Ectomycorrhizal fungal community associated with autochthonous white poplar from Serbia
iForest - Biogeosciences and Forestry, Volume 9, Issue 2, Pages 330-336 (2015)
doi: https://doi.org/10.3832/ifor1370-008
Published: Nov 12, 2015 - Copyright © 2015 SISEF
Research Articles
Abstract
We analyzed the community of ectomycorrhizal fungi of an autochthonous white poplar (Populus alba L.) stand in the Kovilj-Petrovaradin marshes (Serbia), and examined its seasonal dynamics. Ectomycorrhizal types were identified by combining morphological and anatomical descriptions with molecular methods (sequencing of ITS region of ribosomal DNA). In two seasons, 20 ectomycorrhizal types were recorded, from which 11 types were identified to the species level, six were determined to the genus level, two types were determined to the family level and one type remained unidentified. Number of ectomycorrhizal types, number of fine roots, percentage of vital mycorrhizal roots, diversity indexes and abundance of exploration types did not differ significantly between autumn and spring. During both seasons, the most abundant types were: Entoloma sp., Tuber maculatum, Cenococcum geophilum, Tuber rufum and Peziza sp. Due to the high variation of the ectomycorrhizal types-based Shannon-Weaver diversity index in poplar stands, and the fact that poplars form dual mycorrhizal association, this index is not recommended as a reliable index for bioindication in poplar.
Keywords
Ectomycorrhiza, Populus alba, Diversity, Nature Reserve, Seasonal Dynamics, Morphological-Anatomical Characterization, Molecular Identification
Introduction
Poplars are world-wide distributed tree species that combine commercial importance with biotechnological advantages, such as rapid growth, simple in vitro propagation and availability of genetic transformation systems ([30], [22]). They are used in agroforestry systems ([18]), short rotation forestry ([29]) and phytoremediation, as they cycle large amounts of water and grow rapidly by producing large amount of biomass, due to their deep root systems ([44]).
Poplars in plantations and natural stands regularly form dual associations with ectomycorrhizal (ECM) and arbuscular mycorrhizal (AM) fungi ([24]) that are known to prefer different soil depths ([43]). While AM community has rarely attracted the interest of researchers, several prominent ECM species are thoroughly studied in poplars, in particular in white poplar (Populus alba L.), including the most expensive white truffle (Tuber magnatum Pico - [7]).
Seedlings colonized with compatible ECM fungal species and strains are favored in making contacts with water and nutrients, as well as with other organisms in the soil ([33]). In addition to the increased nutrient uptake, mycorrhizas offer numerous other benefits to symbiontic tree species, such as enhanced plant efficiency in absorbing water, reduced fertilization and irrigation requirements, increased drought tolerance, increased pathogen resistance, protection against damage from heavy metals and other pollutants, mitigation of various plant stresses, improvement of seedling growth and survival, and improvement of soil structure by the extramatrical hyphal network ([41], [50], [47]).
The functional compatibility and stress tolerance of ectomycorrhizal types is species specific and depend on both partners. Therefore, information on the ECM community structure can provide valuable information about physiology of forest trees and functioning of forest ecosystems ([35]).
Limited data on ECM community in white poplar from natural stands are available and its seasonal dynamics remain unclear ([21]). The aim of this work was to investigate the ECM community in a case study location represented by an autochthonous white poplar stand in the Special Nature Reserve “Kovilj-Petrovaradin marshes” in Serbia and to analyze seasonal changes in the community between spring and autumn.
Materials and methods
Description of site and sampling
ECM roots were isolated from soil samples collected in the Special Nature Reserve, close to Novi Sad, Serbia (45° 12′ N, 19° 58′ E, elevation 78 m a.s.l.). According to the results from a nearby measuring station (Rimski Šančevi), the average annual precipitation (1951-2010) was 625 mm and the average yearly temperature was 11.4 °C ([52]). Climate is a Dfa subtype of temperate continental climate with July and August as the warmest months (Republic Hydrometeorological Service of Serbia - ⇒ http://www.hidmet.gov.rs/). The sampling site was occasionally flooded and the soil type was classified as fluvisol, loamy form ([27]). The sampling was performed in a naturally grown autochthonous white poplar (Populus alba L.) stand of 50-60 years old trees (100 trees ha-1) mixed with scarcely abundant Acer negundo L. and Robinia pseudoacacia L.
Five mature white poplar trees were randomly selected. Two soil samples per tree were taken in autumn (September 2009) and spring (March 2010) at a distance of about 1 m from the tree trunk. In total, ten soil samples were collected in each season. A soil corer of 274 ml volume and reaching 18 cm depth was used for collecting standardized soil core samples ([34]). Soil core samples were stored at 4 °C for up to one month. One day prior to analysis, soil samples were submerged in water and all fine roots were carefully washed from soil. Vital ECM root tips were separated from old, non-turgescent and non-mycorrhizal (ONN) root tips in water under a dissecting microscope. All fine root tip categories, including all identified types of ECM, were counted as total number in individual soil core sample.
Identification of ectomycorrhizae
ECM types were identified by combining morphological and anatomical approach with molecular methods. Morphological and anatomical characteristics of each ECM root type were assessed by a binocular Olympus SZX 12 (light source: Olympus Highlight 3100, daylight filter) and DIC (Nomarski) microscope Olympus BX 51 (magnification 100-2000×) following Agerer ([2]), Kraigher ([33]), and ECM descriptions published in Agerer ([4]), Agerer et al. ([5]), and Agerer & Rambold ([6]). Based on the presence and abundance of emanating elements, ECM types were also classified into the exploration types proposed by Agerer ([3]).
Molecular identification was based on nucleotide sequencing of ITS regions (Internal Transcribed Spacer) in nuclear ribosomal DNA. This is considered the best molecular marker for fungi identification ([32]). After DNA extraction from 5-20 root tips with a PlantDNeasy® Mini Kit (Qiagen, Hilden, Germany) from each ECM type, the ITS region was amplified using the ITS 1f and ITS 4 primer pair ([19]). DNA fragments were separated in and excised from agarose gel and purified with Wizard® SV Gel and PCR Clean-up System® (Promega Corporation, Madison, WI, USA). Sequencing was performed commercially at Macrogen Inc. (Seoul, Rep. of Korea). Species, genus or family of ECM fungi were determined by comparing with the sequences deposited in the GenBank (⇒ http://www.ncbi.nlm.ni h.gov/genbank/index.html) and Unite ([1]) databases.
Data analysis
Diversity indexes were calculated per sample and per site (i.e., by pooling the ECM community data) following the formulas given by Atlas & Bartha ([8]) and Taylor et al. ([53]):
- Species richness (
d) = (S -1) / log10N, whereSis the number of ECM types andNis the number of all mycorrhizal tips; - Shannon-Weaver’s diversity index (
H) =C/N(NlogN- Σni logni), whereC= 2.3,Nis the number of all mycorrhizal tips andni is the number of mycorrhizal tips of individual ECM type; - Evenness (
e) =H/ logS, whereHis the Shannon-Weaver’s diversity index andSis the number of ECM types; - Equitability (
J) =H/Hmax, whereHis the Shannon-Weaver’s diversity index andHmax is the theoretical maximumHassuming that each ECM type was equally abundant; - Berger-Parker’s evenness index (
BP) = 1 - (Nmax/N), whereNmax is the number of mycorrhizal tips of the most frequent ECM type andNis the number of all mycorrhizal tips.
Data of two soil core samples were joint and single tree was used as a statistical unit. The Student t-test was used to test the significance of differences in the number of ECM types, vital ECM root tips, old, non-turgescent and non-mycorrhizal roots, total fine roots, percentage of vital root tips and abundance of exploration types between autumn and spring. In order to fit the normal distribution, data were transformed as follows: count data were transformed according to square root transformation ([10]), while percentage values were transformed according to arcsine transformation using the Bliss formula ([51]). The non-transformed data are presented in Tabs. 2 and 3. The Mann-Whitney U test was used to test the significance of differences in diversity indexes. All statistical analyses were performed using the package STATISTICA® version 12 (StatSoft Inc., Tulsa, OK, USA).
Results
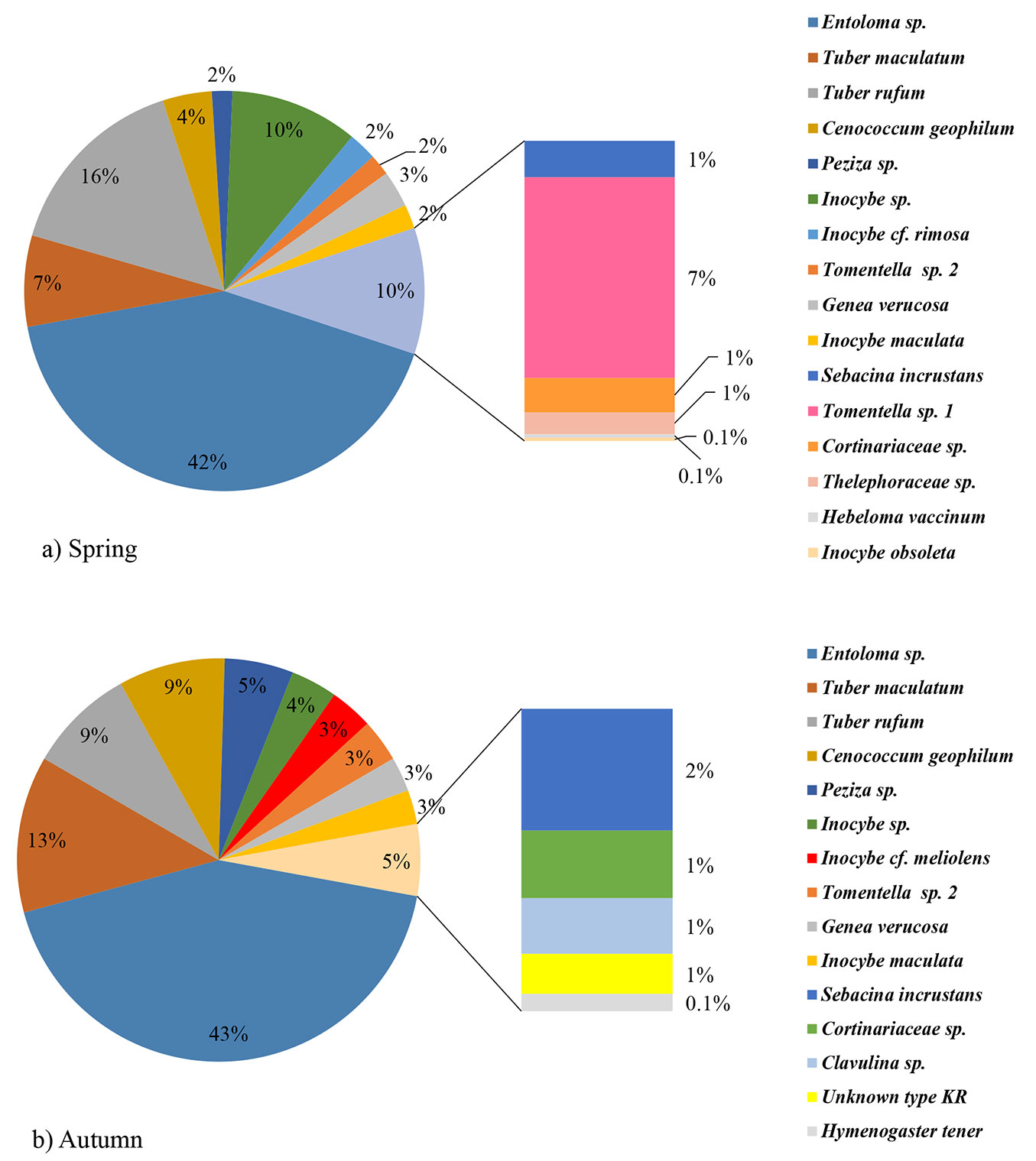
Overall, twenty ECM types were determined at the examined site in the two investigated seasons. Eleven types were identified to the species level, six to the genus level, while other three were determined to the family level or remained unidentified (Unknown type KR - Tab. 1). Cenococcum geophilum, Entoloma sp., Genea verrucosa, Inocybe maculata, Inocybe sp., Peziza sp., Sebacina incrustans, Tomentella sp. 2, Cortinariaceae sp., Tuber rufum and Tuber maculatum were recorded in both seasons. Hebeloma vaccinum, Inocybe cf. rimosa, Inocybe obsoleta, Thelephoraceae sp., and Tomentella sp. 1 were observed only in spring, while Clavulina sp., Hymenogaster tener, Inocybe cf. meliolens and Unknown type KR were recorded only in autumn (Fig. 1a, Fig. 1b).
Tab. 1 - Identification of ectomycorrhizal (ECM) types from a mature white poplar (Populus alba L.) stand at the Special Nature Reserve Kovilj-Petrovaradin marshes (Serbia). Identifications are based on morphological-anatomical characters and nrITS DNA sequence comparison with the international nucleotide sequences databases. GenBank accession numbers are given for the analyzed ECM types. Exploration types were assessed after Agerer ([3]).
| Ectomycorrhizal type | Nucleotide database accession number | Exploration type |
|---|---|---|
| C. geophilum | HG937623 | short distance |
| Genea verrucosa | HG937624 | short distance |
| Hebeloma vaccinum | HG937625 | medium distance fringe subtype |
| Hymenogaster tener | HG937626 | short distance |
| Inocybe cf. meliolens | HG937629 | short distance |
| Inocybe cf. rimosa | HG937627 | short distance |
| Inocybe maculata | HG937628 | short distance |
| Inocybe obsoleta | HG937630 | short distance |
| Sebacina incrustans | HG937631 | short distance |
| Tuber maculatum | HG937633 | short distance |
| Tuber rufum | HG937632 | short distance |
| Clavulina sp. | HG937634 | short distance |
| Cortinariaceae sp. | HG937640 | short distance |
| Entoloma sp. | HG937635 | medium distance smooth subtype |
| Inocybe sp. | HG937636 | short distance |
| Peziza sp. | HG937637 | contact |
| Tomentella sp. 1 | HG937638 | short distance |
| Tomentella sp. 2 | HG937639 | short distance |
| Thelephoraceae sp. | HG937641 | short distance |
| Unknown type KR | - | short distance |
Fig. 1 - Ectomycorrhizal community structure in a mature white poplar stand in Serbia, based on sampling of 10 soil samples in spring (a) and 10 soil samples in autumn (b).
No significant differences between seasons were found as for the number of ECM types, vital ECM roots, old, non-turgescent and non-mycorrhizal roots, total number of fine roots and percentage of vital ECM root tips. However, all values were higher in spring (Tab. 2).
Tab. 2 - Comparison of total and average values (± standard erorr) of number of ectomycorrhizal (ECM) types, vital ECM root tips, old, non-turgescent and non-mycorrhizal roots (ONN), total fine roots and diversity indexes from a mature white stand located in Serbia between spring and autumn. (a): p-values after the t-test; (b): p-values after the Mann-Whitney’s U test.
| Parameter | Spring | Autumn | p-value | ||
|---|---|---|---|---|---|
| Total value per site |
Average value per tree |
Total value per site |
Average value per tree |
||
| Number of ECM types | 16 | 5.10 ± 0.60 | 15 | 4.90 ± 0.20 | 0.838 a |
| Number of vital ECM root tips | 4300 | 430 ± 133.6 | 3030 | 303 ± 65.5 | 0.511 a |
| Number of ONN root tips | 22140 | 2214 ± 511.6 | 19335 | 1933.5 ± 148.2 | 0.686 a |
| Total number of fine root tips | 26440 | 2644 ± 607.7 | 22365 | 2236.5 ± 141.9 | 0.609 a |
| % of vital ECM Root tips | 16 | 16.20 ± 2.90 | 14 | 13.90 ± 3.10 | 0.569 a |
| Species richness index | 4.13 | 1.62 ± 0.20 | 4.02 | 1.63 ± 0.08 | 0.754 b |
| Shannon-Weaver index | 1.95 | 1.01 ± 0.12 | 2.00 | 1.15 ± 0.06 | 0.403 b |
| Evenness | 0.70 | 0.626 ± 0.06 | 0.74 | 0.723 ± 0.03 | 0.174 b |
| Equitability | 1.62 | 1.44 ± 0.13 | 1.70 | 1.67 ± 0.07 | 0.174 b |
| Berger-Parker index | 0.58 | 0.38 ± 0.04 | 0.57 | 0.48 ± 0.04 | 0.117 b |
There were no significant differences between seasons in species richness index, Shannon-Weaver’s index, Evenness, Equitability and Berger-Parker’s index (Tab. 2). Diversity indexes revealed that number of species, relative abundance of individual species, equitability and evenness between species, and dominance rate of the most abundant species did not differ significantly between seasons, i.e., the ECM community structure did not change across seasons.
In both seasons, the same five ECM types dominated and represented about 80% of the total number of ECM root tips. The most abundant ECM type was Entoloma sp. with over 40%, followed by Tuber maculatum, Cenococcum geophilum, Tuber rufum and Peziza sp. Relative abundance of these ECM types was similar in both seasons (Fig. 1a, Fig. 1b).
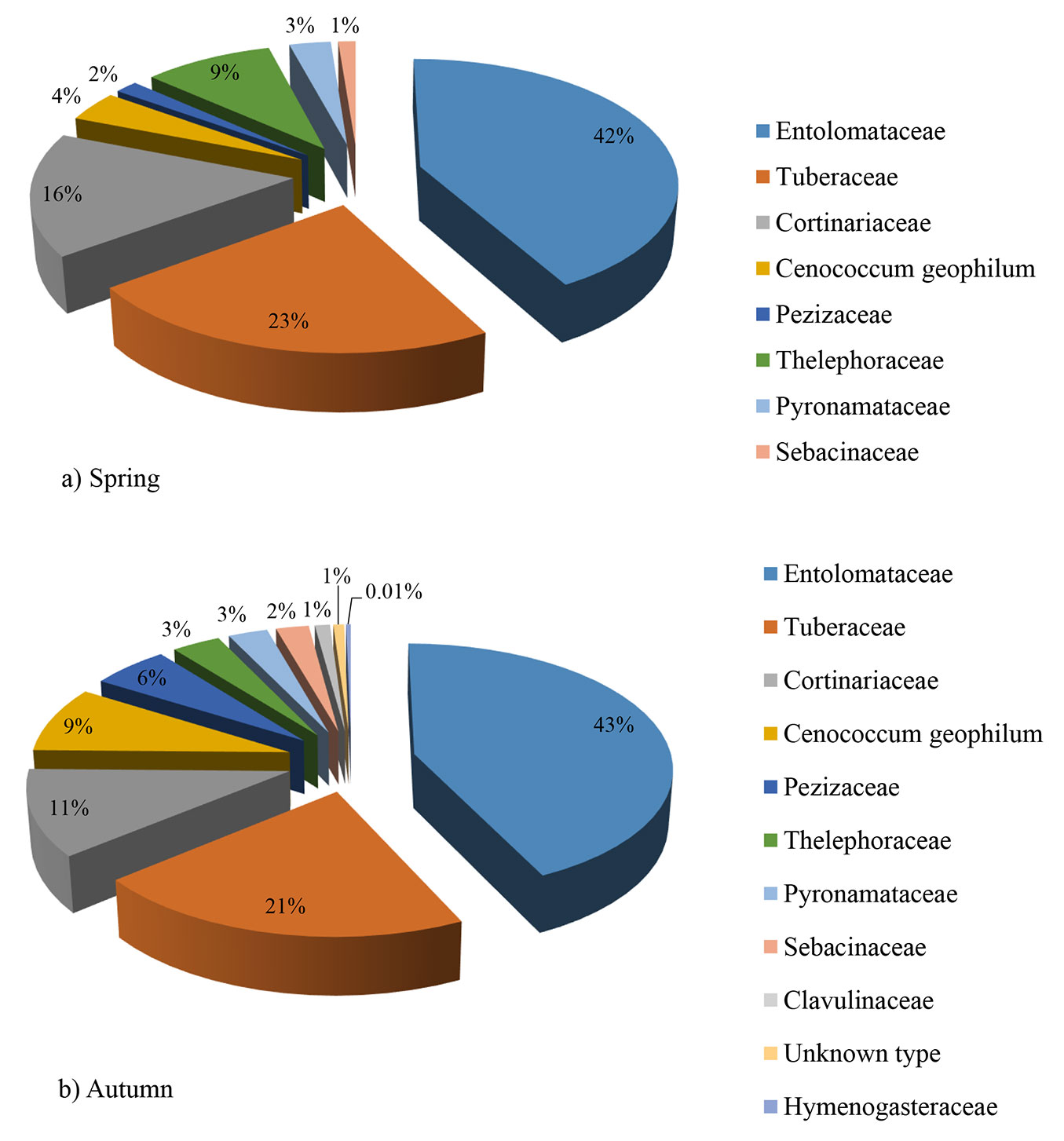
The most abundant ECM fungal family was Entolomataceae, followed by Tuberaceae and Cortinariaceae. In both seasons the three above-mentioned families represented 70% of the total number of all ECM root tips (Fig. 2a, Fig. 2b).
Fig. 2 - Relative abundance of ectomycorrhizal fungal families in a mature white poplar stand in Serbia, based on sampling of 10 soil samples in spring (a) and 10 soil samples in autumn (b)
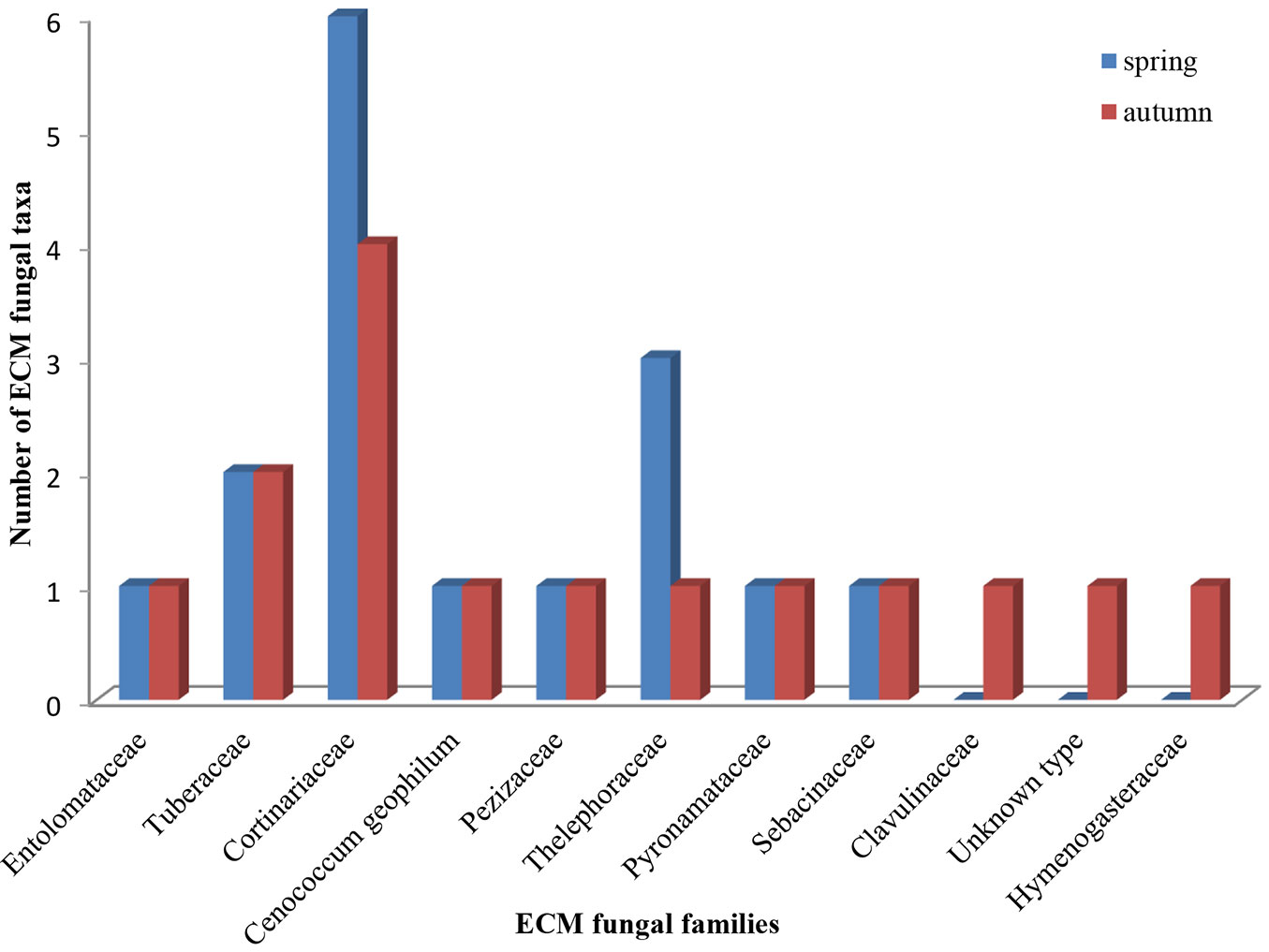
In terms of species richness, the family Cortinariaceae was the richest, with six species in spring and four in autumn (Fig. 3). The number of Basidiomycota ECM symbionts was higher than those of Ascomycota, both in spring (11 vs. 5, respectively) and in autumn (10 vs. 5, respectively). Similarly, the contribution of root tips colonized by Basidiomycota ECM fungi was higher than those colonized by Ascomycota fungi (in spring 68.5 vs. 31.5 %, respectively, in autumn 62 vs. 38%, respectively - data not shown).
Fig. 3 - Number of ectomycorrhizal fungal taxa within fungal families recorded in mature white poplar stand located in Serbia in spring and autumn.
The most abundant exploration type (ET) was short-distance ET followed by medium -distance ET and contact ET. Long-distance ET was not observed. Abundance of each ET did not significantly differ between the seasons (Tab. 3).
Tab. 3 - Relative abundance (± standard error, %) of ectomycorrhiza exploration types in a mature white poplar stand in Serbia in spring and autumn, and significance of Student t-test for the effect of season.
| Exploration type | Spring | Autumn | t-test (p-value) |
|---|---|---|---|
| Contact type | 1.87 ± 1.01 | 2.59 ± 2.59 | 0.754 |
| Short distance | 65.23 ± 9.38 | 60.68 ± 8.42 | 0.648 |
| Medium distance | 32.90 ± 9.09 | 36.72 ± 9.09 | 0.724 |
| Long distance | 0 | 0 | - |
Discussion
The community of ectomycorrhizal fungi in this case study represented by a mature autochthonous white poplar stand located in the Special Nature Reserve “Kovilj-Petrovaradin marshes” (Serbia) is one of the few studies ([21], [25], [27]) on ECM diversity in white poplar and, up to our knowledge, the only one in a mature natural stand.
The average amount of vital ECM root tips (1106 dm-3 in autumn and 1570 dm-3 in spring) was considerably lower than in mature stands of other species such as beech (1460-15 800 dm-3 - [40]) and spruce (4309-6716 dm-3 - [34]). The number of all fine roots per soil volume in poplar stands was even more variable. In this study, such value was 9651 dm-3 in spring and 8163 dm-3 in autumn, while ranged from 2599 dm-3 in control plants irrigated with water to 4573 dm-3 in plants treated with the anti-ozonant ethylenediurea in an ozone-sensitive clone ([28]). Krpata et al. ([36]) counted 17 350-42 630 dm-3 ECM root tips at a site with aspen contaminated by heavy metals. The relatively low number of ECM roots in our white poplar stand reflects the regular flooding occurring in the studied area and the dual colonization with ECM and AM fungi. In the same poplar trees, Katanić et al. ([26]) recorded values of root length colonization with ECM and AM fungi of 16.7 and 8.8 %, respectively.
The 20 ECM types recorded in total are a considerably lower number compared to some previous studies. On individual Populus tremula trees in an old-growth mixed forest, Bahram et al. ([9]) found 122 ECM fungal species. Analyzing ECM community at two sites with 30-40 m tall white poplars during three years, Jakucs ([21]) recorded 70 ECM types, while Krpata et al. ([36]) found 54 ECM types at a heavy metal polluted site with 20 to 25-year-old aspen trees. However, results of our research are in accordance with Visser et al. ([54]), who recorded 22 ECM types in a mixed forest dominated by American aspen, and Kaldorf et al. ([22]), who observed 23 morphotypes in a five-year-old experimental aspen plantation. In addition, at three experimental sites with poplars Karlinski et al. ([23]) found in total 27 ECM fungal taxa. In a white poplar plantation, Katanić et al. ([25]) preliminary recorded 15 ECM types. However, when ECM diversity was studied seasonally, a total of 30 ECM types were observed ([27]). It seems that the number of ECM fine roots and the diversity of ECM types in poplar are highly variable, and the naturalness of the studied Special Nature Reserve does not contribute to increase ECM diversity as compared with other poplars’ sites.
Values of Shannon-Weaver’s diversity index (1.95 in spring and 2.00 in autumn) are in accordance with results obtained by DeBellis et al. ([16]). These authors recorded a similar Shannon-Weaver diversity index of 2.00 in an aspen-dominated plot by using morphological characterization of ECM types, but a considerably higher index was recorded (3.00) when molecular identification tools were applied. Values of the Shannon-Weaver’s diversity index obtained in extreme conditions are controversial. On the one hand, an ozone sensitive poplar clone showed lower values of Shannon-Weaver’s index, namely 1.60 in anti-ozonant protected plants and 1.21 in control plants ([28]). On the other hand, Krpata et al. ([36]) recorded relatively high value of Shannon-Weaver’s diversity index (2.00) in aspen trees grown at a site contaminated with heavy metals. Due to the high variation of the ECM-based Shannon-Weaver’s diversity index for poplar stands, and the fact that poplars form dual mycorrhizal association, this index can not be considered reliable for bioindication, as otherwise proven for spruce ([34]). None of the above-mentioned authors compared fine roots data, species abundance or diversity indexes of poplar stands among seasons. However, in our study differences between seasons did not show any significant effect on diversity of the ECM community.
ECM community associated with white poplars in the Kovilj-Petrovaradin marshes consisted of few abundant and numerous infrequent ECM types, in accordance with previous research ([15], [45]). Koide et al. ([31]) reported abundant ECM types present in both seasons with similar relative abundance, while some rare species were specific for autumn or spring only. In both seasons, five ECM types made up near 80% of all ECM root tips, while 11 or 10 ECM types (in spring and autumn, respectively) contributed to the rest. This finding is in accordance with the evidence that the ECM community varied according to a logarithmic distribution of abundances ([14], [46]).
In the Kovilj-Petrovaradin marshes, the genus Inocybe was the most abundant one, with five ECM types, while genera Tuber and Tomentella had two members. Analyzing the diversity of mycorrhizal fungi in mixed deciduous stand, Lang et al. ([37]) noticed that Tomentella and Inocybe were the most abundant and contributed mostly to the species richness. In the ECM community associated with aspen grown at the site contaminated with heavy metals, the most abundant taxonomic groups were Tomentella (17), Inocybe (6), Cortinarius (5), Hebeloma and Tuber with 3 operational taxonomic units ([36]). At three sites with poplar clones, Karlinski et al. ([23]) recorded the dominance of Cortinariaceae, Thelephoraceae and Tricholomataceae that constituted nearly 90% of the mycorrhizal community.
Inocybe species are well-known colonizers of ECM plants on disturbed or pioneer sites ([42]). Investigating mycorrhizal fungi associated with aspen at three different sites, Cripps ([13]) observed 54 ECM fungi and 14 species belonged to the genus Inocybe. Tomenteloid fungi are the most frequent and widespread ECM partners of deciduous and evergreen tree species in the forests of Europe and North America ([20], [14]). In the white poplar forest adapted to dry conditions of the Hungarian plain, Tomentella group was a minority component of the ECM community ([21]). Similar observations were made in the occasionally flooded Kovilj-Petrovaradin marshes. Investigating the molecular diversity and the ecological specificity of truffles originating from the mid-west of Balkan Peninsula, Marjanović et al. ([39]) recorded 12 species from this group, including Tuber rufum and T. maculatum. Cenococcum geophilum is a cosmopolitan ECM fungus with a wide range of hosts and habitats. It is a frequent and abundant ECM type adapted to environment under stress ([38]) that was recorded in almost half of the samples in our study. Di Pietro et al. ([17]) noted that under extreme stress, ECM formed by Cenococcum geophilum has the ability to survive better than ECM of some other fungi, and that this species is particulary efficient in the protection of fine roots from drought stress. However, during the three-year survey of two ECM communities associated with poplars in dry conditions, Jakucs ([21]) did not observe the presence of C. geophilum, while in our study it was found in an area that is occasionally flooded.
Although the differences were not statistically significant, all parameters investigated showed slightly higher values in spring than in autumn. This is in accordance with Courty et al. ([11]) who recorded the highest number of vital ECM roots in April, while the lowest value was observed in September. The absence of significant differences between autumn and spring in the number of ECM types and diversity indexes in this study is in accordance with the results of De Roman & De Miguel ([15]). In both seasons the same three fungal families were the most abundant (Entolomataceae, Tuberaceae and Cortinariaceae). In contrast, Richard et al. ([48]) found that the relative abundance of two out of three the most abundant families (Russulaceae and Cortinariaceae) showed significant seasonal shifts.
In both seasons, Basidiomycota dominated the examined ECM community, confirming numerous studies on poplars ([12], [13], [36], [23], [28]) where the Basidiomycota group was the most abundant and had more members in comparison to Ascomycota.
The most abundant exploration type in both seasons was short-distance ET and then medium-distance ET. Agerer ([3]) found a relationship between ETs and their potential ecological roles. Jakucs ([21]) noticed that in two forests adapted to similar ecological conditions, dominant ECM types belonged to the same ET. Analyzing ET of ECM community, Rudawska et al. ([49]) concluded that abundance of a particular ET is related to the soil chemistry, since occurrence of contact ET was related to high nutrient content. On the other hand, medium-distance fringe exploration type was abundant at a sites contaminated with heavy metals ([49], [23]). We assume that the similar distribution of ETs observed at our site in spring and autumn results from similar ecological conditions.
Acknowledgements
The study was co-financed by the Slovenian Research Agency through the Research Programme P4-0107 “Forest Biology, Ecology and Technology”, through the Scholarship Ad futura (OMEGA D.O.O., for MK) and project III43007 “Studying climate change and its influence on the environment: impacts, adaptation and mitigation” financed by the Ministry of Education, Science and Technological Development of the Republic of Serbia within the framework of integrated and interdisciplinary research.
References
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Online | Gscholar
Online | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Online | Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
CrossRef | Gscholar
Authors’ Info
Authors’ Affiliation
Saša Orlović
Branislav Kovačević
University of Novi Sad, Institute of Lowland Forestry and Environment, Antona Cehova 13, 21000 Novi Sad (Serbia)
Marko Bajc
Hojka Kraigher
Slovenian Forestry Institute, Večna pot 2, 1000 Ljubljana (Slovenia)
European University, Faculty of Pharmacy, Trg mladenaca 5, 21000 Novi Sad (Serbia)
Corresponding author
Paper Info
Citation
Katanić M, Grebenc T, Orlović S, Matavuly M, Kovačević B, Bajc M, Kraigher H (2015). Ectomycorrhizal fungal community associated with autochthonous white poplar from Serbia. iForest 9: 330-336. - doi: 10.3832/ifor1370-008
Academic Editor
Paola Mairota
Paper history
Received: Jun 04, 2014
Accepted: Jul 10, 2015
First online: Nov 12, 2015
Publication Date: Apr 26, 2016
Publication Time: 4.17 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2015
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 49811
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 41488
Abstract Page Views: 3307
PDF Downloads: 3511
Citation/Reference Downloads: 60
XML Downloads: 1445
Web Metrics
Days since publication: 3656
Overall contacts: 49811
Avg. contacts per week: 95.37
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2016): 6
Average cites per year: 0.60
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Ectomycorrhizal fungal community in mature white poplar plantation
vol. 14, pp. 540-547 (online: 26 November 2021)
Research Articles
Influence of inorganic salts on biomass production, biochemical composition, and bioethanol production of Populus alba
vol. 13, pp. 566-574 (online: 07 December 2020)
Research Articles
Ectomycorrhizae of Norway spruce from its southernmost natural distribution range in Serbia
vol. 12, pp. 43-50 (online: 10 January 2019)
Research Articles
Ectomycorrhizal diversity in a mature pedunculate oak stand near Morović, Serbia
vol. 16, pp. 345-351 (online: 22 November 2023)
Research Articles
Assessment of cadmium tolerance and phytoextraction ability in young Populus deltoides L. and Populus × euramericana plants through morpho-anatomical and physiological responses to growth in cadmium enriched soil
vol. 10, pp. 635-644 (online: 01 June 2017)
Research Articles
Seasonal dynamics of soil respiration and nitrification in three subtropical plantations in southern China
vol. 9, pp. 813-821 (online: 29 May 2016)
Research Articles
The use of branch enclosures to assess direct and indirect effects of elevated CO2 on photosynthesis, respiration and isoprene emission of Populus alba leaves
vol. 1, pp. 49-54 (online: 28 February 2008)
Research Articles
Identification and molecular characterization of LTR and LINE retrotransposable elements in Fagus sylvatica L.
vol. 2, pp. 119-126 (online: 10 June 2009)
Research Articles
Clonal structure and dynamics of peripheral Populus tremula L. populations
vol. 7, pp. 140-149 (online: 13 January 2014)
Research Articles
Stand dynamics and natural regeneration in silver fir (Abies alba Mill.) plantations after traditional rotation age
vol. 7, pp. 313-323 (online: 08 April 2014)
iForest Database Search
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords