Bird composition and diversity in oak stands under variable coppice management in Northwestern Turkey
iForest - Biogeosciences and Forestry, Volume 11, Issue 1, Pages 58-63 (2018)
doi: https://doi.org/10.3832/ifor2489-010
Published: Jan 25, 2018 - Copyright © 2018 SISEF
Research Articles
Abstract
Coppice management results in profound differences in forest structure and composition, which in turn can modify habitat value for bird species. We measured bird species richness and composition at 50 sample plots in pure oak forest stands in northwestern Turkey, which differed in age, cover and height in association with coppice management. We recorded a total of 38 bird species and 699 individuals across all stands. Regression-based multimodel inference showed that structural features of forest stands strongly affect bird diversity and abundance. While canopy cover and tree height affect bird diversity positively, elevation of sampling plots, tree density and tree diameter at breast height (DBH) had a negative effect. In addition, constrained ordination analyses revealed that canopy cover was the most important factor influencing bird species composition. Forest stands that have 42-85% canopy cover, i.e., a few (2009-2580 oak trees) large tall (13.36-15.78 m) trees, were the most preferred habitat by bird species. However, we also found that different bird species favor different stand structural features. Thus, variation in stand structure from maintaining some coppice management across the landscape may be beneficial for rare or endangered species and result in greater landscape level biodiversity.
Keywords
Avian Fauna, Canopy Height, Vegetation Seral Stage, Canopy Cover, Multi-model Inference, Thrace
Introduction
Coppice management was widespread throughout European forests for centuries but was largely abandoned in the second half of the 19th century ([36], [47]), mostly due to the substitution of firewood with fossil fuels ([46]) and changes in silvicultural practices ([43]). In Turkey, coppicing has been a common practice for hundreds of years ([32]). However, the Turkish General Directorate of Forestry abandoned the practice in 2006, and a “close-to-nature” approach has been adopted where conversion to high forest originating from seed is promoted. This approach reflects a shift from a pure timber production objective to a more holistic objective that includes habitat conservation for wildlife ([9]).
Coppicing directly alters the structural features of the forest canopy such as height and cover, and influences the seral stage of the vegetation community. Because it favors vegetative regeneration, coppice management can also result in low genetic diversity of the stands ([7]). Nonetheless, coppicing is still commonly used as a forest management system in southeastern Europe ([36]). In addition, there has been a renewed interest in utilizing coppicing as a tool for biomass production and nature conservation ([47]).
Being high in the food chain, birds are very sensitive to environmental change ([19]). Breeding bird population density and community composition have been shown to vary with changes in coppice age ([17], [18]). Accordingly, forestry practices that alter the age and structure of forests, such as coppicing are highly relevant for bird species conservation ([36]). Bird abundance often increases with forest maturity, altitudinal position, and development (cover and height) of the shrub layer ([14]). However, each species may have a different habitat preference or ecological niche associated with cover and height of trees within forests ([28]).
Mature forests are important for many bird species, especially to cavity-nesting ones ([33]). In European forests, bird species richness is generally higher in unmanaged stands than in less mature managed stands ([39]). Soil fertility, the diversity of vertical structure, and breaks in the forest cover may all influence bird populations ([34]). Tree composition ([12]) and forest structure ([28]) can also strongly affect the composition and diversity of bird species in forests. However, despite harboring fewer species overall, studies have shown that young clear-cuts or small forest gaps support vastly different bird communities and may increase the regional species pool by harboring rare species ([51]). Additionally, bird-community composition changes with successional stages associated with different management regimes ([17]). For example, earlier stages of coppice are suitable for specialized bird species, but the requirements of other more abundant species are met more often in the old coppice ([16]).
According to DeGraaf et al. ([13]), forest structure has a stronger effect on bird diversity than forest cover type or stand size class. There is however little knowledge of the relationship between forest structural characteristics and wildlife abundance in Turkey. Particularly, no information is available on the responses of bird species to conversion of coppice to high oak forest and the structural stages in between. We thus sought to determine bird species richness and composition under oak stands with varying structural characteristics associated with coppice management in Thrace, a region of northwestern Turkey where oak forests and woodlands constitute a large proportion of wildlife habitat. In doing so, we seek to better understand the role of coppicing in supporting bird conservation in the region.
Material and methods
Study area
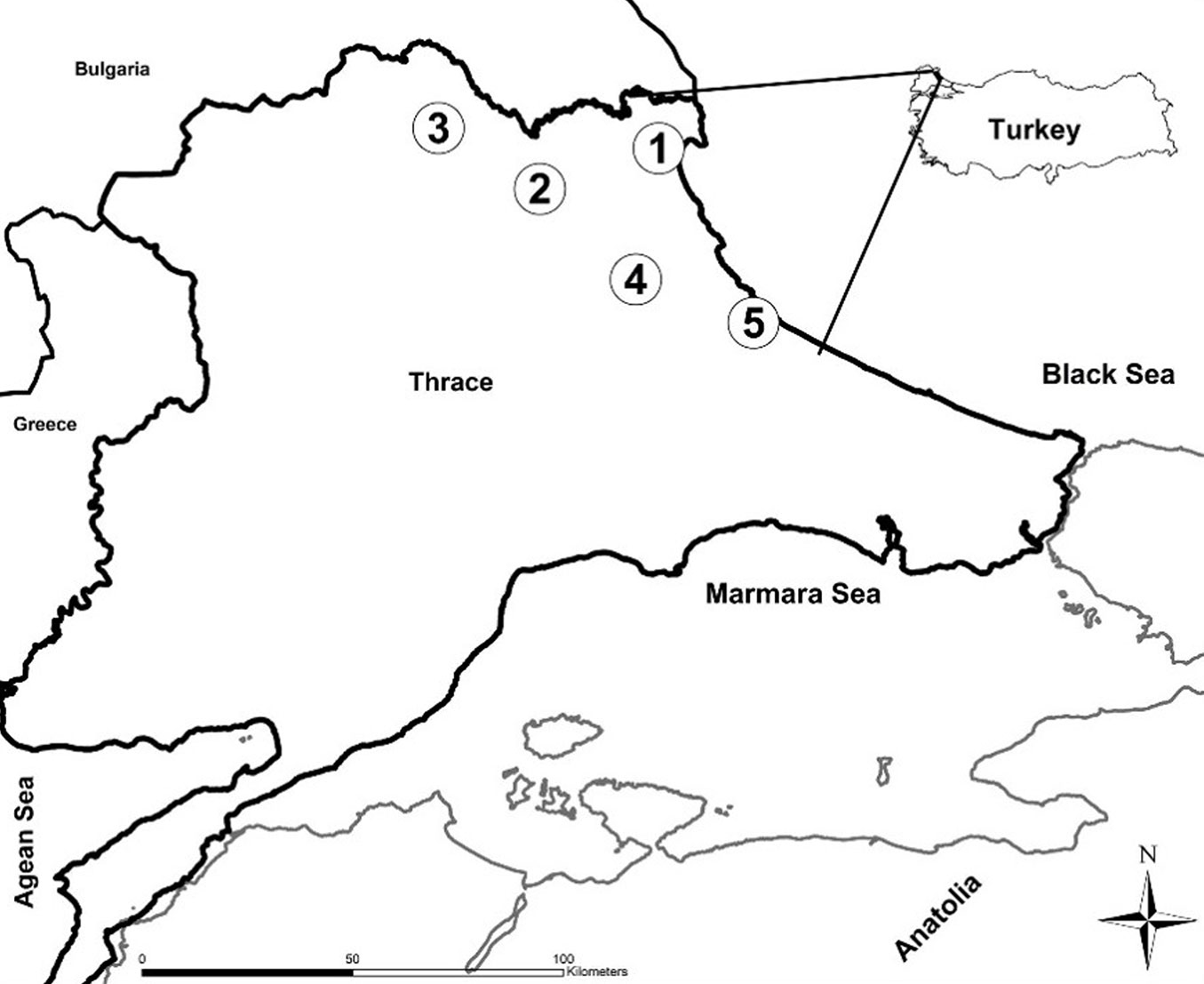
This study was conducted on pure oak stands in northwestern Turkey, within the region of Thrace (Fig. 1). Oak species are the most commonly coppiced trees in Turkey. Forests and woodland dominated by oak cover vast areas (5.2 million ha) of Turkey ([37]). In Thrace oaks constitute 71.7% of forest lands (around 400.000 ha - [32]). Over the past centuries, Thracian forests have suffered heavy destruction, and 85% of old broadleaf forests had changed to coppice forests making it difficult to come across big old oak trees ([15]). However, recent changes imposed by the Turkish Forestry Directorate favor the maintenance of a tall canopy composed of naturally regenerating trees ([9]).
Fig. 1 - Geographic location of the sampling sites in Turkey. (1) Igneada; (2) Demirkoy; (3) Kirklareli; (4) Vize; (5) Catalca.
In our study, five different sites were chosen to capture the variation in oak forests in Thrace. The selected sampling plots all represented coppice originated oak stands, with varying dominance of three major oak species: Sessile oak (Quercus petraea [Mattuschka] Liebl.), Hungarian oak (Quercus frainetto Ten.), and Turkey oak (Quercus cerris L. - [32]). However, the sites differed considerably in environmental and climatic characteristics ([32] - Tab. 1).
Tab. 1 - Main climatic characteristics of sampling sites ([32]).
| Sampling Site | Elevation (m a.s.l.) |
Mean annual precipitation (mm) |
Average annual temp. (°C) |
Annual water deficit (mm) |
|---|---|---|---|---|
| Igneada | 125 | 867 | 13 | 181 |
| Demirkoy | 680 | 1053 | 11 | 84 |
| Kirklareli | 500 | 550 | 14 | 274 |
| Vize | 320 | 720 | 12 | 244 |
| Catalca | 290 | 844 | 14 | 212 |
Bird sampling
For avifauna sampling, we selected a total of 50 plots (100 × 100 m; 1 ha) with ten from each of the different sampling sites (Catalca, Demirkoy, Igneada, Kirklareli and Vize). The minimum distance among sampling plots was 1 km. Sampling was conducted two times: late April and then early August of 2010. Bird observations were done during the morning from sunrise to 11:00 and from 15:00 to sunset. If a plot was counted in the morning in the spring, the second counting procedure for same plot was made after 15:00 in early August. In our analysis, we used total number of bird counts for each sampling plots. Each count was made by directly observing and hearing sounds of birds in 15 minutes intervals in the center of the sampling plots. In addition, we recorded habitat types, tree species, elevation and canopy cover. Observations were obtained via a single observer point count methodology ([4]). The species identification was based on several field guides ([48]). The bird species were categorized into guilds according to their feeding preferences as carnivore, carnivore-insectivore, granivore, granivore-frugivore, insectivore, insectivore-frugivore, insectivore-granivore, insectivore-granivore-frugivore or omnivore based on Perrins ([40]) to analyze relationships between stand type and feeding guilds.
Stand structure measurements
In the past, our sample plots were managed by clear cut coppicing in 20 years rotations. However, the exact history of the rotations and the clear-cut schedules were unknown for the study sites. Accordingly, a complete inventory was taken of each stand to characterize the tree development stage (mostly July and August) in 2008, 2009, and 2010 ([32]). We selected our sampling plots from these vegetation plots. Plot coordinates and elevation were determined by GPS. In each sample plot (20 × 20 m quadrats), we recorded tree species, measured tree density, DBH (diameter at breast height), height and cover (canopy). All data were converted to per hectare basis except cover. Cover was assessed as one of four categories: 0-10%, 11-40%, 41-70 and 70-100%. Our stand structure sampling protocol is described in much greater detail in Makineci et al. ([32]).
Data analysis
We calculated bird species richness as the total number of species recorded at the plots, and species diversity using the Shannon-Weaver index ([30]). Response of bird species richness and diversity to individual stand structural attributes (canopy cover, height, tree density, DBH and elevation etc.) were assessed using multimodel inference ([6]) within a mixed modeling framework ([49]). Initially, a global model was obtained with all stand structural attributes and elevation (as co-variate) which were included as fixed effects using the “lme” and “nlme” functions of the nlme package ([41]) within the R software environment for statistical computing and graphics ([42]). Site was also included in the models as a random effect to account for the five separate administrative regions our data was collected from. A log transformation was used on the response in order to meet normality when necessary and Poisson distribution was used for species richness. All of the fixed effect variables were standardized prior to analysis to allow for comparison of effect sizes among parameter estimates. Models were validated by checking the distribution of the residuals graphically to ensure a random distribution. Top models, i.e., those with a difference in AICc of no more than 4 from the best model, were determined using the “dredge” function of MuMIn package ([3]) in R. Model averaging ([6]) was then performed using the top models with the MuMIn function “model.avg” to obtain the averaged coefficient values and relative importance of all fixed effects within the top models. Because all of the tree species in our sites were deciduous oaks, we did not expect the bird community to vary with tree species composition. Nonetheless, we initially assessed whether oak species composition was related to bird diversity and composition based on the correlation between bird species richness and diversity with the proportion of one oak species versus another graphically and statistically using Spearman’s rank correlation with the “cor” function in R. If the effect was not significant, tree species composition was not considered further in the analyses.
In order to assess the association of individual species with the different stand types we performed indicator species analysis using a multi-level pattern analysis approach ([11]) using the R package “indicspecies”. Multi-level pattern analysis allows for the association of a species to be made to one or more groups (i.e., clusters) of sites based on both the presence and the absence of the species within the groups. This is particularly important for our study because to understand the role of woodland management in bird conservation, we need to assess the dependence of individual species to multiple forest stand types created by coppicing and logging activities. In order to achieve this, we first used cluster analysis to characterize stand types representing the variation in tree structural attributes (i.e., cover, height, density, DBH) across our study plots. K-means clustering ([20]) was conducted with the R base function “kmeans”. The resulting stand types were then used as groupings for the species indicator analysis.
Finally, we conducted Constrained Correspondence Analysis (CCA) to assess the compositional response of the bird community to the structural attributes (i.e., cover, height, density, DBH) of our forest stands ([27]). In order to partial out any variation in bird composition associated with the administrative regions and elevation, we also included these factors as conditions in our CCA using the “cca” function of the vegan package ([38]) in R. The distribution of sites belonging to the different stand structural types determined from the cluster analysis was displayed on the ordination plot using the “ordiellipse” function in vegan. This multivariate approach allowed us to create an ordination that best represents the relative influence of individual stand structural attributes on the avian community as a whole.
Results
Description of sampled bird assemblage
Thirty-eight bird species and 699 individuals were recorded during the survey in our sampling plots (Tab. S1 in Supplementary material). Nine feeding guilds were determined, i.e., carnivore (3 species), carnivore-insectivore (1 species), granivore (2 species), granivore-frugivore (2 species), insectivore (8 species), insectivore-frugivore (8 species), insectivore-granivore (7 species), insectivore-granivore-frugivore (5 species), and omnivore (2 species - Tab. S1). The most observed species were Fringilla coelebs (207 birds), Parus major (80 birds) and Sitta europea (64 birds - Tab. S1).
Effect of stand structure on bird richness and diversity
The relative strength of the effect of the stand structural variables on bird species richness and diversity in decreasing order of importance were canopy cover, elevation, tree density, tree height, and mean tree DBH (Tab. 2, Tab. S2 in Supplementary material). Bird species richness and diversity increased with both canopy cover and tree height, while decreasing with elevation, tree density, and tree DBH (Tab. 2, Tab. S2). There was no significant (P >0.1) difference in bird species richness and diversity among plots dominated by the different oak species (Quercus petraea, Q. frainetto and Q. cerris). The proportion of oak species across sites was thus not included in further analyses.
Tab. 2 - Averaged coefficient values, estimate (β) and standard error (SE), and relative importance (RI) of fixed effects from multiple top regression models predicting the influence of site factors on bird species richness and diversity. See Tab. S2 in Supplementary material for details on top models.
| Effect | Richness | Diversity | ||||
|---|---|---|---|---|---|---|
Est. β |
SE | RI | Est. β |
SE | RI | |
| CC | 0.13345 | 0.07960 | 1.00000 | 0.11492 | 0.07514 | 1.00000 |
| E | -0.00087 | 0.00034 | 0.85605 | -0.00083 | 0.00032 | 0.78870 |
| TD | -0.00002 | 0.00001 | 0.44373 | -0.00001 | 0.00001 | 0.37331 |
| TH | 0.02636 | 0.02691 | 0.37235 | 0.01814 | 0.02468 | 0.29726 |
| DBH | -0.00312 | 0.02669 | 0.25492 | -0.00350 | 0.02228 | 0.23728 |
Variation in stand structural types
Four distinct stand types (A, B, C and D) were obtained by the cluster analysis (Tab. 3). Both type A and C consisted of relatively few, large and tall oak trees, but type A displayed much greater canopy cover overall (Tab. 3). Type B and D both consisted of mainly small short trees (Tab. 3). Type B also had relatively few trees and low canopy cover (Tab. 3). In contrast, Type D had nearly six times as many trees and displayed high canopy cover (Tab. 3).
Tab. 3 - Summary of structural attributes and description of four stand types determined by cluster analysis of plots using mean tree cover, density, diameter at breast height (DBH), and height.
| Stand type |
Average Cover (%) |
Density (trees ha-1) |
DBH (cm) |
Height (m) |
Description |
|---|---|---|---|---|---|
| A | 85 | 2580 | 18.10 | 15.78 | High canopy cover, few large tall trees |
| B | 22 | 4898 | 4.60 | 3.60 | Low canopy cover, few small short trees |
| C | 42 | 2009 | 17.42 | 13.36 | Moderate canopy cover, few large tall trees |
| D | 78 | 28537 | 3.95 | 4.75 | High canopy cover, many small short trees |
Bird species associated with different stand structural types
Twenty-four bird species were observed only in one stand type (A, B, C, or D), eight species in two stand types (AB, AD, BC, or CD) and six bird species in three stand types (ABC, ACD, or BCD - Tab. S4). For A and C stands we recorded the highest number of bird species: 20 bird species were found in A, 19 bird species in C. In B stands we observed 11 bird species, while the lower number of bird species (only 8) was observed in D stands. Three of them were observed only in D stands, whereas the others were also observed in A, B and C stands. In addition, in A and C stands 632 individuals (90%) from 28 insectivorous bird species were recorded (Tab. S3 in Supplementary material).
According to multilevel pattern analysis, seven bird species were indicative species. B. buteo, P. collybita, T. melba and T. viscivorous were indicative species of stand type A, G. glandarius of stand type C, C. corax of stand type D, and S. europaea were indicative species of A and D stand types (Tab. 4, Tab. S4).
Tab. 4 - Association of a bird species with a particular stand type is indicated by 1. (*): 0.05<p≤0.10; (**): p≤0.05.
| Species | Stand Type | Prob. | |||
|---|---|---|---|---|---|
| A | B | C | D | ||
| Buteo buteo | 1 | 0 | 0 | 0 | * |
| Corvus corax | 0 | 0 | 0 | 1 | ** |
| Garrulus glandarius | 0 | 0 | 1 | 0 | * |
| Phylloscopus collybita | 1 | 0 | 0 | 0 | * |
| Sitta europaea | 1 | 0 | 0 | 1 | ** |
| Tachymarptis melba | 1 | 0 | 0 | 0 | * |
| Turdus viscivorus | 1 | 0 | 0 | 0 | * |
We found that widely distributed species were Fringilla coelebs, Parus major, Turdus merula, Dendrocopos medius, Picus canus and Lanius collurio, observed in three stand types (Tab. S4). These species were also highly abundant across our plots with 207 Fringilla coelebs, 80 Parus major and 64 Sitta europaea counted during the sampling.
Variation in bird assemblage composition with stand structure
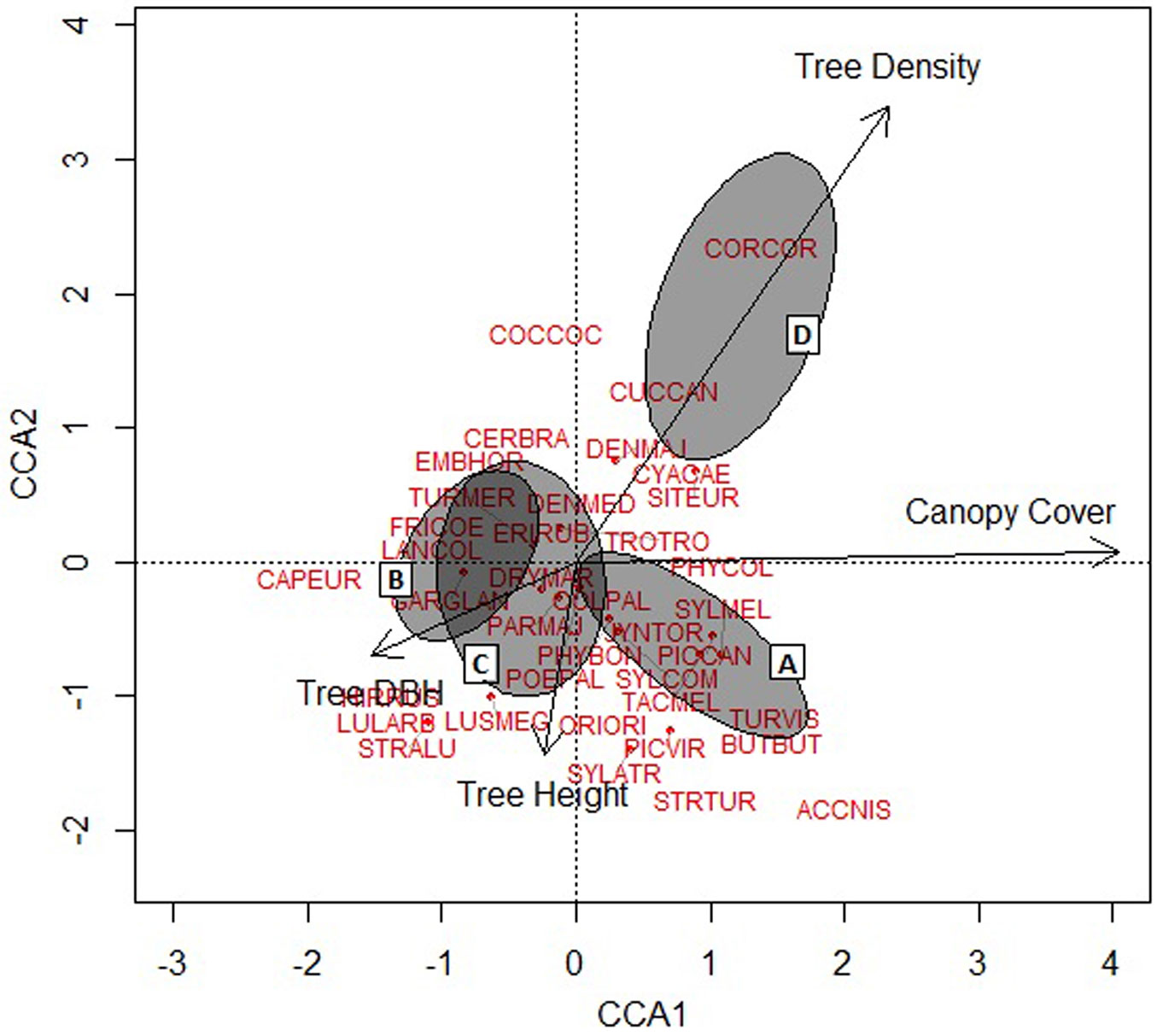
The stand structural variable that most strongly influenced bird assemblage composition was canopy cover followed by tree density (Fig. 2). Tree DBH and height had relatively less impact on bird composition. Our indicative bird species Buteo buteo (BUTBUT), Corvus corax (CORCOR), Sitta europaea (SITEUR), Phylloscopus collybita (PHYCOL), Tachymarptis melba (TACMEL) and Turdus viscivorous (TURVIS) were positively affected by canopy cover and tree density, whereas Garrullus glandarius (GARGLA) was affected negatively by this variable, but positively by tree DBH. Picidae (woodpeckers) species were clustered in the center. Coccothraustes coccothraustes (COCCOC) and Cuculus canorus (CUCCAN) were strongly associated with tree density. In contrast, the species Caprimulgus europaeus (CAPEUR), Lullula arborea (LULARB), Hirundo rustica (HIRRUS), Strix aluco (STRALU) and Luscinia megarhynchos (LUSMEG) were associated with tree height (Fig. 2).
Fig. 2 - Bird species distribution in relation to individual stand structural attributes produced by Constrained Correspondence Analysis (CCA). The distribution of study sites on the ordination are shown by ellipses representing the 90% confidence interval boundary for each stand type (A, B, C, and D). The length and direction of the arrows indicate the relative influence of individual stand structural attributes on the composition of the bird community.
Discussion
Our findings demonstrate that stand structure, especially canopy cover, has a strong influence on bird species composition and diversity. The highest diversity of birds in our study area was found on stands that included tall trees and high canopy cover (~42-85% corresponding to A and C stand types). Tree DBH, height, and vertical and horizontal stratifications influence bird diversity and abundance ([1]). Other vegetation characteristics, like maturity of trees or undergrowth density, may also play an important role in bird community dynamics ([14]), since the vegetative structure determines where and how species use resources ([5]). We found that canopy cover, height and DBH are all important for increasing and sustaining bird diversity. Large trees provide nesting habitat for many birds especially for resident species and cavity nesters ([23]). Keten et al. ([24]) showed that insect diversity and abundance increased with tree DBH and tree height in coppiced Thracian oak forests. Thus, high insect abundance and diversity may be responsible for the greater bird diversity and numbers observed at tall and dense stands in our study. For example, of the seven species which showed significant associations with stand type, Sitta europaea, Phylloscopus collybita, Tachymarptis melba and Turdus viscivorous are insectivorous suggesting that insect abundance at these stands may be important for their distribution.
Winkler ([51]) found that bird species richness, density and diversity in Sopron Mountains of Hungary were the lowest in the earliest successional stage and the highest in mature stands. In our study, early coppice stands (D stands) with a high number of stems (~30.000 trees ha-1) harbored very few bird species, most of which are generalists. The low number of bird species observed in short dense stands in our study might be due to the lack of a well developed understory shrub layer, as the development of the shrub layer is highly important for bird diversity and abundance ([8]). B stands in our study represent low canopy cover with few small short trees. Bird species that prefer open forested habitat like Caprimulgus europaeus ([50]), and intermediate pine forests like Streptopelia turtur ([2]) were the most seen bird species in these stands.
In contrast, species such as Phylloscopus collybita preferred stands with high canopy cover and tree density (A stands). Hinsley et al. ([22]) also found P. collybita to be associated with mature trees and larger wood with dense canopy cover. P. collybita nests on the ground in the breeding season (Rodrigues & Crick ([44]) and may prefer the more abundant plant litter of mature stands with lack of disturbance.
Our findings further show that bird assemblage composition and the presence of specific bird species as well guilds are strongly associated with the developmental or successional stage of the vegetation. For example, predator species such as Accipiter nisus and Buteo buteo preferred high canopy cover and tall trees potentially because their preys (e.g., mice) are found in greater abundance in old oak stands ([25]). Many of the bird species in our study were only associated with a single stand type. For example, Erithacus rubecula, Corvus corax and Columba palumbus were only found at young coppice with short trees and high stem density. D stands represents dense (28.537 trees ha-1) but short trees (most <1 m in height). E. rubecula breeds in hedgerows ([21]), while C. palumbus breeds in wood of all sizes ([21]). Thus D stands in our study may provide breeding habitat to these species, and feeding area for C. corax which feed and scavenge in open areas ([45]).
Mistletoes are common in Thracian oak forests particularly where moderate canopy cover and large tall trees are found ([26]). Cramp & Perrins ([10]) indicated that the most important birds for yellow mistletoe dispersal are Turdus viscivorus L. and Garrulus glandarius L., which were indicative species of stand A and C in our study. This suggests that these stands provide food for these bird species.
Among the most abundant species observed in our study were Turdus merula, Fringilla coelebs and Parus major, which are thought to be generalist. Our results show that these species can be found at A, B and C stands but not in D stands. Thus, these species show flexibility in habitat preference and only tend to avoid the earliest seral stages. Fuller & Warren ([18]) also found that T. merula, F. coelebs and P. major prefer older coppice forests.
While overall species diversity is favored by late succession stands with large tall trees, maintaining variation in canopy structure is crucial to increasing bird diversity across the landscape as a whole, given that different canopy stages hold importance for different bird species and guilds. MacColl et al. ([29]) suggest that a mosaic of stand ages across the landscape is beneficial to a wide range of forest bird species and that management should consider the requirements of all age-classes of birds at different times of the year. Mullerova et al. ([35]) measured the conservation value of forests based on the occurrence of red-list species, which were considerably reduced after coppice abandonment. Hence, as suggested by Mullerova et al. ([35]), the re-establishment or maintenance of some areas under coppice management is crucial to prevent biodiversity loss.
Conclusions
Although we did not record any very rare or threatened bird species at our early seral stage stands, we suggest that continuing coppice management is beneficial for biodiversity conservation to some extent and that complete abandonment of coppicing and associated vegetation structure may result in local extinction or a decrease in abundance of some narrowly distributed and rare species in Turkey. While greater bird abundance may be observed in mature forests, maintaining young coppice stands may increase the overall landscape-scale bird diversity. Coppicing a limited proportion of the landscape does not hinder biodiversity conservation, as it is becoming clearer that the preservation of historical forms of management that molded forest composition may be crucial for the conservation of many rare species ([31]).
Acknowledgement
This study was supported by The Scientific and Technological Research Council of Turkey (TUBITAK), Project Number: TOVAG-107O750. We thank Dr. Servet Caliskan, Dr. Hatice Yilmaz and Dr. Ohran Sevgi for their valuable contributions. This study was also supported by the Scientific Research Projects Coordination Unit of Istanbul University (Project number:UDP-34590)
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Istanbul University, Faculty of Forestry, Forest Entomology and Protection Department, Istanbul (Turkey)
Duzce University, Faculty of Forestry, Wildlife Ecology and Management Department, Duzce (Turkey)
Abant Izzet Baysal University, Faculty of Agriculture and Natural Science, Wildlife Ecology and Management Department, Bolu (Turkey)
Istanbul Technical University, Eurasia Institute of Earth Sciences, Istanbul 34469 (Turkey)
Emrah Ozdemir
Istanbul University, Faculty of Forestry, Forest Yield and Biometry Department, Istanbul (Turkey)
Istanbul University, Faculty of Forestry, Soil Science and Ecology Department, Istanbul (Turkey)
Duzce University, Faculty of Forestry, Forest Biometry and Management Department, Duzce (Turkey)
Corresponding author
Paper Info
Citation
Beskardes V, Keten A, Kumbasli M, Pekin B, Yilmaz E, Makineci E, Ozdemir E, Zengin H (2018). Bird composition and diversity in oak stands under variable coppice management in Northwestern Turkey. iForest 11: 58-63. - doi: 10.3832/ifor2489-010
Academic Editor
Massimo Faccoli
Paper history
Received: May 09, 2017
Accepted: Nov 13, 2017
First online: Jan 25, 2018
Publication Date: Feb 28, 2018
Publication Time: 2.43 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2018
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 49593
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 42118
Abstract Page Views: 2933
PDF Downloads: 3377
Citation/Reference Downloads: 18
XML Downloads: 1147
Web Metrics
Days since publication: 2843
Overall contacts: 49593
Avg. contacts per week: 122.11
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2018): 10
Average cites per year: 1.25
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Short Communications
Estimation of canopy attributes of wild cacao trees using digital cover photography and machine learning algorithms
vol. 14, pp. 517-521 (online: 17 November 2021)
Research Articles
The estimation of canopy attributes from digital cover photography by two different image analysis methods
vol. 7, pp. 255-259 (online: 26 March 2014)
Research Articles
Estimation of forest cover change using Sentinel-2 multi-spectral imagery in Georgia (the Caucasus)
vol. 13, pp. 329-335 (online: 07 August 2020)
Technical Advances
Thermal canopy photography in forestry - an alternative to optical cover photography
vol. 8, pp. 1-5 (online: 07 May 2014)
Research Articles
Quantifying the vertical microclimate profile within a tropical seasonal rainforest, based on both ground- and canopy-referenced approaches
vol. 15, pp. 24-32 (online: 27 January 2022)
Research Articles
Is methane released from the forest canopy?
vol. 4, pp. 200-204 (online: 03 November 2011)
Research Articles
Developing stand transpiration model relating canopy conductance to stand sapwood area in a Korean pine plantation
vol. 14, pp. 186-194 (online: 14 April 2021)
Research Articles
Mapping the vegetation and spatial dynamics of Sinharaja tropical rain forest incorporating NASA’s GEDI spaceborne LiDAR data and multispectral satellite images
vol. 18, pp. 45-53 (online: 01 April 2025)
Research Articles
Canopy temperature variability in a tropical rainforest, subtropical evergreen forest, and savanna forest in Southwest China
vol. 10, pp. 611-617 (online: 17 May 2017)
Research Articles
Assessment of land sensitivity to degradation using MEDALUS model - a case study of Grdelica Gorge and Vranjska Valley (southeastern Serbia)
vol. 15, pp. 163-170 (online: 07 May 2022)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword