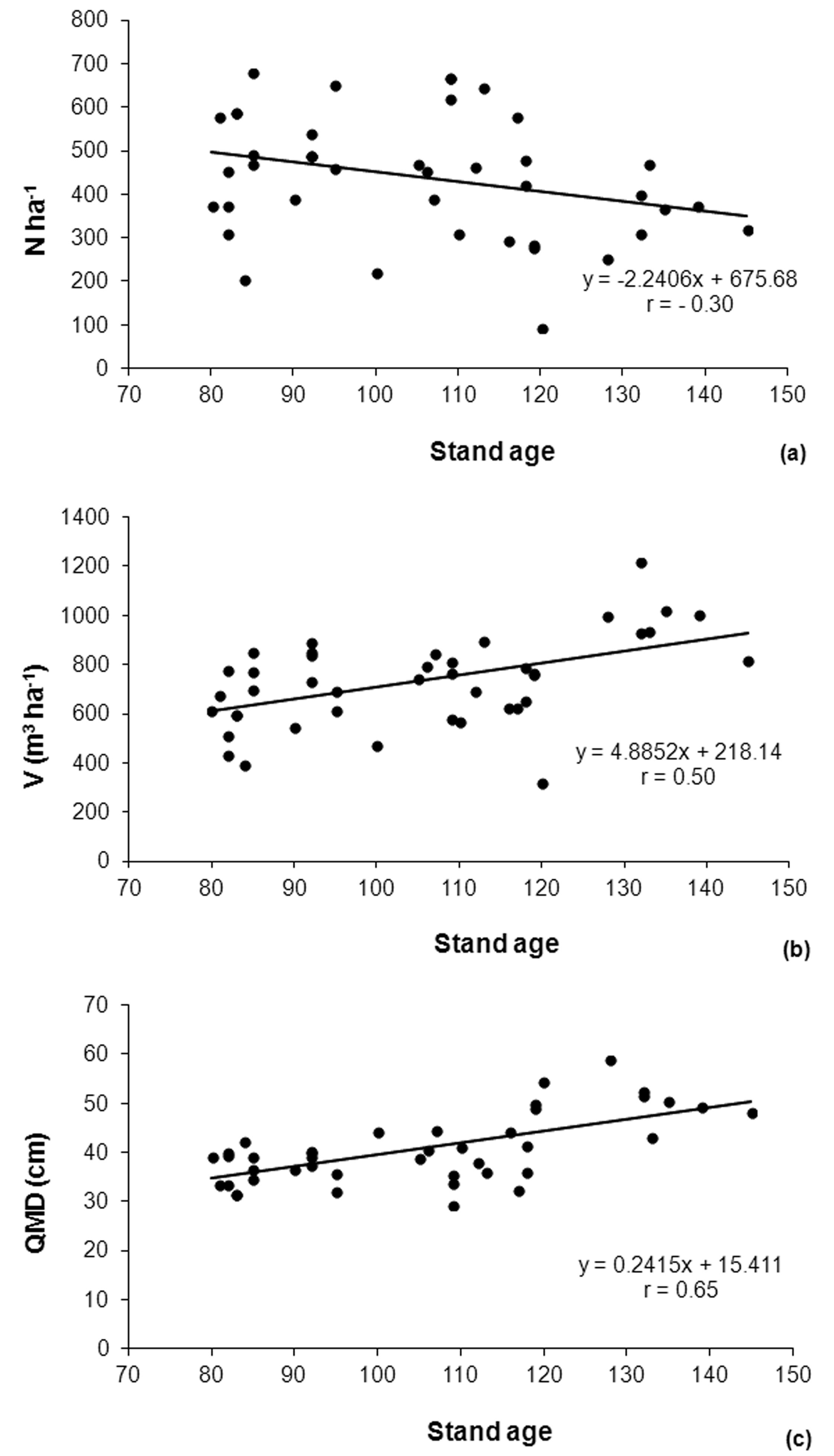
Significant relationships (p < 0.01) were found between fir stand age and all the examined structural parameters. With increasing age firs from the original plantation showed a general decreasing trend for N (r = -0.30), while V (r = 0.50) and QMD (r = 0.65) showed an increasing trend. Nevertheless, number of trees per hectare, volume per hectare and QMD of fir were very variable within the same age class (Fig. 3), depicting a very diversified situation. QMD had the highest correlation coefficient (r = 0.65) probably because it was less influenced by stand density compared to V ha-1.
Fig. 3 - Variation of main stand parameters of the original fir stands with increasing age. (a): number of trees per hectare; (b): volume per hectare; (c): quadratic mean diameter of trees (QMD).
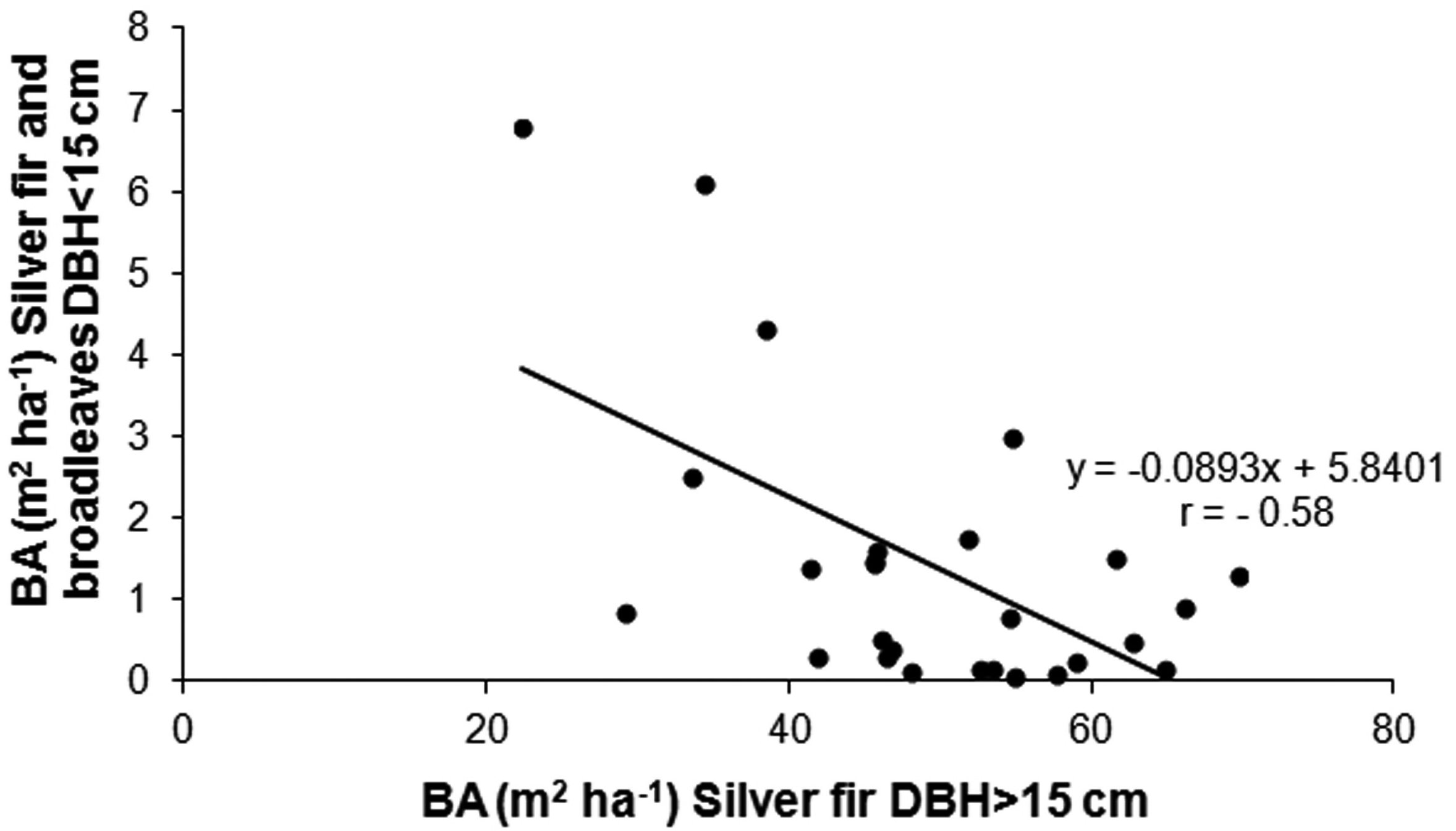
The relationship between BA ha-1 of the original silver fir component and BA ha-1 of the young firs and broadleaves (r = -0.58, p < 0.01) showed that the density of the regeneration stratum was inversely related to the density of the upper fir layer (Fig. 4).
Fig. 4 - Relationship between the basal area (BA) of the original silver fir stands and the basal area of fir and broadleaves in the lower layer (DBH < 15 cm).
Number of trees with DBH > 50 cm varied between 20 and 40 trees per hectare. Number of trees with DBH > 70 cm varied between 0 and 16 trees per hectare (Tab. 1).
Tab. 1 - Average number per hectare (standard deviation in brackets) of trees with DBH > 50 cm and DBH > 70 cm.
| Stand age | DBH > 50 cm | DBH > 70 cm |
|---|---|---|
| 80-89 | 20 (13) | 14 (-) |
| 90-99 | 25 (20) | 0 |
| 100-109 | 26 (20) | 16 (-) |
| 110-119 | 23 (16) | 14 (8) |
| 120-129 | 21 (14) | 14 (7) |
| 130-139 | 27 (26) | 10 (7) |
| 140-149 | 40 (17) | 0 |
Gap dynamics
From 1983 to 2007 the number of gaps and their total area increased, following the increasing stand age (Tab. 2). Gap fraction was 1.2 % in 1983, 1.7 % in 1997 and 2.9 % in 2007.
Tab. 2 - Number of gaps, mean gap size, total gap area and gap fraction detected in 1983, 1997 and 2007.
| Characteristics | 1983 | 1997 | 2007 |
|---|---|---|---|
| Number of gaps | 39 | 98 | 128 |
| Mean gap size (ha) | 0.03 | 0.02 | 0.02 |
| Standard deviation of gap size (ha) | 0.03 | 0.02 | 0.02 |
| Maximum gap size (ha) | 0.11 | 0.18 | 0.12 |
| Total gap area (ha) | 1.24 | 1.79 | 3.02 |
| Gap fraction | 1.2 | 1.7 | 2.9 |
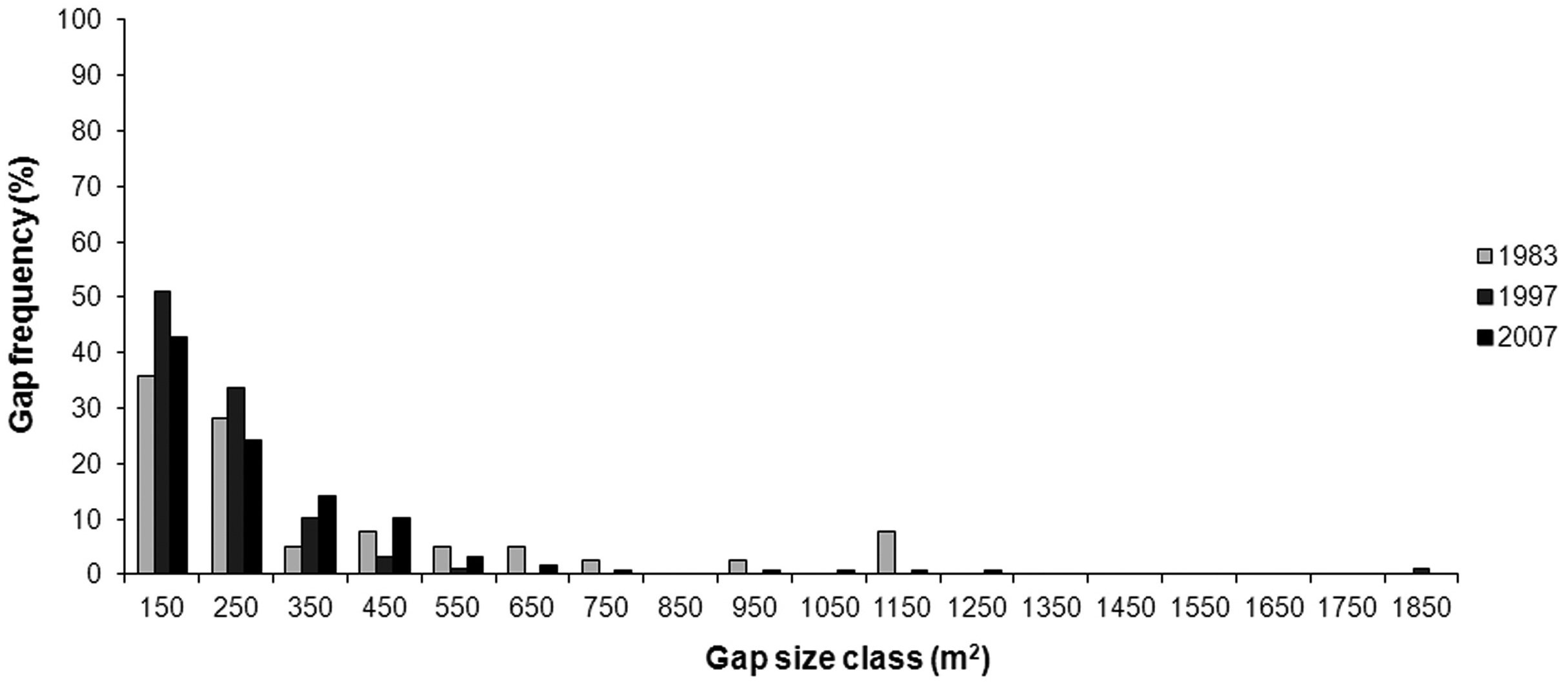
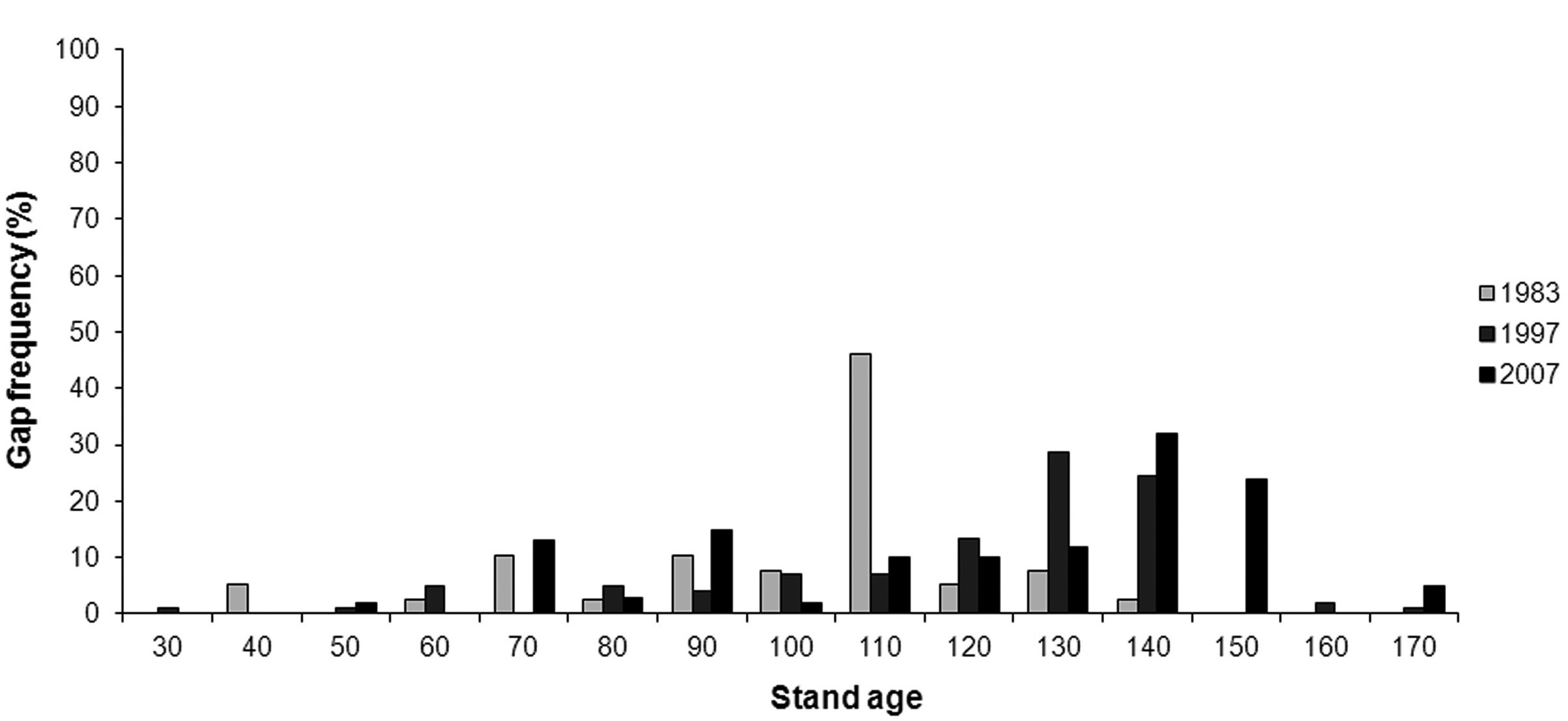
Most gaps had an area less or equal to 250 m2 in all three surveyed years (Fig. 5); these gaps were 64 % of total number of gaps mapped in 1983, 85 % in 1997 and 67 % in 2007. Small gaps (≤ 250 m2) covered a total of 3 477 m2 (28 % of total gap area) in 1983, 11 408 m2 (64 %) in 1997 and 11 429 m2 (38 %) in 2007. The majority of all digitized gaps occurred in stands older than 100 years (Fig. 6).
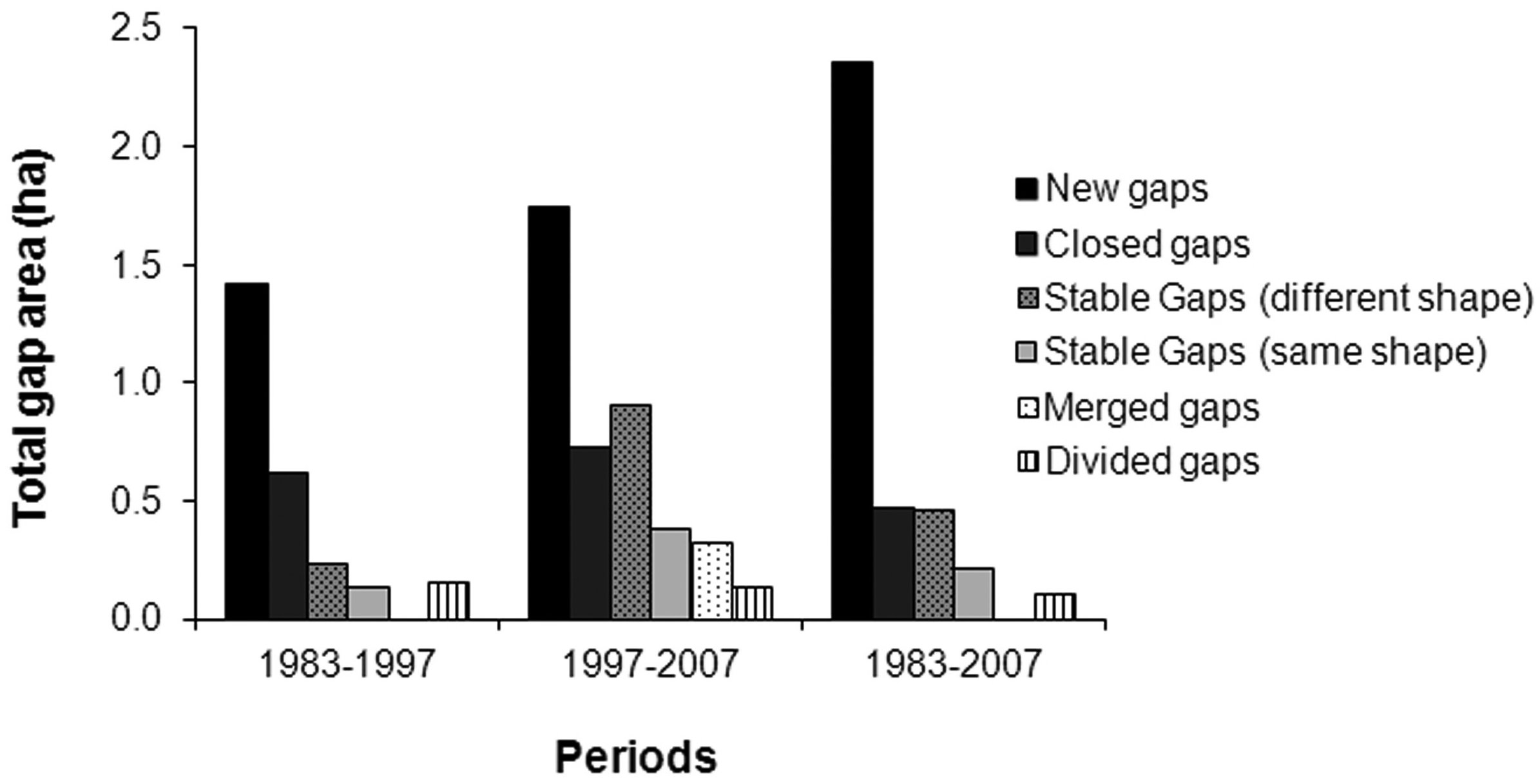
Between 1983 and 2007 a total of 107 new gaps were formed (total area: 2.4 ha), 20 gaps were closed (total area: 0.5 ha) and 21 gaps remained stable (total area: 0.7 ha). In 1997-2007 seven gaps merged (total area: 0.3 ha) and six gaps were divided (total area: 0.1 ha - Fig. 7).
Natural regeneration
The average number of seedlings and saplings per hectare in all surveyed gaps was 16 110 ± 16 646 (standard deviation); mean height was 0.7 ± 2.1 m.
Overall, fir was the dominant species (> 80 %) in the regeneration layer, but over 68 % were seedlings and 23 % were in the < 0.5 m height class. Broadleaves had a smaller number of individuals per hectare, but on average 25 % of these were in the > 2 m height class (Tab. 3).
Tab. 3 - Average number of seedlings and saplings per hectare for all surveyed gaps (standard deviation in brackets).
| Heigth class | Silver fir | Sycamore maple |
Beech | Chestnut | Other broadleaves |
Other conifers |
|---|---|---|---|---|---|---|
| Seedlings | 8833 (2330) |
1309 (1196) |
9 (15) |
18 (15) |
62 (41) |
0 |
| < 0.5 m | 2936 (1631) |
168 (77) |
141 (55) |
53 (53) |
159 (70) |
0 |
| 0.5 - 1 m | 424 (46) |
0 | 203 (41) |
18 (31) |
35 (41) |
9 (15) |
| 1 - 2 m | 433 (232) |
9 (15) |
80 (27) |
0 | 27 (34) |
18 (22) |
| > 2 m | 354 (110) |
176 (188) |
319 (27) |
44 (55) |
230 (55) |
44 (15) |
| Total | 12980 (3655) |
1662 (552) |
752 (118) |
133 (22) |
513 (89) |
71 (18) |
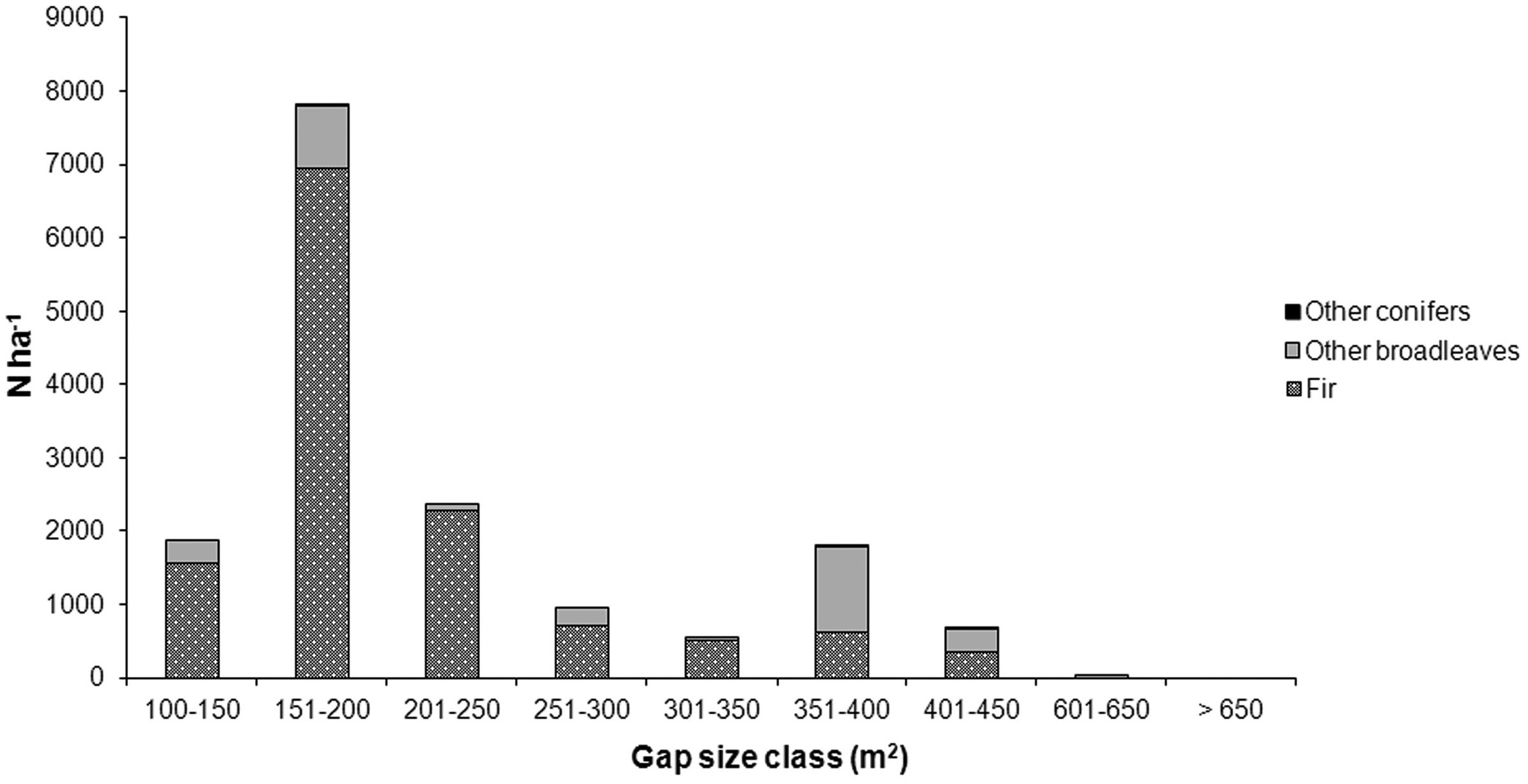
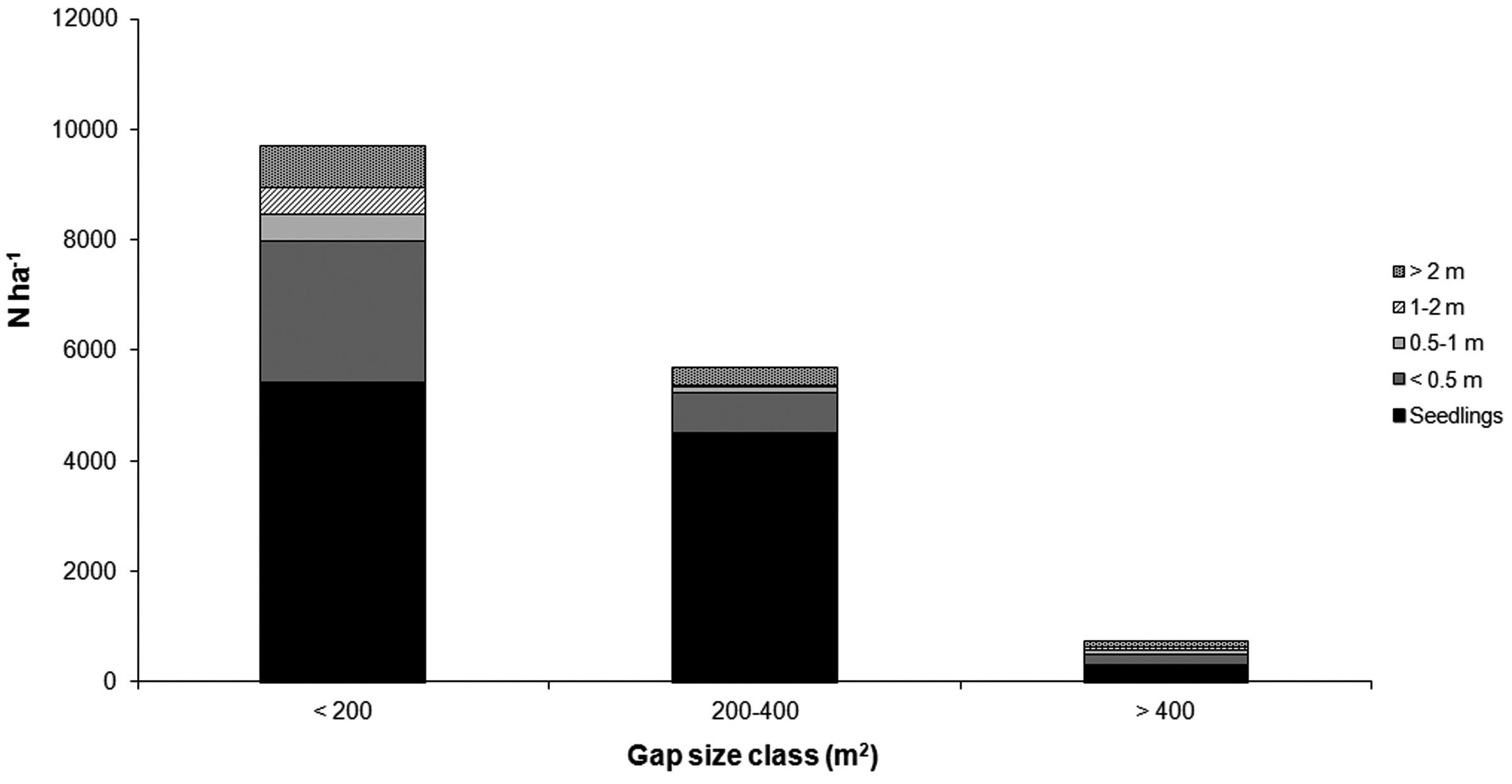
Most seedlings and saplings grew in gaps < 200 m2 (15 gaps out of 30 - Fig. 8). Fir was the prevailing species in most gaps, especially in gaps ranging from 100 to 250 m2. Other broadleaves (beech and sycamore ma- ple) were often associated with fir and they were relatively more abundant in 350-400 m2 gaps (Fig. 8).
Mean number of seedlings and saplings (combined in the five height classes) did not differ significantly (Mann-Whitney U-test) among the three gap size classes (< 200 m2, 200-400 m2 and > 400 m2), except for fir seedlings and fir < 0.5 m which were significantly more abundant in the smaller gap size (Tab. 4, Fig. 9).
Tab. 4 - Average number of seedlings and saplings per hectare (standard deviation in brackets) in relation to height classes and gap size (n = number of surveyed gaps; means with different letters are statistically different (α ≤ 0.05) using Mann-Whitney U-test).
| Heigth class |
Gap size | All | Silver fir | Sycamore maple |
Beech | Chestnut | Other broadleaves |
Other conifers |
|---|---|---|---|---|---|---|---|---|
| Seedlings | < 200 m2 (n=15) |
5420 (906) |
5323a (905) |
71a (100) |
0a | 0a | 27a (-) |
0a |
| 200-400 m2 (n=11) |
4501 (1135) |
3395ab (1427) |
1043a (1191) |
9a (19) |
18a (15) |
35a (41) |
0a | |
| > 400 m2 (n=4) |
309 (188) |
115b (41) |
195a (151) |
0a | 0a | 0a | 0a | |
| < 0.5 m | < 200 m2 (n=15) |
2555 (1491) |
2246a (1410) |
106a (46) |
71a (15) |
35a (38) |
97a (100) |
0a |
| 200-400 m2 (n=11) |
716 (227) |
557b (279) |
44a (55) |
53a (27) |
18a (15) |
44a (15) |
0a | |
| > 400 m2 (n=4) |
186 (236) |
133b (230) |
18a (15) |
18a (15) |
0a | 18a (31) |
0a | |
| 0.5-1 m | < 200 m2 (n=15) |
495 (93) |
336a (55) |
0a | 124a (55) |
9a (15) |
27a (46) |
0a |
| 200-400 m2 (n=11) |
115 (81) |
44a (41) |
0a | 53a (27) |
9a (15) |
9a (15) |
0a | |
| > 400 m2 (n=4) |
80 (46) |
44a (77) |
0a | 27a (27) |
0a | 0a | 9a (15) |
|
| 1-2 m | < 200 m2 (n=15) |
469 (188) |
354a (173) |
9a (15) |
80a (27) |
0a | 18a (31) |
9a (15) |
| 200-400 m2 (n=11) |
44 (41) |
35a (31) |
0a | 0a | 0a | 9a (15) |
0a | |
| > 400 m2 (n=4) |
53 (70) |
44a (55) |
0a | 0a | 0a | 0a | 9a (15) |
|
| > 2 m | < 200 m2 (n=15) |
752 (201) |
239a (116) |
150a (55) |
256a (41) |
35a (31) |
62a (41) |
9a (15) |
| 200-400 m2 (n=11) |
318 (46) |
88a (15) |
27a (27) |
27a (46) |
9a (15) |
133a (-) |
35a (31) |
|
| > 400 m2 (n=4) |
97 (31) |
27a (27) |
0a | 35a (38) |
0a | 35a (15) |
0a |
Fig. 9 - Average number per hectare of seedlings and saplings in relation to gap size and height classes.
Sycamore maple was more frequent in the seedling stage and in the < 0.5 m and > 2 m height classes; seedlings were more abundant in 200-400 m2 gaps. Beech was more abundant in the > 2 m height class, but did not show differences between the three gap sizes, except for seedlings which concentrated in the 200-400 m2 gaps. Other broadleaves were present in all height classes without clear trends in gap size distribution. Chestnut and other conifers were generally less frequent. The latter were absent in the < 0.5 m and 0.5 - 1 m height classes.
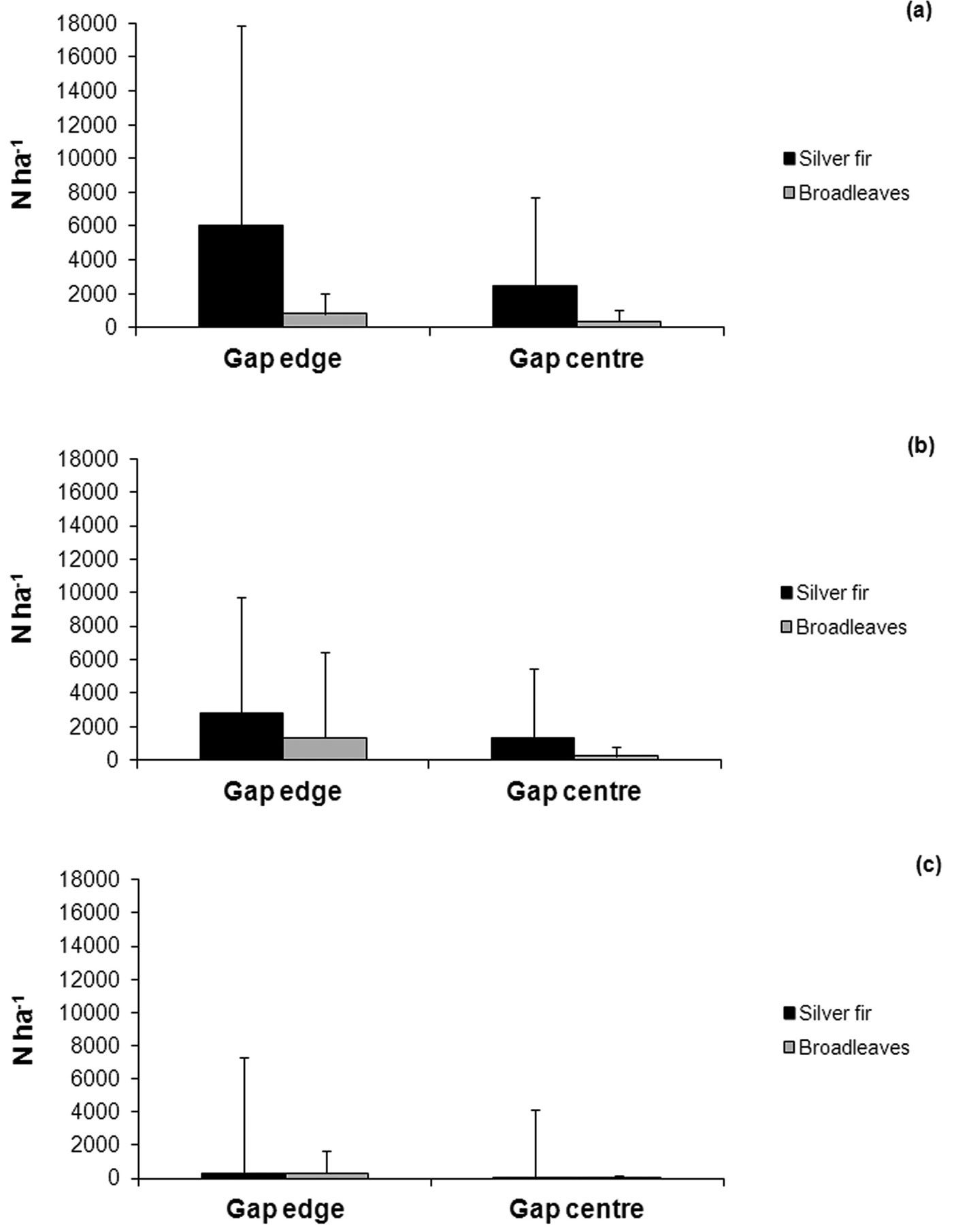
No significant difference (Mann-Whitney U-test) was observed in fir and broadleaves regeneration density between gap centers and gap edges (Fig. 10).
Fig. 10 - Average number per hectare and standard deviation of seedlings and saplings in relation to gap size (< 200 m2 (a), 200-400 m2 (b), > 400 m2 (c)) and within-gap position.
Impact from wild ungulates was most obvious in the < 0.5 m and 0.5-1 m height classes, both on fir and broadleaves, where 15 to 75 % of all individuals showed unambiguous signs of grazing. As expected, seedlings were generally not affected, while there was still some damage in the 1-2 m height class (11 to 33 %). Based on our data, it was not possible to identify any clear relationship between gap size, tree species and damage intensity.
Discussion
Structural characteristics and natural regeneration in old-growth stands with fir have been extensively studied in Europe ([32], [15], [57], [90]). Some authors have compared managed stands with stands were active management has stopped for relatively long periods of time, examining the consequences of management cessation on stand structure, composition and old-growth characters ([53], [12], [91], [64], [86]).
The aging fir plantations in Vallombrosa have not been managed for over 35 years after traditional rotation age. Following management suspension these plantations have developed some peculiar characteristics: wherever stand density has not diminished after the death of single trees or small groups of trees, fir stands reached a high standing volume (over 1000 m3 ha-1 at age 120+), which alone is not a typical feature of old-growth ([7]); wherever fir density was lower, a layer of trees with DBH < 15 cm filled in the structure. Fifteen different broadleaved species were recorded in this layer, in varying proportions and usually mixed with fir. This indicates that these stands are evolving towards a mosaic of stands with different age, structure and composition, where fir regeneration alternates with beech and other broadleaves, with patterns similar to those of natural beech-fir forests ([71], [95]).
On average, the number of large living trees (DBH > 70 cm) recorded in our survey is about half the density of large living trees reported by Nilsson et al. ([68]) in old-growth forests in the nemoral forest region of central Europe (30 ha-1). Nevertheless, positive effects on biodiversity have already been detected: changes in breeding bird assemblages have been recently reported for this area of the Apennines (e.g., common treecreper, Certhia familiaris; black woodpecker, Dryocopus martius - [88]) and attributed to an evident increase in bark feeders directly dependent on the presence of large aging trees ([36], [89], [60]).
Although these fir stands studied are definitely anthropogenic, gap dynamics is driven by the same mechanism that creates canopy openings in natural temperate forests, i.e., the death of single trees or small groups of trees resulting in fine-scale gap dynamics ([80], [93], [62], [76], [33], [10], [96], [75]). In particular, the prevalence of small gaps (< 250 m2) was similar to gap size reported for mixed old-growth forests with beech and fir ([70], [32], [52], [90]).
Total gap area was a small percentage (2.9 % in 2007) of total surveyed area. This value is lower than the gap area generally observed in old-growth forests with fir ([52]). For example, in an old-growth forest dominated by beech and silver fir in the Dinaric Mountains of Bosnia and Herzegovina, Nagel & Svoboda ([66]) reported a percentage in canopy gaps ranging from 12 to 17.2 %. The gap fraction in Vallombrosa was similar to that (3.1 %) reported by Dobrowolska & Veblen ([32]) for mixed natural stands with silver fir in the Jata Reserve (Poland), described as stands with structural features corresponding to “optimal and terminal phases of stand development (sensu [55]) in which mature trees are beginning to die and create gaps”.
Many studies have focused on the importance of treefall gap openings in determining species compositions and controlling tree regeneration processes ([99], [100], [56], [10]). Our results showed that gap size had an influence on the total number of seedlings and saplings, but seems to have relatively limited influence on species diversity and on the developmental stage of the regeneration. In our survey, surrounding forest matrix was homogenous for all inventoried gaps, consisting of pure fir stands with very limited presence of broadleaves (mainly beech and sycamore) reaching the dominant layer (< 2 % of the total number of trees). Thus, seed source had a limited influence on species diversity in the regeneration layer. Only young firs (seedlings and fir < 0.5 m) appeared to be affected by gap size, being significantly more abundant in gaps < 200 m2. These findings are in accordance with what reported by Albanesi et al. ([1]), who found that fir seedling density was higher in small (185 m2) than in medium (410 m2) gaps created by cutting trees in a 90-years-old planted silver fir stand in the southern Apennines. However, the apparently limited influence of gap size and position within the gaps on tree species diversity may reflect the relatively small range of gap sizes observed in this study. The sampled gaps were at least 10 years old but interpretation of results must be cautious because time since gap creation can affect both the density of young trees and the relative heights of different tree species.
Our results on wild ungulate impact on natural regeneration was consistent with a previous survey carried out by Casanova et al. ([22]) who found that browsing damage to fir regeneration in the Vallombrosa Forest varied between 30 and 95 % of individuals. It is well known that young firs are subjected to heavy browsing by wild ungulates ([2], [34], [43], [44], [63], [84]). Increasing evidence of such impact is being reported in the Alpine region ([63], [23], [21]) but few studies have been carried out in the Apennines. However, despite the outstanding density of wild ungulates in the Vallombrosa Forest, we observed a diffused understory of seedlings and saplings of various species wherever the density of the dominant fir layer was reduced or gaps had opened up. This confirms that natural regeneration is a highly variable process in both space and time, and browsing could be only one of the factors driving seedling establishment and subsequent growth and survival ([84]).
Conclusions
Due to their management history, pure even-aged fir stands beyond their traditional rotation age (100 years) did not exist until recently in the Apennines. Unprecedented dynamics are taking place in these stands, influencing both their future development and their capacity of sustaining important ecosystem services and biodiversity conservation. This is particularly important in the case of the Vallombrosa fir stands for their role as a Natura 2000 site. Our results show that in the last decades a gradual shift from the simplified structure of the fir plantations to stands with diverse and complex structures is taking place. These changes are favoring an increase in the overall biodiversity, in line with the aims of the protected area. However, factors such as increasing wild ungulate pressure, significant changes in climatic parameters and further aging of the remaining fir trees, might contribute in accelerating or shifting this trend towards different outcomes. Thus, these processes must be carefully monitored in order to assess whether the protection goals are being met.
The fact that no active management has been carried out in the forest in the last decades led to a trade-off between wood production, which has decreased, and biodiversity which is increasing. This trade-off could be partly reduced by active management in the younger fir stands (< 80 years) where moderate thinnings may help in increasing individual fir stability and may favor the natural diffusion of broadleaves and fir regeneration. Furthermore, some small gaps could be opened up artificially in the older fir stands having uniform and dense structures. According to our results, gap size should range between 100 and 200 m2 to favor the natural recruitment of both fir and other species.
Recent changes in management goals have put in a new perspective the natural dynamics taking place in aging, pure even-aged fir stands in the Apennines. These stands are becoming important elements for biodiversity conservation while slowly acquiring a monumental and old-growth character which should be valued and protected.
Acknowledgments
We wish to thank two anonymous reviewers for their helpful suggestions and comments on an early version of the manuscript.
This study was partially supported by the University of Florence, Fondi di ricerca di Ateneo 2010 “La rinaturalizzazione dei soprassuoli di abete bianco di origine artificiale in seguito all’applicazione del Piano di Gestione della Riserva Naturale di Vallombrosa (FI)” (Scientific coordinator: Susanna Nocentini).
References
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Davide Travaglini
Silvia Fiorentini
Chiara Lisa
Susanna Nocentini
Department of Agriculture, Food and Forestry Systems, University of Florence, v. S. Bonaventura 13, I-50145, Florence (Italy)
Corresponding author
Paper Info
Citation
Bottalico F, Travaglini D, Fiorentini S, Lisa C, Nocentini S (2014). Stand dynamics and natural regeneration in silver fir (Abies alba Mill.) plantations after traditional rotation age. iForest 7: 313-323. - doi: 10.3832/ifor0985-007
Academic Editor
Emanuele Lingua
Paper history
Received: Mar 04, 2013
Accepted: Dec 19, 2013
First online: Apr 08, 2014
Publication Date: Oct 01, 2014
Publication Time: 3.67 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2014
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 46314
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 38896
Abstract Page Views: 2137
PDF Downloads: 3912
Citation/Reference Downloads: 24
XML Downloads: 1345
Web Metrics
Days since publication: 3673
Overall contacts: 46314
Avg. contacts per week: 88.27
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Feb 2023)
Total number of cites (since 2014): 11
Average cites per year: 1.10
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Effects of gap size and within-gap position on seedlings establishment in silver fir stands
vol. 1, pp. 55-59 (online: 28 February 2008)
Research Articles
Short- and long-term natural regeneration after windthrow disturbances in Norway spruce forests in Bulgaria
vol. 11, pp. 675-684 (online: 23 October 2018)
Research Articles
Optimizing silviculture in mixed uneven-aged forests to increase the recruitment of browse-sensitive tree species without intervening in ungulate population
vol. 11, pp. 227-236 (online: 12 March 2018)
Research Articles
Species interactions in pure and mixed-species stands of silver fir and European beech in Mediterranean mountains
vol. 14, pp. 1-11 (online: 02 January 2021)
Research Articles
Regeneration of Abies pinsapo within gaps created by Heterobasidion annosum-induced tree mortality in southern Spain
vol. 7, pp. 209-215 (online: 27 February 2014)
Research Articles
Post-fire recovery of Abies cephalonica forest communities: the case of Mt Parnitha National Park, Attica, Greece
vol. 11, pp. 757-764 (online: 15 November 2018)
Research Articles
Post-fire effects and short-term regeneration dynamics following high-severity crown fires in a Mediterranean forest
vol. 5, pp. 93-100 (online: 30 May 2012)
Research Articles
Modelling natural regeneration of Oak in Saxony, Germany: identifying factors influencing the occurrence and density of regeneration
vol. 16, pp. 47-52 (online: 16 February 2023)
Research Articles
Methods for predicting Sitka spruce natural regeneration presence and density in the UK
vol. 12, pp. 279-288 (online: 23 May 2019)
Research Articles
Density and spatial distribution of beech (Fagus sylvatica L.) regeneration in Norway spruce (Picea abies (L.) Karsten) stands in the central part of the Czech Republic
vol. 9, pp. 666-672 (online: 12 March 2016)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword