Effects of black locust and black pine on extremely degraded sites 60 years after afforestation - a case study of the Grdelica Gorge (southeastern Serbia)
iForest - Biogeosciences and Forestry, Volume 9, Issue 2, Pages 235-243 (2015)
doi: https://doi.org/10.3832/ifor1512-008
Published: Aug 22, 2015 - Copyright © 2015 SISEF
Research Articles
Abstract
The selection of tree species can affect the success of afforestation in the rehabilitation of degraded forest sites and forest restoration. In general, black locust (Robinia pseudoacacia L.) and black pine (Pinus nigra Arnold.) represent the most commonly used species in the afforestation of soils that have been degraded by erosion. As far as the extent of the ameliorative effects of black locust and black pine are concerned, it was found that they may play an important role in the selection of species for the afforestation of extremely degraded sites. This study is aimed at determining the potential of black locust and black pine to affect several soil properties, erosion control and C stock, thus creating favourable site conditions for the restoration of previous forest vegetation. This research was conducted in the Grdelica Gorge in south east Serbia, where eight sample plots with an average size of 0.47 ha were established 60 years ago on terrain afforested with black locust and black pine. In each sample plot, we measured the diameter at breast height of all black locust and black pine trees, and the height of 10 black locust and 10 black pine trees in each diameter class. In addition, samples of mineral soil (from depths of 0-5, 5-10 and 10-20 cm) were taken at 4 randomly selected soil profiles in each sample plot, and 8 samples of litter (30 × 30 cm) were also collected. Additionally, laboratory analyses of the physical and chemical properties of the soil and litter were performed in 2 replicates. The obtained results showed that: (1) at the 0-5 cm depth, there was no statistically significant difference in the reaction of the soil solution, although a significant difference in the reaction of the soil solution between the soils under the two species was observed at soil depths greater than 5 cm; (2) there was a significantly higher N content under black locust in the 0-5 cm soil layer; (3) the reduction of soil loss under black locust is statistically significant in all observation periods; (4) black pine is more efficient in C storage. Our results demonstrate that black locust has the potential to improve soil properties and reduce soil loss caused by erosion, while its favourable impact does not decrease over time, making it more suitable for afforestation on degraded land in the examined area.
Keywords
Afforestation, Black Locust, Black Pine, Soil Properties, Soil Losses, Carbon Stock
Introduction
Deforestation as a result of anthropogenic pressure leads to barren land whose condition marks it as an extremely unfavorable site ([39]). The consequential accelerated soil erosion on bare land does not usually allow for natural forest regeneration ([4]). Under the harsh conditions of these sites, reforestation is an instrument used to rehabilitate degraded forest sites and restore forests. In addition to preventing erosion and rapid surface runoff ([13], [64], [8], [5]), afforestation is used to provide other beneficial effects on the environment, including the improvement of microclimatic conditions in degraded sites, boosting of the conditions which allow for an increase in biodiversity ([63], [15]) and carbon sequestration ([49], [31], [61], [37]). Moreover, the economic effect of afforestation on degraded lands is not negligible ([13]), and among its other benefits this represents an important ecosystem service.
The selection of tree species can affect the type and intensity of the afforestation effects. In fact, different species selected for afforestation produce a range of different impacts on floristic diversity. Interestingly, previous research has shown that afforestation using different pine species (Pinus sp.) can have a negative impact on floristic diversity, species composition and the prospect of progressive succession ([1], [59], [34], [4]). However, a number of other ameliorative effects of afforestation with these species, such as erosion control ([7]), the improvement of certain soil properties such as organic matter content, N content, C/N ratio, readily available P and K ([42], [44]) as well as atmospheric carbon sequestration in biomass and soil ([49], [40]) should not be overlooked.
Cao et al. ([7]) presented the results of the improvement in soil nutrient conditions, particularly in total and available N and organic matter, 10 years after afforestation, reporting that shallow-rooted species, such as black locust, are more suitable for the afforestation of degraded and erodible soils. Regarding the afforestation of lignite spoils with black locust and black pine, Panagopoulos & Hatzistathis ([42]) reported a larger amount of organic matter and a higher N content in the afforested soils compared to naturally revegetated soils. In fact, 14 years after afforestation, N content was 15% higher under black locust than under black pine. Research of the vegetation restoration effects on severely eroded barren land ([66]) showed 4 to 6 times higher organic soil matter and total N in the afforested soils 12 years after vegetation restoration. Qiu et al. ([44]) reported significantly increased C and N contents in the surface soils under black locust compared to grassland, which increased the difference in soil organic C and total N between the surface and deeper soils.
In this study, it was hypothesized that black locust and black pine can mitigate soil degradation in extreme site conditions. In particular, the investigation was aimed at determining the impact of black locust and black pine on certain soil properties, erosion control and C stock, as well as their potential to create favorable site conditions for the restoration of forest vegetation on degraded land.
Materials and methods
Study area
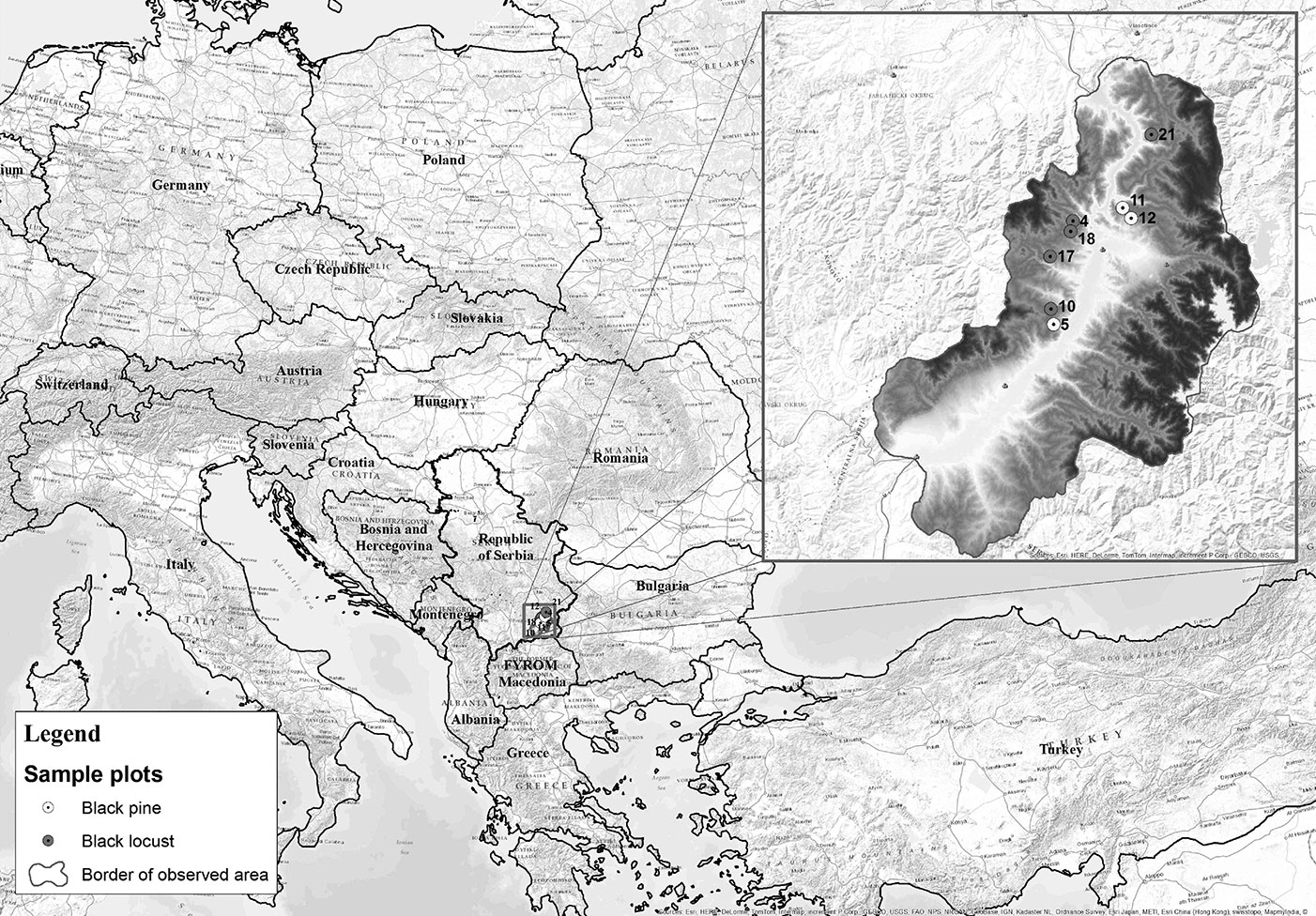
The area of the Grdelica Gorge extends between 42° 22′ and 42° 55′ N latitude and between 21° 19′ and 20° 00′ E longitude, on an area of 1784.34 km2 (Fig. 1). This area is characterized by a well developed hydrographic network consisting of 137 streams, with a total catchment area of 1700.33 km2. A combination of pronounced altitudinal differences over short distances and dissected relief with steep slopes contributes to its increased erosion potential.
Regarding climatic conditions, the investigated area has a continental climate with a mean annual air temperature of 10.9 °C and a total annual precipitation of 672 mm, based on observations between 1949 and 2011 ([46]).
In the case of the Grdelica Gorge, the harsh conditions of its deforested sites are the result of an erodible parent rock whose highly disturbed and fractured layers display varying degrees of weathering. Furthermore, skeletal and dry soils with a low pH have developed on the steep slopes, that led to conditions enhancing the erosion process. Interestingly, research of soil properties and soil erosion in the area of the Grdelica Gorge ([54]) has shown that erosion particularly occurs on soils developed on crystalline schist.
The composition of species in the area of the Grdelica Gorge is characterized by the presence of rare and fragmentary represented forest communities, as well as relict and endemorelict examples. More precisely, the most common forest type at altitudes up to 600 m a.s.l. in the area is the association of Turkey oak and Hungarian oak (Quercetum frainetto-cerris Rud. 1949). In addition, Montane beech forest (Fagetum moesiacae montanum Jov. 1953) is also present in this region at higher elevations, from 800 to 1300 m ([28]). However, in the valley of the South Morava River, the most common communities are forests of pedunculate oak and broom (Genisto elatae-Quercetum roboris Horv. 1938) and forests of poplar and willow (Salici-Populetum albae Drees. 1936 - [57]). These associations inhabited the degraded sites at the time before deforestation occurred.
Degradation in the area of the Grdelica Gorge is a result of both natural and anthropogenic factors. Since the mid-nineteenth century, both forest destruction for agricultural purposes and negative anthropogenic impacts in this area have intensified ([14]). In particular, agriculture on deforested areas was extensive and short-term in character, as after only a few years the soils were impoverished and subsequently abandoned, resulting in the deforestation and degradation of new areas of land ([60]). In fact, the combination of environmental factors and strong anthropogenic impacts (deforestation) led to accelerated erosion and severe damage by frequent torrential floods ([45]).
The first recorded erosion control works in the Grdelica Gorge were conducted in 1907, with the most extensive afforestation of the area carried out between 1956 and 1958 ([30]).
Due to their pioneering qualities, the two most commonly used tree species for the afforestation of extremely degraded sites in the Grdelica Gorge have been black locust (Robinia pseudoacacia L.) and black pine (Pinus nigra Arnold.). The pit planting method was applied in the very dense planting of black locust, i.e., 10 000 seedlings per hectare, whereas the number of black pine seedlings per hectare reached 2500. One section of these areas is the research object of this study, focusing on a comparative analysis of the effects of afforestation with black locust and black pine over a 60 year period. To this purpose, forests of black locust and black pine of the same age (58-60 years old) and with similar environmental conditions (climatic, orographic, edaphic, etc.) were selected as study sites.
Data collection
A total of eight sample plots (sp) were established in areas afforested with black locust and black pine in the period from 1956 to 1958. Specifically, five sample plots (sp4, sp10, sp17, sp18 and sp21) were established in black locust forests and three in forests of black pine (sp5, sp11 and sp12). The sample plot sizes ranged from 0.35 ha to 0.50 ha, with an average size of 0.47 ha, as the configuration of the terrain (slope, aspect, etc.) has often hampered the establishment of plots of uniform size. The sample plots were established on crystalline schists at varying stages of weathering, with layers that were generally highly disturbed and fractured. The observed soil type was leptosol ([65]), which belongs to the textural class of sandy loam, with a light mechanical composition and a weakly expressed or unexpressed structure. In order to reduce the experimental error, all sample plots were placed in areas with slopes > 20%, on warm exposures (S, SW and SE), within stands 58 -60 years old that were never subjected to any silvicultural practice. In addition, no signs of plant diseases or insect attacks were observed in the plots. However, exposure and slope at the selected sites were the least favorable for the establishment of vegetation ([52], [41], [61]).
Structural and producti characteristics of
In all plots, the diameter at breast height (d) of all trees was measured, and the height (h) of 10 trees in each of the 5 cm increment diameter classes was recorded. To assess the structure and the production per ha of the study stands, the following parameters were derived: the number of trees (N) and their distribution by diameter classes, basal area (G), height curves, volume (V), volume increment (Iv) and mean dimensions of representatives trees (dg, hg). The volume of specific tree for each species was calculated using a double-entry tree volume table-model type V = f(d, h) and the current volume increment using the increment percentage method. The increment percentage (piv) was obtained using the model type piv = f(dg, hg, N, s), where s represents the share by volume of the tree species in a mixture ([3]). Under such conditions, s = 1.0, since the sampled stands were pure black locust or black pine forests.
Soil and litter properties
A total of four soil profiles were opened in each sample plot and 8 samples of litter were collected (30 × 30 cm), with soil sampling carried out at fixed depths of 0-5 cm, 5-10 cm and 10-20 cm.
Basic physical and chemical properties of air-dry soil were determined using the following methods: the traditional pipette method was used for particle size analysis ([51], [26]); bulk density (BD) was measured on soil cores after drying at 30 °C to a constant weight ([25]); soil pH was determined in distilled water with a solid-liquid (S/L) ratio of 1: 2.5 ml g-1, hydrolytic acidity (H meq/100 g) using Kappen’s method (extraction by CH3COONa, titration with 0.1M NaOH); the sum of exchangeable basis (S) also using Kappen’s method (extraction by 0.1M HCl, titration with 0.1M NaOH - [29]) and the total capacity of cation adsorption (T) and degree of base saturation (V%) - were also calculated ([20]). Soil organic carbon (C) was measured using the Tjurin method ([36]) and total nitrogen (N) using the Kjeldahl method ([6], [24]). After extraction, the available P and K were determined by the Egner-Riehm method ([9]). All analyses were performed in 2 replicates.
Soil loss
The calculation of soil loss was performed using the Erosion Potential Method (EPM - [17]), which was developed in Serbia and is still in use, mainly in the countries of the former Yugoslavia and the Western Balkans ([70], [58], [50]), but also in Iran ([55], [2]). In this study, the level of erosion was assessed based on annual soil loss (Wyear) immediately before afforestation (Wyear0), 10 years after afforestation (Wyear10) and 60 years after afforestation (Wyear60).
Carbon stock in biomass and soil
The assessment of carbon stock in living biomass, dead wood, litter and soil was obtained using the equations recommended by the IPCC ([23]), while the carbon stock in living biomass (Bt) was obtained using the following equation (eqn. 1):
where Vt is the total bole volume (m3 ha-1), Dw is the wood density (Mg dry mass m-3 green; 0.58 for black pine and 0.74 for black locust - [53]), BEF2 is the biomass expansion factor (dimensionless), which is the conversion factor to expand under-bark bole biomass to include non-merchantable biomass such as bark and branches (1.4 - [23]), CF is the conversion factor from dry biomass to carbon (Mg C [Mg dry mass]-1; 0.5 - [23]), and R is the conversion factor to include below-ground biomass (dimensionless; 0.32 for black pine and 0.26 for black locust - [23]).
The carbon stock in dead wood was assumed at a maximum of 25% of the total carbon stock in living biomass, with the maximum value estimated in this way divided by five, given that the process of decomposition takes 5 years ([23]). In addition, the carbon stock in litter was estimated as the entire dry matter of litter ([23]), whereas the carbon stock in soil (SCD) was calculated using the following equation (eqn. 2):
where SCD is the soil carbon density for the j-th layers (l) of the sampling site (Mg C ha-2), SOCcontent is the soil organic carbon content for a single sampled depth (% of mass or g C kg-1), BD is the soil mass of the undisturbed volume of a single sampled depth (t m-3), Depth is the thickness of the sampled layer (m), frag is the volume of the coarse fragments in the single sampled depth (%).
Carbon stock was estimated in living biomass, dead wood, litter and soil in areas under black locust and black pine.
Data processing
Normal distribution of sampling data was inferred by standard skewness and kurtosis. Differences in the ameliorative effects of afforestation between black locust and black pine was tested using the Student’s t-test and ANOVA (α = 0.05). In addition, the basic physical and chemical properties of the soil in the areas afforested with black locust and black pine were compared, with soil losses and carbon stock estimated both in total and in different carbon pools (living biomass, dead wood, litter and soil). The post-hoc Fisher’s LSD test and Pearson’s correlation analysis were applied to test the strength of the relationship between soil pH, total capacity of cation adsorption (T), base saturation (V%) and clay content.
Results
Structural and producti characteristics of
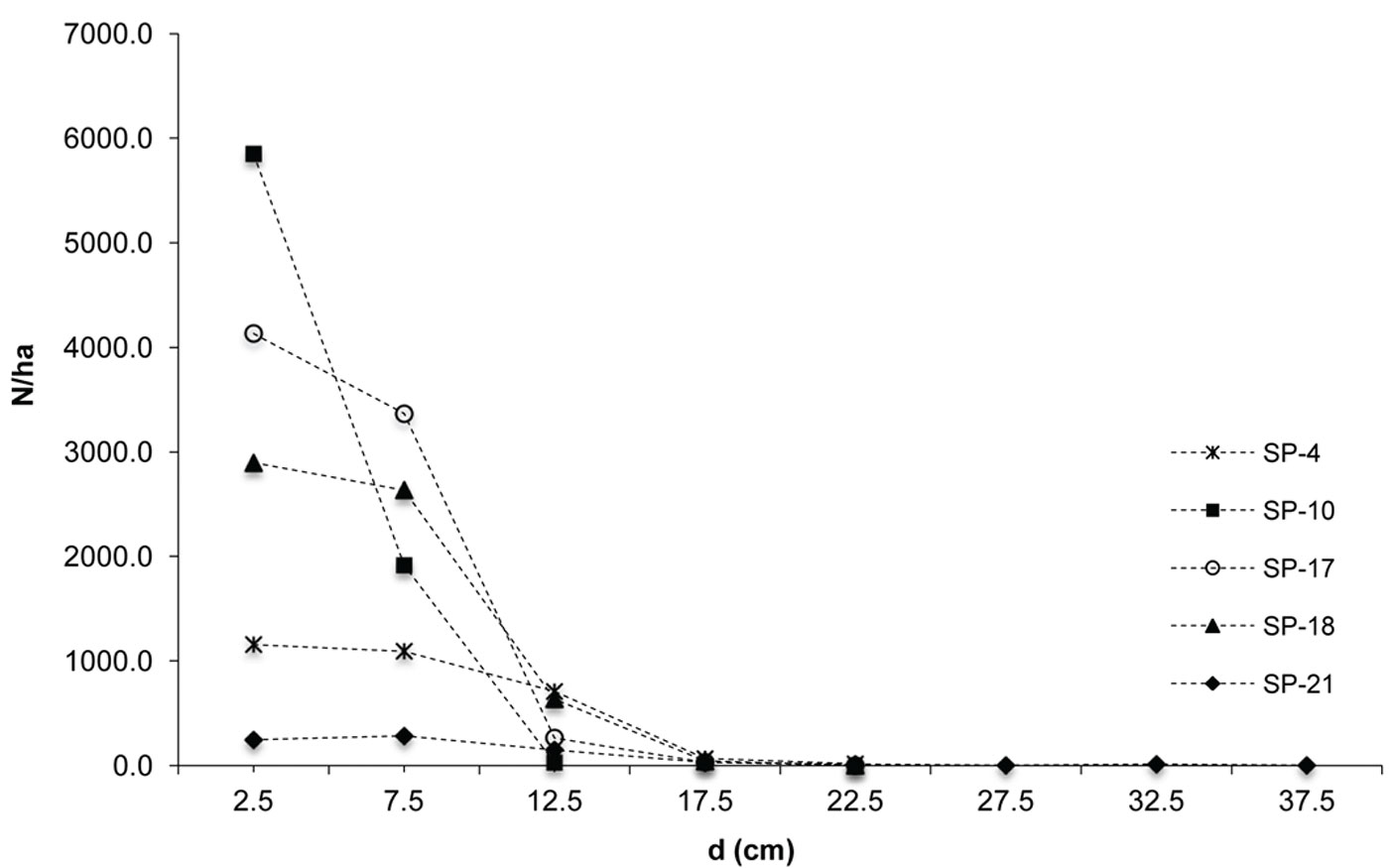
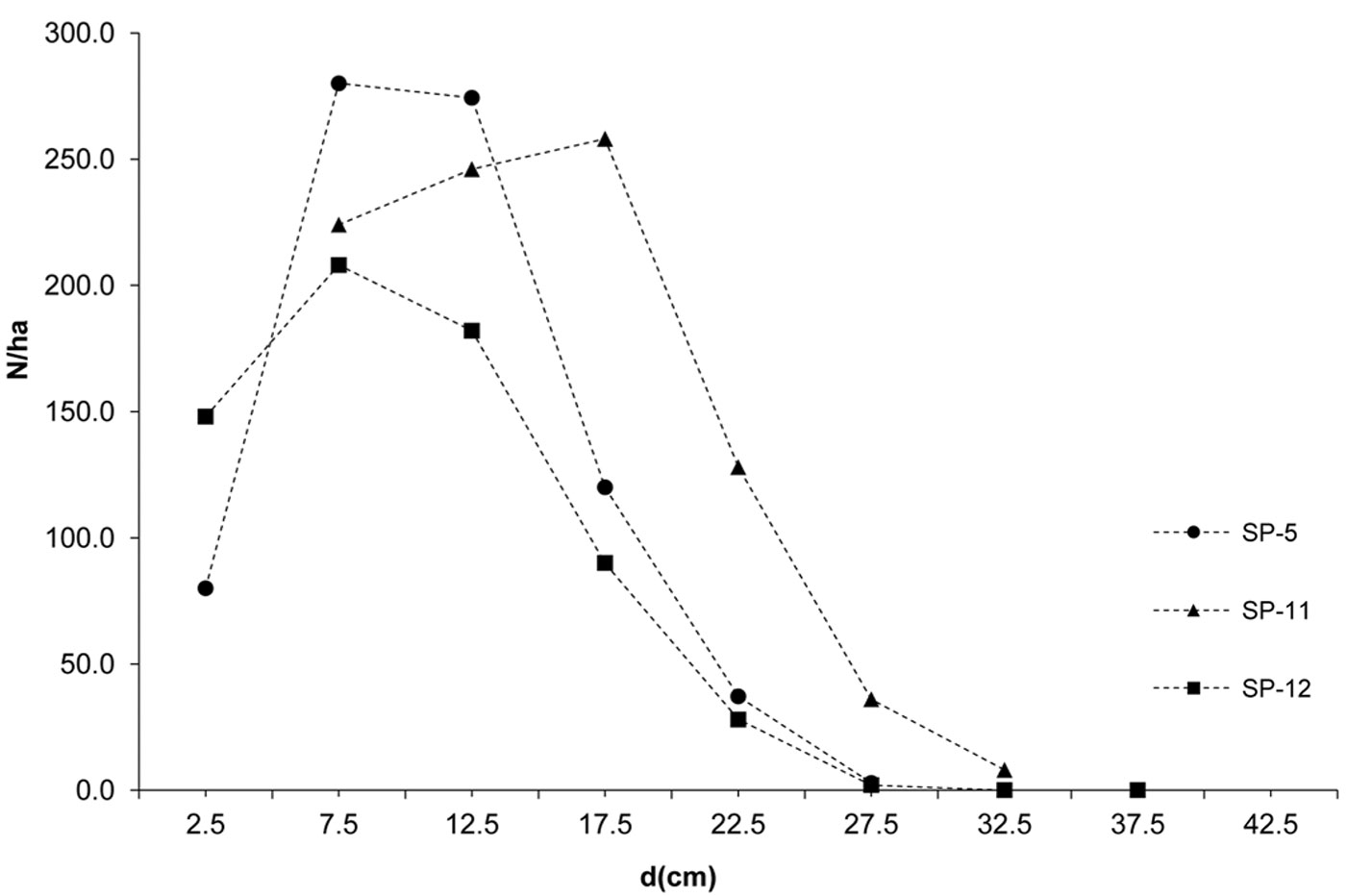
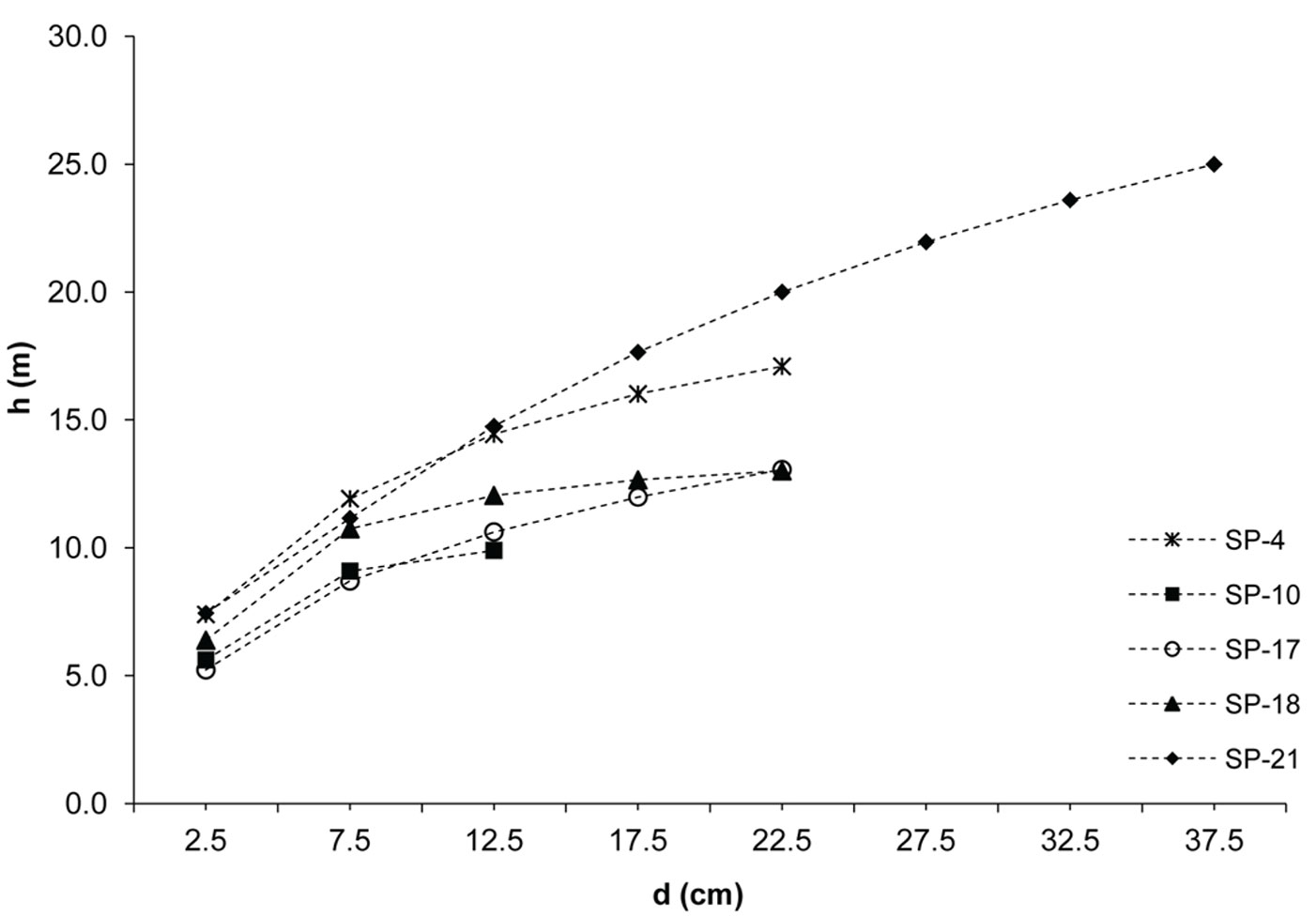
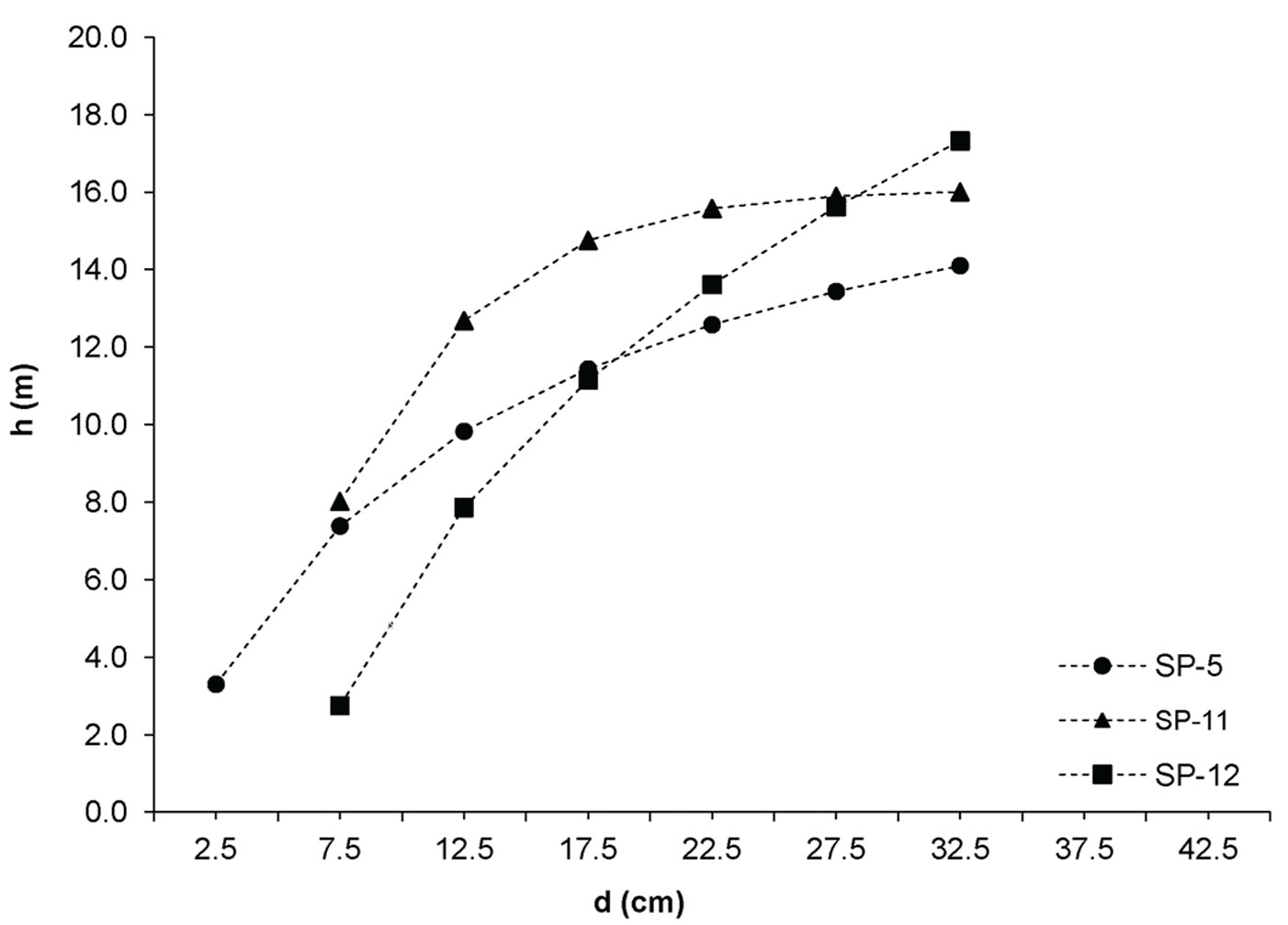
The investigated forests of black locust and black pine showed significantly differences in the production parameters (Tab. 1). Although the number of black pine trees was 5.4 times smaller, this species achieved 189.6% larger basal area, 30.8% higher volume and 30.9% higher current volume increment as compared with black locust. Moreover, the dimensions of the mean representative trees were also greater in the pine stands, being the mean diameter 128.2% greater and the mean height 40.7% higher than in the black locust plots. In addition, structural differences were also observed, especially with regard to diameter structure. Black locust trees were mainly represented in the thinner diameter classes with a very small variation, ranging from the 2.5 cm to 22.5 cm (with the exception of sp21, where only 1.9% of trees were represented in the diameter class > 22.5 cm). Diameter distribution was left-skewed for black locust (Fig. 2) and right-skewed for black pine (Fig. 3), the latter species also showing a larger frequency of slightly larger diameter classes (from 7.5 cm to 32.5 cm). In addition, the height curves of black locust (Fig. 4) were slightly steeper than those obtained for black pine (Fig. 5).
Tab. 1 - Characteristics of black locust and black pine stands. (1): Differences in the absolute amount; (2): Differences in percetage; (SD): Standard deviation.
| Species | Parameter | N(trees ha-1) |
G(m2 ha-1) |
dg(cm) |
hg(m) |
V(m3 ha-1) |
Iv(m3 ha-1) |
|---|---|---|---|---|---|---|---|
| Black locust | Average | 5116.6 | 15.4 | 7.1 | 9.1 | 77.6 | 5.5 |
| SD | 3122.93 | 6.74 | 2.17 | 2.6 | 31.73 | 2.22 | |
| Min | 740 | 5.8 | 4.4 | 6.6 | 43.5 | 2.4 | |
| Max | 7960 | 22 | 10 | 13 | 111.2 | 7.9 | |
| Black pine | Average | 794.1 | 44.6 | 16.2 | 12.8 | 101.5 | 7.2 |
| SD | 121.3 | 50.44 | 0.49 | 1.53 | 26.5 | 1.79 | |
| Min | 658 | 13 | 15.9 | 11.2 | 74.4 | 5.4 | |
| Max | 900 | 102.8 | 16.9 | 14.3 | 127.4 | 9 | |
| Difference | 1 | -4322.5 | 29.2 | 9.1 | 3.7 | 23.9 | 1.7 |
| 2 | - 544.3 | 189.6 | 128.2 | 40.7 | 30.8 | 30.9 |
Soil and litter properties
A developed soil horizon A in all sample plots is an important indicator of progressive soil processes. Taking into consideration that the investigated stands were of the same age and that afforestation was carried out on soils with very sparse and stunted vegetation or no vegetation at all, the presence of the horizon A is indicative of the ameliorative effects of afforestation on the process of pedogenesis.
Soil reaction (pH in water) in the 0-5 cm layer was weakly to moderately acidic, while it was moderately to strongly acidic in deeper layers. Although the 0-5 cm soil layer was moderately humous (3-5%), the lower layers of the profile were only mildly humous (1-3%).
Concerning the nutrient content in the studied soil, the following values were recorded: the total N ranged from 0.44 to 0.16%, indicating a soil well supplied with N, while the content of available P was very low and the available K was medium to high.
Tab. 2 shows the basic soil characteristics of the areas afforested with the two different species. No significant differences between the soil samples of the two species were found with respect to clay content, hydrolytic acidity (Y1), humus content, C, C/N, available P and available K. However, differences were statistically significant when the total N content, the total capacity of cation adsorption (T) and base saturation (V%) were considered. In particular, the total capacity of cation adsorption (T) and base saturation (V%) were significantly higher in the soils under black pine as compared with those under black locust. Moreover, the values of the above parameters decreased with depth, especially in the soil layers deeper than 5 cm under black locust. Furthermore, the 0-5 cm soil layer under black locust was significantly richer in total N compared to the soils afforested with black pine, whereas no significant differences were observed in the 5-10 cm layer.
Tab. 2 - Basic soil properties at different depths in black locust and black pine stands. (*): p < 0.05; (**): p < 0.01.
| Soil Properties |
Black locust | Black pine | ||||
|---|---|---|---|---|---|---|
| 0-5 cm | 5-10 cm | 10-20 cm | 0-5 cm | 5-10 cm | 10-20 cm | |
| Clay % | 8.40 ± 2.8 | 11.40 ± 4.1 | 11.30 ± 2.8 | 6.30 ± 0.4 | 16.40 ± 3.6 | 17.60 ± 3.5 |
| pH | 6.00 ± 0.2 | 5.56 ± 0.1 | 5.34 ± 0.2 | 6.30 ± 0.4 | 6.02 ± 0.3* | 5.80 ± 0.2* |
| Y1 | 15.20 ± 3.3 | 15.00 ± 2.2 | 17.00 ± 1.4 | 10.50 ± 3.4 | 12.60 ± 2.6 | 13.50 ± 2.3 |
| T | 20.51 ± 4.0 | 14.30 ± 2.9 | 12.25 ± 0.9 | 26.96 ± 2.1** | 24.26 ± 0.7** | 25.03 ± 1.8** |
| V (%) | 49.54 ± 17.9 | 26.34 ± 12.4 | 9.82 ± 0.7 | 74.18 ± 9.8* | 66.20 ± 6.6** | 63.92 ± 5.1** |
| Humus | 5.34 ± 1.3 | 2.14 ± 0.4 | 1.51 ± 0.3 | 4.37 ± 1.4 | 2.78 ± 0.6 | 2.04 ± 0.3 |
| C (%) | 2.82 ± 0.8 | 1.24 ± 0.2 | 0.88 ± 0.2 | 2.58 ± 0.8 | 1.62 ± 0.3 | 1.18 ± 0.8 |
| N (%) | 0.305 ± 0.05* | 0.165 ± 0.02 | - | 0.213 ± 0.04 | 0.177 ± 0.07 | - |
| C/N | 10.10 ± 1.3 | 8.60 ± 0.8 | - | 11.80 ± 2.3 | 9.50 ± 1.4 | - |
| P (mg/g) | 2.45 ± 1.7 | 1.65 ± 1.6 | - | 3.40 ± 1.2 | 4.70 ± 3.2 | - |
| K | 33.40 ± 7.7 | 31.20 ± 8.0 | 19.40 ± 6.8 | 23.10 ± 8.6 | 23.60 ± 14.8 | 18.20 ± 11.6 |
As for soil pH in the 5-10 cm and 10-20 cm layers, black locust soil samples showed values significantly lower than those of pine soil samples, while no significant differences were found for the 0-5 cm layer samples. Soil pH under black locust considerably decreases with depth, with values of 6.00 ± 0.2, 5.56 ± 0.1 and 5.34 ± 0.2 in the 0-5 cm, 5-10 cm and 10-20 cm layers, respectively. Similarly, soil pH under black pine decreases with depth, although this is less pronounced: at depths of 0-5, 5-10 and 10-20 cm, the pH value was 6.30 ± 0.4, 6.02 ± 0.3 cm and 5.80 ± 0.2, respectively (Tab. 3).
Tab. 3 - Soil pH in the layers at different depths in black locust and black pine stands. (1): “×” in the same column denotes the absence of differences between the average values of the tested groups; “×” in different vertical planes denotes the existence of differences between the average values of the tested groups.
| Soil layer | Black locust | Black pine | ||
|---|---|---|---|---|
| Average | Differences1 | Average | Differences1 | |
| 0-5 cm | 6.00 | × - | 6.30 | × - |
| 5-10 cm | 5.56 | - × | 6.02 | × × |
| 10-20 cm | 5.34 | - × | 5.80 | - × |
| Stats | F = 12.36; p = 0.005 | F = 2.88; p = 0.099 | ||
Litter properties have a significant impact on the soil. When the basic properties of litter of the two species used for afforestation were compared (Tab. 4), it was found that the pH and N content were significantly higher in the black locust litter than in the black pine litter. However, humus, C contents and C/N ratio were higher in the black pine litter.
Tab. 4 - Properties of litter in black locust and black pine stands. (ns): not significant.
| Soil property | Black locust | Black pine | Prob. |
|---|---|---|---|
| Average amount of forest litter (kg m -2) | 1.975 ± 0.84 | 2.695 ± 1.75 | ns |
| pH | 6.54 ± 0.1** | 5.31 ± 0.3 | < 0.01 |
| Humus (%) | 60.68 ± 8.6 | 82.19 ± 4.0** | < 0.01 |
| C (%) | 35.17 ± 4.9 | 47.50 ± 2.3** | < 0.01 |
| N (%) | 1.662 ± 0.2** | 1.003 ± 0.2 | < 0.01 |
| C/N | 21.2 ± 2.0 | 50.6 ± 17.4* | < 0.05 |
Soil loss
In areas under black locust, estimates of soil loss have been significantly reducing since the period of afforestation, the period of the first ten years after afforestation and in the subsequent period up to the present day (53.80, 15.68 and 4.32 Mg km2 yr-1, respectively). Analogously, soil loss estimates in black pine stands were significantly reduced from 49.81 to 12.08 Mg km2 yr-1 in the first 10 years after afforestation. However, the reduction of soil loss in the subsequent period up to present (5.02 Mg km2 yr-1) was not statistically significant (Tab. 5).
Tab. 5 - Estimated soil losses in areas reforested with black locust and black pine. (1): “×” in the same vertical plane denotes the absence of differences between the average values of the tested groups; “×” in different vertical planes denotes the existence of differences between the average values of the tested groups.
| Wyr | Black locust | Black pine | ||
|---|---|---|---|---|
| Average | Differences1 | Average | Differences1 | |
| Wyr0 | 53.80 | × - - | 49.81 | × - |
| Wyr10 | 15.68 | - × - | 12.08 | - × |
| Wyr60 | 4.32 | - - × | 5.02 | - × |
| Stats | F = 80.21; p = 0.000 | F = 40.81; p = 0.000 | ||
Carbon stock in biomass and soil
The sequestration of atmospheric carbon in biomass and soil is one of the ecosystem services provided by afforestation and is of particular importance for degraded and harsh habitats. In this study, the estimate of the total C stock was 107.2 ± 56.0 Mg ha-1 in black locust and 131.5 ± 33.2 Mg ha-1 in black pine. In particular, the largest amount of C stock was found in the living biomass: 45.4 ± 28.1 Mg ha-1 in black locust and 57.3 ± 6.4 Mg ha-1 in black pine (Tab. 6). Although no significant differences in carbon stocks were found between the two species considered, the ability of such species to sequester C was apparent.
Tab. 6 - C stock in biomass and soil in black locust and black pine stands. (ns): not significant.
| C pools | Black locust | Black pine | Prob. | ||
|---|---|---|---|---|---|
| Mg ha-1 | % | Mg ha-1 | % | ||
| Living biomass | 45.4 ± 28.1 | 43 | 57.3 ± 6.4 | 43 | ns |
| Dead wood | 17.4 ± 12.0 | 16 | 22.3 ± 2.6 | 17 | ns |
| Litter | 19.8 ± 10.0 | 18 | 27.2 ± 19.2 | 21 | ns |
| Soil | 24.6 ± 5.6 | 23 | 24.6 ± 13.7 | 19 | ns |
| Total C stock | 107.2 ± 56.0 | 100 | 131.5 ± 33.2 | 100 | ns |
Discussion
The extreme nature of the Grdelica Gorge habitat is equally reflected both in the natural conditions of specific sites in this area and the anthropogenic factors that contribute to the creation of the harsh conditions. In particular, the natural factors that make the sites of the Grdelica Gorge extreme are its highly dissected relief, the uneven distribution of rainfall, the occurrence of high intensity rainstorms, as well as early autumn and late spring frosts. Additional factors are the parent rock highly susceptible to weathering and the soils having generally a light texture and weakly pronounced structure. Such natural conditions combined with negative anthropogenic impacts foster erosion processes in the soils of the Grdelica Gorge. Uncontrolled and unplanned deforestation, together with inappropriate land use practices in the area, have contributed to the manifestation of the Grdelica Gorge’s extreme habitat.
Afforestation in the area of the Grdelica Gorge was primarily conducted for the purpose of erosion control. This study is an attempt to comprehend the ameliorative effects of such afforestation after 60 years and to compare the effects of the two most commonly species used for contrasting the erosion. In this respect, we focused onto the impact of black locust and black pine on soil properties, soil losses caused by erosion and C stock in the restored forest ecosystem, as well as on their structural and productive characteristics. Our results suggest that certain indicators of the ameliorative effects of afforestation may differ depending on the species selected.
Structural and producti characteristics of forests
Given the uniform habitat conditions and the lack of previous silvicultural treatments, black pine performed significantly better than black locust. In addition to the biological characteristics of the species, this could be due to the current high density of black locus stands, reaching over 5000 trees per hectare, with a very slow differentiation in diameter. Indeed, this is reflected by the high frequency observed in thinnest diameter classes and the high degree of tree slenderness (hg/dg over 1.2) found in the black locust stands, caused by an intense height increment. In this context, the thinning of black locust forests is a necessary silvicultural measure to be taken urgently. The reduction in the number of trees would promote the expansion of tree canopies and roots, with an expected positive impact on the stabilization of these soils.
Soil and litter properties
Previous research confirmed that afforestation does reduce the soil pH ([42], [18], [27]). Our results indicate that soil layers deeper than 5 cm are significantly more acidic under black locust than under black pine, while no significant differences in acidity were found for 0-5 cm soil layers. Although the soils under the black locust and black pine are of same type, localities afforested with black locust are slightly more acidic than those with black pine. This may be partly due to the fact that when the afforestation was conducted, slightly more acidic localities were planted with black locust rather than with black pine. Moreover, a significantly higher pH value was found in the 0-5 cm soil layer when compared to the 5-10 cm and 10-20 cm soil layers of black locust stands, while in black pine stands no significant differences in pH values were found between adjacent soil layers. The higher soil pH of shallow layers under black locust trees can be explained by the litter characteristics, whose pH is almost neutral (6.54 ± 0.1), whereas the litter of black pine is acidic (5.31 ± 0.3), and the difference in acidity is statistically significant (p < 0.001). In addition, black locust litter has a higher decomposition rate ([56]), and the mode of litter decomposition of black locust affects the quality of the organic matter that accumulates in the mineral soil layer ([10]). Given its pH-neutrality, fast decomposition and the incorporation of quality organic matter into mineral soil layers, we can assume that such characteristics may contribute to an increase in pH of the surface soil layer under black locust.
In this study the degree of base saturation (V%) was positively correlated with soil pH under both black locust (r = 0.961, p = 0.024) and black pine (r = 0.996, p < 0.001). It has been reported that pH reaction is a good indicator of V% in moderately acidic to neutral soils ([19]). The soil pH under black pine is higher and, therefore, their base saturation is also expected to be higher. As a result, the total capacity of cation adsorption is significantly higher in soils under black pine. In the case of soils under black locust, the total capacity of cation adsorption is correlated with pH (r = 0.961, p = 0.039) and clay content (r = 0.998, p = 0.002), while in soils under black pine the total capacity of cation adsorption is not correlated with these parameters.
The soils under black locust had a significantly higher total N content than those under black pine, and the total N content in the studied area decreased with soil depth. In fact, nitrogen fixation by black locust trees may explain the higher total N soil content, as well as the properties of the species’ litter, the mode of its transformation ([10]) and its mineralization rate ([48], [56]). In particular, the litter of black locust contained significantly more N (1.662 ± 0.2%) than that of black pine (1.003 ± 0.2%). Tateno et al. ([56]) reported that the litter of black locust aged 20-30 years contained 1.49% of N.
The C/N ratio is an indicator of the transformation rate of organic matter. According to our results, the C/N ratio was 21.2 ± 1.9 in the black locust litter, which is considerably more favorable than the ratio of 31.9 reported by Tateno et al. ([56]). Furthermore, the C/N ratio in black locust litter was significantly smaller than that in the litter of black pine (50.6 ± 17.4). A smaller C/N ratio (<20) indicates a faster litter decomposition and mineralization of organic N ([48]), which is then incorporated into the surface soil layer, resulting in a higher N content of soils in black locust stands.
Soil loss
The characteristics of vegetation affect soil erosion and water runoff ([66], [68], [8], [12], [21]). According to Zhou et al. ([69]), soil erosion shows a negative linear correlation with the vegetation cover. The results of this study showed that the estimated soil losses in the afforested areas were drastically reduced. Comparing the estimates of soil loss immediately before afforestation ([54]) with those of 10 years later, a reduction by 76% and 71% was estimated in the soils under black pine and black locust, respectively. In the subsequent period up to the present day, the soil loss under black pine has been reduced by 58%, while it decreased by 72% under black locust. Durán Zuazo et al. ([12]) reported a 58-98% reduction in soil loss in areas covered with different types of vegetation compared to areas with no vegetation, while Chirino et al. ([8]) suggested a decrease up to 70-95%. In this study, a significant reduction of soil losses in the first 10 years after afforestation of black pine stands was observed, while such reduction was not statistically significant in the subsequent period up to present. However, the reduction of soil loss under black locust is statistically significant in all the observation periods. Therefore, it can be concluded that black locust gradually and constantly contributes to a reduction of soil loss. The efficiency of this species in preventing the erosion may be related to the improvement of several soil properties, such as N content or C/N, thus reducing soil erodibility. The beneficial impact of black locust on soil properties increases with stand age ([44]), making it a very effective species for continuous erosion control over a long time period.
The selection of species for the afforestation of barren and/or severely eroded land may have beneficial effects on erosion control by reducing the soil and nutrient loss ([47], [66]). Zhang et al. ([67]) emphasize the efficiency of black locust against erosion, while Cao et al. ([7]) reported that black locust was an effective species in the conservation of underdeveloped land. Wang et al. ([62]) explored the impact of soil erodibility on nutrient losses, reporting that total N loss is mainly controlled by the rate of runoff and sediment yield (soil loss). In this study, the reduction of soil loss and the increase in total N indicate the effectiveness of black locust in erosion control.
Carbon stock in biomass and soil
Afforestation, forest restoration and suitable forest management practices are powerful strategies for the mitigation of climate change through atmospheric C sequestration in biomass and soil ([33], [35], [22]). The potential of carbon sequestration depends on the type of afforestation, environmental conditions and modes of management ([16]). According to Lal ([32]), total carbon stock under a 27-year-old oak forest is 117 t ha-1, and 227 t ha-1 under a 69-year old forest of oak and beech. The total carbon stock in the area of the Grdelica Gorge (107.2 t ha-1 for black locust and 131.5 t ha-1 for black pine) is remarkable, considering that the afforestation was carried out on abandoned and degraded land. Based on our results, there is no significant differences either in the total C stock or in the C stock of carbon pools between black locust and black pine forests (Tab. 6). However, the percentages of C stock in pools differ between black pine and black locust. The share of C stock in living biomass is 43% in both black locust and black pine. In black locust the share of C stock in dead wood is 16%, whereas in black pine this share accounts for 17%. Ouimet et al. ([40]) reported that the share of C stock in living biomass accounted for as much as 71% of the total C stock in 22-year-old plantations of Pinus resinosa Ait.
In this study, the share of the total C stock found in litter and soil of black locust stands differed from that of black pine stands. The C stock in litter was greater in the afforestation with black pine (21% vs. 18%), whereas the soil C stock was greater in the afforestation with black locust (23% vs. 19%). The greater share of C stock in the litter of black pine can be associated with the slower decomposition of the black pine litter which accumulates on the soil surface ([10]).
According to Dixon et al. ([11]), forest soils contain approximately 40% of the total above-ground carbon and represent an important pool for its sequestration ([27], [32]). The greater share of C stock in soil under black locust (23%) as compared with that under black pine (19%) may be associated with the soil properties under the former species, which affect the carbon sequestration performances of the soil. As a broadleaved, nitrogen-fixing species, black locust provides a greater accumulation of carbon in the soil ([43]). Given that the rate of litter decomposition of nitrogen-fixing species is higher than in other species ([38]), this may increase the rate of C accumulation in the soil under black locust ([43]). Moreover, in areas covered with black locust where erosion processes are less pronounced, the conditions for C sequestration tend to be more favorable.
Conclusion
The results of this study indicate that under extremely harsh habitat conditions, the tree species analyzed have different impacts on several soil properties, as well as on the erosion control and the C stock. Black locust performed better than black pine, leading to the following beneficial effects: (i) the N enrichment of the surface soil and a higher total N soil content; (ii) a decrease in the acidity of the surface soil layers and an increase in pH of the 0-5 cm layer; (iii) a long-term erosion control through a continuous reduction of soil loss; (iv) an improvement of soil conditions for C sequestration, and a greater share of the total C stock in soil. On the other hand, black pine achieved significantly higher production (basal area, volume, current volume increment, mean diameter, mean height), thereby showing a greater efficiency in C sequestration, particularly in living biomass.
Regarding forest ecosystem restoration, black locust is more efficient than black pine, in that its impact on the soil creates favorable conditions, thus promoting the re-establishment of the original forest. Considering that afforestation in the study area was primarily aimed at erosion control and was conducted on highly exhausted soil damaged by erosion, the ameliorative effects of the selected species, especially those of black locust, are obvious. In conclusion, it can be stated that black locust is a more suitable species for the afforestation of degraded land in the examined area.
Knowledge of the impact of different species on the mitigation of harsh habitat conditions can provide guidelines for the selection of species to be used in future afforestation activities aimed at the restoration of degraded forest sites. Moreover, an appropriate selection of species for afforestation of degraded land may help land use managers in achieving optimal benefits and ecosystem services.
Acknowledgements
We are grateful to the Ministry of Education and Science of the Republic of Serbia for their financial support through the projects: “Climate Change and Its Impact on the Environment - Monitoring, Adaptation and Mitigation”, reference no. 043007 and “Sustainable management of total forest potential in the Republic of Serbia”, reference no. 37008. The authors would also like to thank two anonymous reviewers for their helpful suggestions.
References
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Damjan Pantić
Snežana Belanović Simić
Dragan Borota
Matilda Djukić
Danijela Djunisijević-Bojović
University of Belgrade, Faculty of Forestry, Kneza Višeslava 1, 11030 Belgrade (Serbia)
PE “Vojvodinašume“, Preradovićeva 2, 21131, Petrovaradin (Serbia)
Corresponding author
Paper Info
Citation
Lukić S, Pantić D, Simić SBć, Borota D, Tubić B, Djukić M, Djunisijević-Bojović D (2015). Effects of black locust and black pine on extremely degraded sites 60 years after afforestation - a case study of the Grdelica Gorge (southeastern Serbia). iForest 9: 235-243. - doi: 10.3832/ifor1512-008
Academic Editor
Tamir Klein
Paper history
Received: Nov 24, 2014
Accepted: May 01, 2015
First online: Aug 22, 2015
Publication Date: Apr 26, 2016
Publication Time: 3.77 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2015
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 52628
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 43726
Abstract Page Views: 3358
PDF Downloads: 4145
Citation/Reference Downloads: 29
XML Downloads: 1370
Web Metrics
Days since publication: 3801
Overall contacts: 52628
Avg. contacts per week: 96.92
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2016): 14
Average cites per year: 1.40
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Short Communications
Influences of Black Locust (Robinia pseudoacacia L.) afforestation on soil microbial biomass and activity
vol. 9, pp. 171-177 (online: 16 February 2015)
Research Articles
Carbon storage and soil property changes following afforestation in mountain ecosystems of the Western Rhodopes, Bulgaria
vol. 9, pp. 626-634 (online: 06 May 2016)
Short Communications
Variation in soil carbon stock and nutrient content in sand dunes after afforestation by Prosopis juliflora in the Khuzestan province (Iran)
vol. 10, pp. 585-589 (online: 08 May 2017)
Research Articles
Effects of tree species, stand age and land-use change on soil carbon and nitrogen stock rates in northwestern Turkey
vol. 9, pp. 165-170 (online: 18 June 2015)
Research Articles
Soil stoichiometry modulates effects of shrub encroachment on soil carbon concentration and stock in a subalpine grassland
vol. 13, pp. 65-72 (online: 07 February 2020)
Research Articles
Estimation of fuel loads and carbon stocks of forest floor in endemic Dalmatian black pine forests
vol. 13, pp. 382-388 (online: 01 September 2020)
Research Articles
Allometric equations to assess biomass, carbon and nitrogen content of black pine and red pine trees in southern Korea
vol. 10, pp. 483-490 (online: 12 April 2017)
Research Articles
Changes in the properties of grassland soils as a result of afforestation
vol. 11, pp. 600-608 (online: 25 September 2018)
Research Articles
Spatial and temporal variation of drought impact on black locust (Robinia pseudoacacia L.) water status and growth
vol. 8, pp. 743-747 (online: 18 June 2015)
Research Articles
Effects on soil characteristics by different management regimes with root sucker generated hybrid aspen (Populus tremula L. × P. tremuloides Michx.) on abandoned agricultural land
vol. 11, pp. 619-627 (online: 04 October 2018)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword