Effect of the plantation age on the use of Eucalyptus stands by medium to large-sized wild mammals in south-eastern Brazil
iForest - Biogeosciences and Forestry, Volume 8, Issue 2, Pages 108-113 (2015)
doi: https://doi.org/10.3832/ifor1237-008
Published: Jul 21, 2014 - Copyright © 2015 SISEF
Research Articles
Abstract
In São Paulo State, in Southeastern Brazil, the Eucalyptus plantations have been replacing large areas which were formerly occupied by pastures used for livestock production. Such land use change may affect the habitat use by wildlife in these anthropic landscapes. In this region, the commercial Eucalyptus plantations of the paper and cellulose industry usually take from 6 to 7 years to be harvested. During its production cycle, the Eucalyptus stands vary from an open savanna-like environment just after plantation, when plants still resemble bushes, to a forest-like environment with densely distributed 18-meter high trees. Previous studies show that the Eucalyptus plantations in Southeastern Brazil are used by generalist species including medium and large sized mammals. However, the possible influence of such dramatic temporal environmental heterogeneity on the wildlife habitat use in Eucalyptus plantations is still unknown. In this study, which follows a classic stratified design, we evaluate the influence of the Eucalyptus stand age on the local patterns of distribution and abundance of middle to large-sized wild mammals. Our results show an increase not only in their species richness, but also in their frequency of occurrences along the commercial cycle of the Eucalyptus plantations with a steep decline in both before harvest. This pattern may be related to weed control practices which significantly reduce the understory vegetation, in especial at the end of the commercial cycle while preparing for harvest. Future studies should prioritize the possible variation on the trophic structure in the Eucalyptus plantations along commercial cycles as a response of wildlife friendly silvicultural/agricultural management practices.
Keywords
Forestry, Silvicultural Landscapes, Anthropic Environments, Wildlife, Temporal Heterogeneity
Introduction
Patterns of wildlife distribution and abundance have suffered alterations worldwide due to human occupation and related activities ([55]). The agriculture expansion has replaced the original vegetation and fragmented primary environments, thus affecting wildlife patterns of habitat use ([22], [15]). However, studies in agricultural landscapes have shown the use of man-made habitats by the wildlife, such as coffee plantations in Mexico ([38]), banana and coconut plantations in Costa Rica ([24], [23]), cocoa and Eucalyptus plantations in Brazil ([16], [32]) and subsistence agriculture in Nepal ([2]).
A great spatial heterogeneity due to different strata and structural complexity exists in native forests, which results in a significant β-diversity ([37], [44]). In an agricultural landscape, a considerable loss of biodiversity occurs where monocultures are spatially more homogenous. However, the environment of Eucalyptus plantations varies dramatically along their commercial cycle. At the first stage, it assumes a bushy structure that later evolves into a forest, which is totally clear-cut at the end of 6-7 years ([56], [61]). Therefore, these artificial forests present great temporal heterogeneity which may affect patterns of abundance and distribution of resident wildlife, not only for small mammals ([34]), but also for their predators ([60]). In fact, recent studies show these forests are capable of maintaining a resident wildlife ([46], [32], [13], [34]). This study aims at assessing the temporal variation of the specific composition and relative abundance of middle to large-sized mammal species found in Eucalyptus plantations in the State of São Paulo, Southeastern Brazil.
Eucalyptus plantations currently occupy over one million hectares in São Paulo, the richest and most developed state in Brazil. Their main commodities are the pulp and paper ([1]). Together with livestock production, the ethanol agroindustry primarily based on sugarcane and the pulp and paper silvicultural industry primarily based on Eucalyptus are the main agroindustrial sectors of São Paulo state. However, unlike the other two agroindustrial sectors, pulp and paper industries follow Brazilian environmental laws relatively well, due to market pressure posed by certification organizations such as the Forest Stewardship Council (FSC - [59]). Accordingly, the assessment of the distribution of middle to large-sized mammals in Eucalyptus plantations may give significant advances to both the environmental certification process of this sector and the elaboration of public policies which can result in the inclusion of such landscapes in the context of wildlife conservation.
Materials and Methods
Study Area
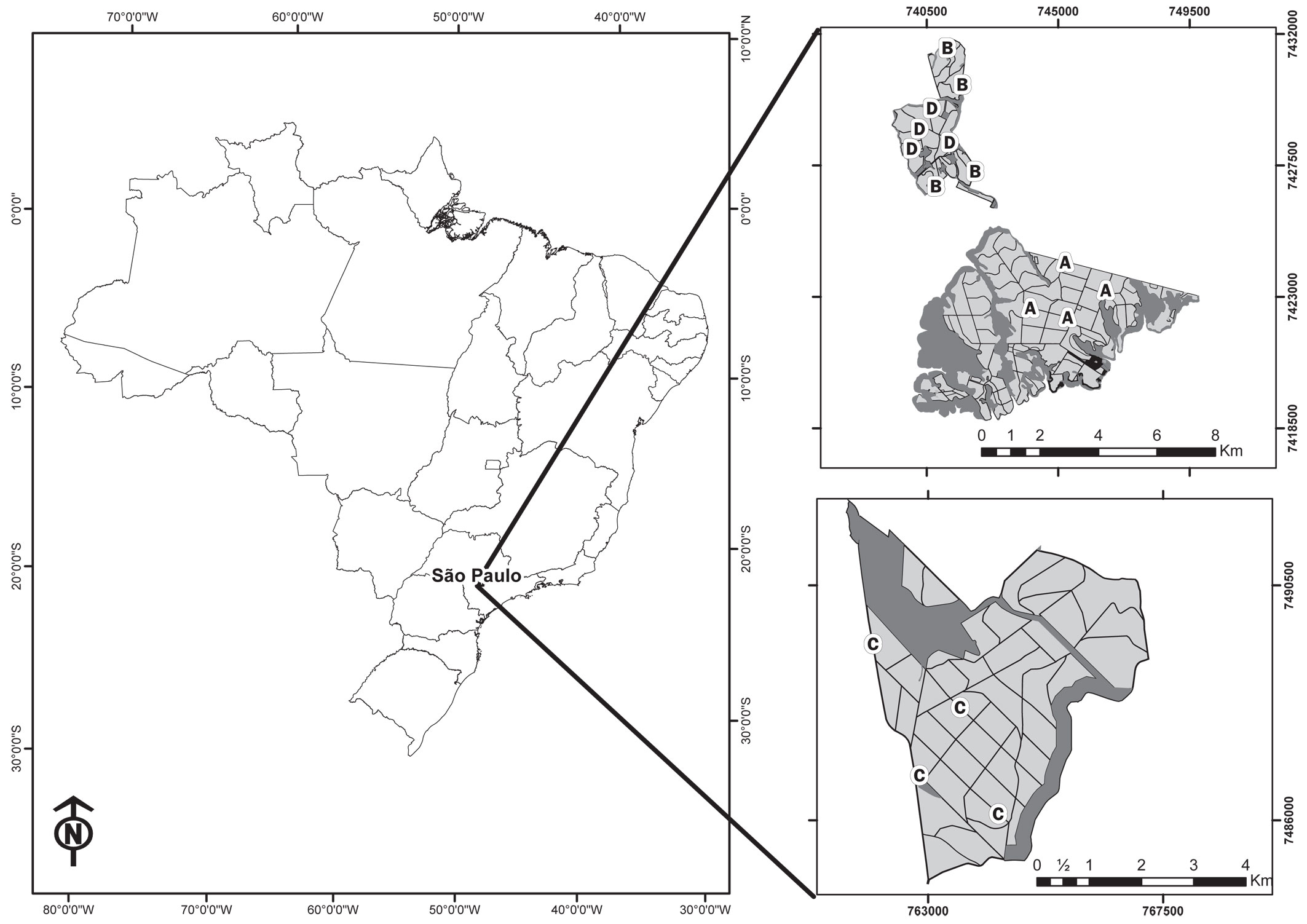
This study was conducted at three Eucalyptus commercial plantations located between Barra Bonita and Jurumirim reservoirs, Paranapanema River Basin, State of São Paulo, Southeastern Brazil (Fig. 1). The native vegetation cover in the region was composed of “Cerradão” (forested savannah) and semidecidual Atlantic forest ([57]). The climate is tropical (Köppen) with rainy summers and dry winters with average temperatures between 19.7 and 21.5 °C ([7]).
Fig. 1 - Location of study sites in Brazil and São Paulo State and the distribution of sampling sites on Eucalyptus stands in the plantation farms assessed in this study. (A): zero to one year old stands; (B): two to three year old stands; (C): four to five year old stands; (D): six to seven year old eucalypt stands.
The historical use of these lands dates back to the 18th century with the deforestation for pasture establishment. In the late 19th century, coffee plantations dominated the region, being replaced by sugarcane in the early 20th century. At the end of the 20th century, sugarcane began to be gradually replaced by Eucalyptus plantations, which currently covers approximately 40% of the Alto Paranapanema river basin, with only about 10% of remaining native vegetation left ([28]).
Sampling methodology
The sampling period of this study was from August 2007 to February 2008, including seven two-day campaigns carried out every month. Sand plot for track counts ([62], [42], [48]) and camera-traps ([26], [27]) were used to detect middle to large-sized mammals. Sand plots were cleaned and camera-traps activated on the first day of the campaign, and data collection took place on the second day. The study area was divided into four age ranges of Eucalyptus stands: (i) 0-1 year; (ii) 2-3 years; (iii) 4-5 years; and (iv) 6-7 years (Fig. 1).
The surveys within sand plots took place using trails which were naturally formed by the space between the Eucalyptus tree rows, starting at the gravel roads that divided the stands. Sand plots were placed every 25 meters along each trail, summing up ten sand plots per trail. Four trails were established for each Eucalyptus stand age range. Each trail was considered as a sample unit, totaling 16 sample units. The trails where the sand plots were placed received one camera-trap each, placed 75 meters from the trail entrance. The minimal distance between sampling units was one kilometer. As we considered frequency of occurrence as the dependent variable, independently of the actual species abundances, we could consider the sampling units as independent.
Analytical methodology
Mammals registered in the field surveys were grouped in the following trophic categories (adapted from [49], [50], [20], [11], [12]): (a) carnivores; (b) herbivores; (c) omnivores and (d) insectivores. A list of detected species and their frequency of occurrence have been determined (taxonomic classification followed [63]). Sampling sufficiency was assessed by species incidence curves, with 100 randomizations in a rarefaction procedure (Sobs) by the Mao Tao method ([8]). Specific richness was estimated by the Bootstrap method ([53]). Species incidence curves were adjusted according to the Pearl-Reed trend model ([18]).
Data was tested for normality and homoscedasticity by Kolmogorov-Smirnoff test ([30]) and Levene’s test ([29]), respectively. Eucalyptus stand age ranges were mutually compared regarding species richness and frequency of occurrence for all species, and by trophic categories by Analysis of Variance (ANOVA - [25]) and Analysis of Means (ANOM - [40]). Venn diagrams ([39]) were used for the detection of overlaps among distinct stand ages regarding the species and trophic categories.
Results
A total of 15 species of middle to large-sized mammals of 10 families was detected, including the following exotic species: cattle (Bos taurus), domestic dog (Canis lupus (= familiaris)) and the European hare (Lepus spp. - Tab. 1). This represents 86% of the total estimated species richness by Bootstrap, varying from 75.9% in 6-7 years-old stands to 90.6% in 2-3 years-old stands (Tab. 2). The following trophic categories have been detected: herbivores, carnivores, omnivores and insectivores. Their detected and estimated species richness varied from 71.5% (insectivores in 0-1 and 6-7 years-old and carnivores in 0-1 year-old stands) to 95.2% (omnivores in 2-3 years-old stands - Tab. 2).
Tab. 1 - Detected mammal species, their taxonomy, trophic categories and numbers of occurrences by eucalypt stand-age range.
| Order | Family | Species | Trophic categories |
Stand Age Range (years) | |||
|---|---|---|---|---|---|---|---|
| 0-1 | 2-3 | 4-5 | 6-7 | ||||
| Cingulata | Dasypodidae | Dasypus novemcinctus (Linnaeus, 1758) | Omnivore | 1 | 2 | 8 | 1 |
| Euphractus sexcinctus (Linnaeus, 1758) | Insectivore | 0 | 0 | 2 | 0 | ||
| Pilosa | Myrmecophagidae | Myrmecophaga tridactyla (Linnaeus, 1758) | Insectivore | 2 | 5 | 2 | 1 |
| Lagomorpha | Leporidae | Lepus sp. (Linnaeus, 1758) | Herbivore | 7 | 0 | 1 | 0 |
| Sylvilagus brasiliensis (Linnaeus, 1758) | Herbivore | 0 | 1 | 0 | 0 | ||
| Artiodactyla | Tayassuidae | Pecari tajacu (Linnaeus, 1758) | Herbivore | 1 | 0 | 0 | 0 |
| Cervidae | Mazama sp. (Rafinesque, 1817) | Herbivore | 1 | 3 | 4 | 0 | |
| Bovidae | Bos taurus (Linnaeus, 1766) | Herbivore | 0 | 1 | 0 | 1 | |
| Carnivora | Felidae | Leopardus tigrinus (Shreber, 1775) | Carnivore | 1 | 0 | 0 | 0 |
| Puma concolor (Linnaeus, 1771) | Carnivore | 0 | 0 | 2 | 0 | ||
| Canidae | Canis lupus (=familiaris) (Linnaeus, 1758) | Omnivore | 1 | 7 | 0 | 0 | |
| Cerdocyon thous (Linnaeus, 1766) | Omnivore | 5 | 4 | 6 | 0 | ||
| Chrysocyon brachyurus (Illiger, 1815) | Omnivore | 5 | 8 | 9 | 0 | ||
| Mustelidae | Eira barbara (Linnaeus, 1758) | Omnivore | 0 | 0 | 1 | 0 | |
| Procionidae | Nasua nasua (Linnaeus, 1766) | Omnivore | 0 | 0 | 1 | 0 | |
Tab. 2 - Species-incidence curve with Bootstrap procedure. (robs): observed species richness; (rest): estimated species richness; (robs/rest·100): percentage of the estimated species richness actually detected.
| Trophic category |
Stand age | r obs | r est | robs/rest·100 (%) |
|---|---|---|---|---|
| Herbivores | 0-1 | 3 | 3.6 | 83.4 |
| 2-3 | 3 | 3.7 | 81 | |
| 4-5 | 2 | 2.3 | 87 | |
| 6-7 | 1 | 1.3 | 77 | |
| Subtotal | 5 | 5.8 | 86.2 | |
| Omnivores | 0-1 | 4 | 4.7 | 85 |
| 2-3 | 4 | 4.2 | 95.2 | |
| 4-5 | 5 | 5.7 | 88 | |
| 6-7 | 1 | 1.3 | 77 | |
| Subtotal | 6 | 6.7 | 89.5 | |
| Insectivores | 0-1 | 1 | 1.4 | 71.5 |
| 2-3 | 1 | 1 | 1 | |
| 4-5 | 2 | 2.4 | 83.3 | |
| 6-7 | 1 | 1.4 | 71.5 | |
| Subtotal | 2 | 2.4 | 83.3 | |
| Carnivores | 0-1 | 1 | 1.4 | 71.5 |
| 2-3 | 0 | 0 | 0 | |
| 4-5 | 1 | 1 | 1 | |
| 6-7 | 0 | 0 | 0 | |
| Subtotal | 2 | 2.5 | 80 | |
| Total | 0-1 | 9 | 11 | 82.1 |
| 2-3 | 8 | 8.9 | 90.6 | |
| 4-5 | 10 | 4.4 | 87.7 | |
| 6-7 | 3 | 4 | 75.9 | |
| Grand-Total | 15 | 17.4 | 86 |
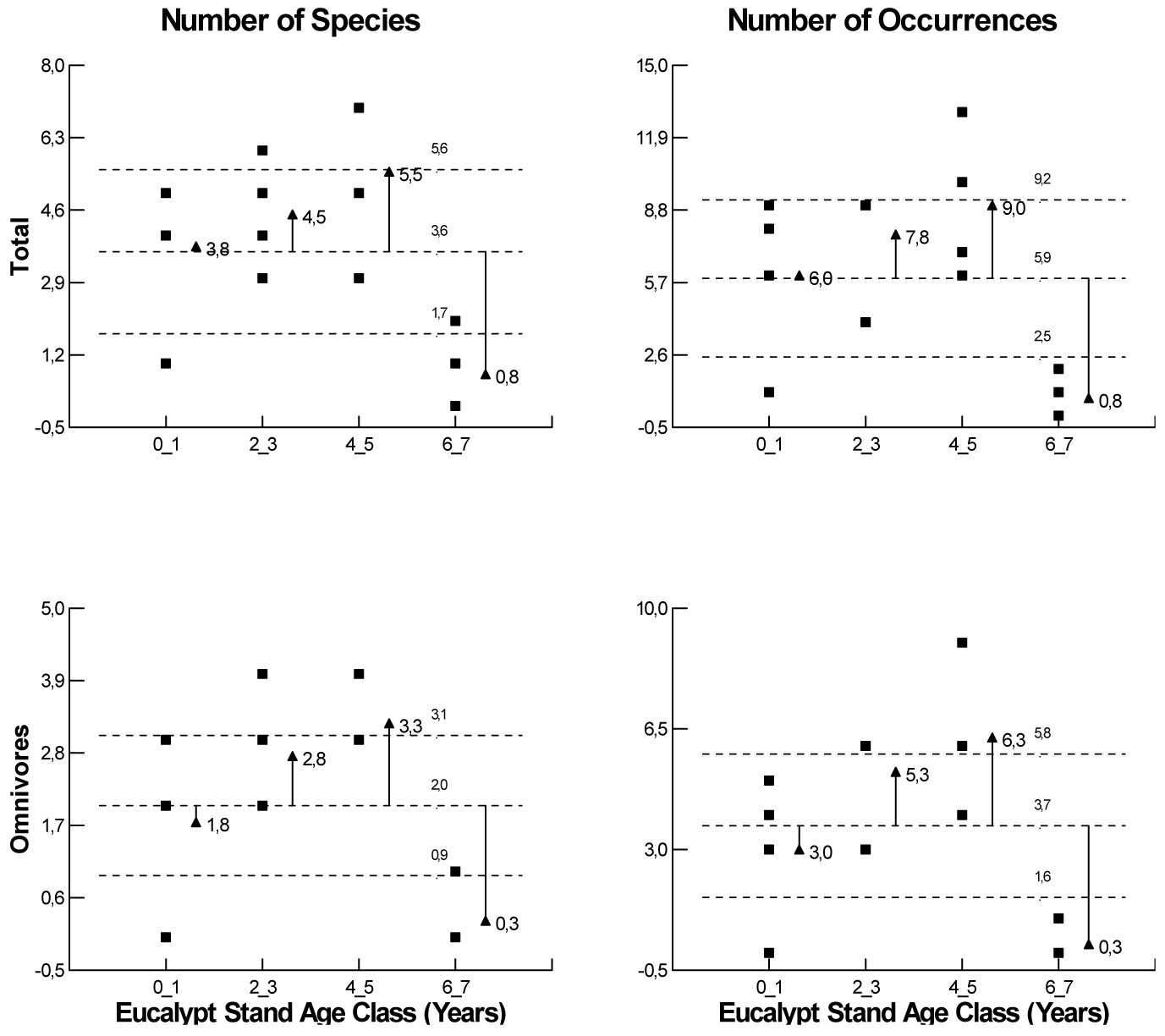
Data set was homoscedastic and normally distributed (p < 0.05). There was a significant variation in total species richness (F=6.81, df=3, p=0.006) and frequency occurrences (F=7.07, df=3, p=0.005), in relation to plantation age. However, such variation has only been detected in omnivores (F=9.33, df=3, p=0.002; F=9.95, df=3, p=0.001), respectively, for species richness and frequency of occurrence (Fig. 2).
Fig. 2 - Analysis of Means (α<0.05), according to Ott ([40]) for species richness and frequency of occurrence for the whole species group and for omnivorous species by distinct Eucalyptus stand ages.
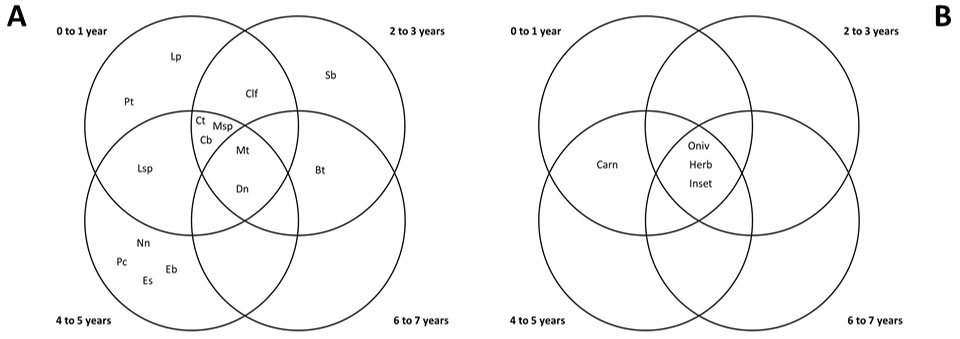
Seven species occurred exclusively at a single stand age: Leopardus tigrinus and Pecari tajacu at 0-1 year-old; Sylvilagus brasiliensis at 2-3 year-old; and Euphractus sexcinctus, Nasua nasua, Eira barbara and Puma concolor at 4-5 year-old stands. Two species occurred in all stand ages: Myrmecophaga tridactyla and Dasypus novemcinctus. All trophic categories, with the exception of carnivores (absent in 2-3 and 6-7 year-old stands) occurred at all Eucalyptus stand ages (Fig. 3).
Fig. 3 - Venn’s Diagrams showing overlaps of species (A) and trophic categories (B) occurrence in Eucalyptus plantations of distinct ages. (Dn): Dasypus novemcinctus; (Es): Eupractus sexcinctus; (Mt): Myrmecophaga tridactyla; (Lsp): Lepus sp.; (Sb): Sylvilagus brasiliensis; (Pt): Pecari tajacu; (Msp): Mazama sp.; (Bt): Bos taurus; (Lp); Leopardus tigrinus; (Pc): Puma concolor; (Clf): Canis lupus familiaris; (Ct): Cerdocyon thous; (Cb): Chrysocyon brachyurus; (Eb): Eira barbara; (Nn): Nasua nasua; (Omn): Omnivores; (Carn): Carnivores; (Herb): Herbivores; (Inset): Insectivores.
Discussion
The total species composition found in this study is similar to other studies carried out in the same region ([52], [6], [3]). Although relatively small, the present sampling effort was sufficient to detect 86% of the total estimated number of species of middle to large-sized mammals that use local Eucalyptus plantations in central-south state of São Paulo, in south-eastern Brazil. However, methodological bias for terrestrial species possibly prevented the detection of primates ([51]), as well as the capybara (Hydrochoerus hydrochaeris - [58]) and the Neotropical otter (Lontra longicaudis - [41], [43]), respectively arboreal, semi-aquatic and aquatic species.
The manned-wolf (Chrysocyon brachyurus) and the crab-eating fox (Cerdocyon thous) were the most abundant species at the present study. Both species are omnivorous with generalist diet including plant matter, invertebrates and small vertebrates. Such a diet provides them with a wide feeding niche, allowing them to feed on different resources according to availability ([14]). Armadillos (Dasypus spp.) and giant ant-eaters (Myrmecophaga tridactyla) were also abundant, possibly for eating insects (in particular ants and termites) usually abundant in Eucalyptus plantations ([5], [35]). As a matter of fact, ants are considered the most important “plague” of Eucalyptus plantations in southeastern Brazil, demanding a rigorous control by the pulp and paper industry ([47]). In addition to ants and termites, other opportunistic invertebrates and small vertebrate species can be found in Eucalyptus plantations possibly due to its relatively fast growth (i.e., biomass production - [4], [45], [36]). These species are possibly food resources for middle to large-sized mammals ([9], [54]).
Herbivores, in especial the European hare (Lepus spp.) are relatively abundant in the early stages of Eucalyptus plantations (0-1 year), possibly favored by the abundance of remaining grass still present as the former landscape matrix was formed by exotic pasture land (predominantly Brachyarya = Urochloa spp.). However, later (2-5 years), omnivores and insectivores become more numerous possibly because of the presence of some understory vegetation used by arthropods and small vertebrates. However, in the final stage of commercial Eucalyptus stands (6-7 years) all trophic categories drastically decrease in number of species and frequency of occurrence. At this stage mechanical trimmings become more frequent, resulting in the removal of the understory vegetation in order to facilitate harvest ([10], [61]). Such intense weed control is usually overdone in order to avoid legal problems with local environmental agencies as even an economically viable understory vegetation can be considered as native revegetation that requires especial license to be cut (see Lei No. 11.428, from 22nd December 2006; Resolução CONAMA No. 338, from 23 February 2007; Resolução CONAMA No. 392, from 25 June 2007; Portaria DEPRN No. 08, from 20 November 1989 and Decreto Estadual 53.027-2008). Such management practice seems rather ineffective for both the pulp and paper industry and the environmental quality as the maintenance of some understory vegetation is undoubtedly better for the wildlife than no vegetation at all. In addition, a less intense weed control could result in cost reduction for the pulp and paper industry and its consumers.
Conclusions
The detected temporal variation in the use of Eucalyptus stands by middle to large-sized mammals is possibly related to the relatively high temporal heterogeneity of this environment as well as to the silvicultural practices along its commercial cycle ([19]). According to the “continuum model” ([33], [17], [19], [31]) animal species are distributed as a continuum in the landscape, spatially and temporally, according to the distribution of resources in a pattern that resembles a dégradé more than on a well-defined patchwork pattern. In such model, species will move, use, and therefore, occur on a determined environment at a certain moment depending on its resources availability and the species’ ability to access them ([33]). The present species are relatively common in silvicultural landscapes. However, their use of resources associated with the landscape matrix (i.e., Eucalyptus plantations themselves) are directly associated to the silvicultural practices along commercial plantation cycles. Such principle should be considered by the certification process of the pulp and paper industry ([21]) in order to promote and maintain a sustainable conservation value for silvicultural landscapes in south-eastern Brazil.
Acknowlegdements
We would like to thank Júlio César Costa, Gustavo Sigrist Betini, Sabrina Koester-Gobbo and other colleagues for the assistance in the field. This study received financial support from FAPESP (Proc. No. 2006/60954-4), CNPq (Proc. No. 142732/ 2007-9) and Eucatex S/A. LMV has a productive research fellowship from CNPq.
References
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Universidade Federal de São Carlos - UFSCar (Brazil)
Universidade de Santo Amaro - UNISA (Brazil)
Universidade Sagrado Coração - USC (Brazil)
Laboratorio de Ecologia Isotópica - CENA, Universidade de São Paulo, Caixa Postal 96, Piracicaba, SP 13416-000 (Brazil)
Corresponding author
Paper Info
Citation
Timo TPC, Lyra-Jorge MC, Gheler-Costa C, Verdade LM (2015). Effect of the plantation age on the use of Eucalyptus stands by medium to large-sized wild mammals in south-eastern Brazil. iForest 8: 108-113. - doi: 10.3832/ifor1237-008
Academic Editor
Gianfranco Minotta
Paper history
Received: Jan 06, 2014
Accepted: May 05, 2014
First online: Jul 21, 2014
Publication Date: Apr 01, 2015
Publication Time: 2.57 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2015
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 57755
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 46991
Abstract Page Views: 4605
PDF Downloads: 4553
Citation/Reference Downloads: 77
XML Downloads: 1529
Web Metrics
Days since publication: 4195
Overall contacts: 57755
Avg. contacts per week: 96.37
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2015): 41
Average cites per year: 3.73
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Diversity and distribution patterns of medium to large mammals in a silvicultural landscape in south-eastern Brazil
vol. 11, pp. 802-808 (online: 14 December 2018)
Review Papers
Large-scale effects of forest management in Mediterranean landscapes of Europe
vol. 6, pp. 342-346 (online: 29 August 2013)
Research Articles
The impact of seed predation and browsing on natural sessile oak regeneration under different light conditions in an over-aged coppice stand
vol. 9, pp. 569-576 (online: 04 April 2016)
Research Articles
Do different indices of forest structural heterogeneity yield consistent results?
vol. 15, pp. 424-432 (online: 20 October 2022)
Research Articles
Effects of different silvicultural measures on plant diversity - the case of the Illyrian Fagus sylvatica habitat type (Natura 2000)
vol. 9, pp. 318-324 (online: 22 October 2015)
Short Communications
Gliding patterns of Siberian flying squirrels in relation to forest structure
vol. 12, pp. 114-117 (online: 11 February 2019)
Research Articles
Effects of forest management on bird assemblages in the Bialowieza Forest, Poland
vol. 8, pp. 377-385 (online: 02 October 2014)
Research Articles
Fluctuation of the ecological niche of Moringa peregrina (Forssk.) Fiori with topoclimatic heterogeneity in southern Iran
vol. 16, pp. 53-61 (online: 16 February 2023)
Short Communications
Variation in soil carbon stock and nutrient content in sand dunes after afforestation by Prosopis juliflora in the Khuzestan province (Iran)
vol. 10, pp. 585-589 (online: 08 May 2017)
Research Articles
Variability of ant community composition in cork oak woodlands across the Mediterranean region: implications for forest management
vol. 10, pp. 707-714 (online: 27 July 2017)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword