Seven Ulmus minor clones tolerant to Ophiostoma novo-ulmi registered as forest reproductive material in Spain
iForest - Biogeosciences and Forestry, Volume 8, Issue 2, Pages 172-180 (2015)
doi: https://doi.org/10.3832/ifor1224-008
Published: Aug 13, 2014 - Copyright © 2015 SISEF
Research Articles
Collection/Special Issue: 3rd International Elm Conference, Florence (Italy - 2013)
The elms after 100 years of Dutch Elm disease
Guest Editors: A. Santini, L. Ghelardini, E. Collin, A. Solla, J. Brunet, M. Faccoli, A. Scala, S. De Vries, J. Buiteveld
Abstract
The Spanish elm programme began in 1986 in response to the devastating impact of Dutch elm disease on natural elm stands and urban trees. Its main objectives were to conserve remaining genetic resources and select and breed tolerant native elm genotypes. After 27 years of work conducting susceptibility trials on thousands of elm genotypes, the first seven tolerant Ulmus minor trees are now being registered by the Spanish Environmental Administration. This paper presents the results of the susceptibility tests on these clones and their distinctive genetic, morphological and phenological features. In all susceptibility trials the commercial “Sapporo Autumn Gold” clone, which is highly tolerant to O. novo-ulmi, was used as a control. The registered clones were named “Ademuz”, “Dehesa de la Villa”, “Majadahonda”, “Toledo”, “Dehesa de Amaniel”, “Retiro” and “Fuente Umbría”. The most tolerant clone was “Dehesa de Amaniel”, as its wilting values were below 5% during the two consecutive inoculation trials performed in Madrid. “Fuente Umbría”, tested over four consecutive years in Guadalajara and Palencia, was the Spanish clone with the most reliable tolerance level to O. novo-ulmi. The “Ademuz” and “Majadahonda” clones had the highest ornamental scores and are promising trees for use in urban environments and tree breeding for ornamental quality. These two genotypes showed a later bud burst phenology than the other U. minor clones, demonstrating suitability to areas with late frost events. The Spanish programme aims to substantially increase the range of tolerant native elms through new selections and crossings to gain a better understanding of the genetic basis of resistance.
Keywords
Dutch Elm Disease, Breeding, Plant Release, Resistance, Invasive Species
Introduction
In the first half of the 20th century, the first Dutch elm disease (DED) pandemic caused a massive loss of elms in Europe and North America. The much more aggressive O. novo-ulmi Brasier took the place of the causal agent, Ophiostoma ulmi (Buisman) Nannf., in the second half of the century. The second pathogen has caused the disappearance of adult elms in many European and North American locations ([3]). O. novo-ulmi is almost impossible to control through chemical, biological or silvicultural methods due to its high virulence and highly effective transmission via small beetles of the Scolytus and Hylorgopinus genera ([43]). Tolerant elm genotype selection and breeding has been the most successful strategy for elm recovery, particularly in urban environments ([25], [27], [34], [37]). The Spanish elm breeding and conservation programme began in 1986 as the result of an agreement between the Spanish Environmental Administration and the Technical University of Madrid School of Forestry Engineering. Its two main objectives were to conserve remaining elm genetic resources and to transmit their variability to future generations of tolerant elms obtained through breeding; i.e., hybridisation of selected progenitors (native or tolerant Asian elms) to obtain tolerant trees with the appearance of native elm species.
The first elm breeding programme began in the Netherlands in 1928 ([10]) and was followed by several programmes in the United States and various European countries ([23]). Asian elms, including Ulmus pumila, U. chenmoui, U. davidiana var. japonica and U. wallichiana, have been the main sources of resistance in the Dutch, American and Italian elm breeding programmes ([10], [29], [27], [4]). As a result of crossing these species with native elms, a wide range of hybrid clones of varying tolerance levels and genetic backgrounds is now available on the market. The Spanish programme took advantage of the knowledge, methodologies and plant materials previously developed by the Dutch and Italian programmes. In the first 14 years, U. pumila was used as the main source of resistance, giving rise to 10 crossings tolerant to O. novo-ulmi ([33]). The tolerance of these crossings was tested in clone replicates (N > 16) over several years at various locations, and clone adaptation to different environments in Spain was evaluated. Five crossings with Asian background were recently selected to be released onto the market for ornamental use.
In the 1990s the Spanish programme included some native elms, mainly U. minor, in the O. novo-ulmi susceptibility trials. In the following decade the programme focused mainly on selecting native elms. This new strategy complied with European and Spanish legislation governing the quality and genetic background of forest reproductive materials for production and marketing. In the European Union, forest reproductive materials are governed by Council Directive 1999/105/CE, and Annex I of the directive lists the permitted forest species. Although Ulmus species are not included in Annex I, Article 3.2 of the directive allows particular Member States to add to the list. In Spain, several regions expressed the need to certify the plant origin and genetic quality of some forest species not included in Annex I that are traditionally used in reforestation programmes. As a result, Annex XII of Spanish Royal Decree-Law 289/2003 listed 24 additional forest species, including U. glabra and U. minor ([11]). The major spread of U. pumila in Spain and its extensive hybridisation with the native U. minor ([5]) led to conservation concerns for the native species. To preserve the genetic integrity of the Spanish elms, artificial hybrids in the genus Ulmus were not included in Annex XII of Royal Decree-Law 289/2003. This means that hybrids with Asian background cannot be marketed for forest use in Spain, although they can be used for urban planting.
Progress in selecting elms was slow due to the long periods required to propagate trees and evaluate their tolerance with a scientifically sound base, as plant material needs to be at least four years old ([36]). In the case of selecting pure U. minor material, a further difficulty was the very limited number of native elms exhibiting some degree of tolerance to O. novo-ulmi, which was around 0.5% in comparison to 2-5% for hybrids with Asian background (unpublished results). Fortunately, susceptibility trials performed in the last 10 years provided some native individuals with low leaf wilting values. After 27 years of activity, the Spanish programme continues to breed and conserve Spanish elms with the ultimate goal of recovering their forest and ornamental uses.
Native elms can be registered by the Environmental Administration as “qualified forest reproductive material” when they show low (0-30%) crown wilting or symptoms similar to the tolerant “Sapporo Autumn Gold” clone after two consecutive years of artificial inoculation with O. novo-ulmi. At least six replicates of the tested clone must be inoculated. Replicates are grown in a plot in which a susceptible control clone has to exhibit more than 70% wilting symptoms. When a tested clone is registered, it can be propagated, marketed and used for forest purposes. The “qualified” category is provisional and after 10 years it becomes “controlled material” and acquires a permanent category ([11]). Before clones are registered as “controlled material”, they must meet the same requirements as “qualified material” when tested at a second location. This paper reports the selection and the features of the first U. minor clones registered for forest use in Spain. The potential use of these clones and the future of the Spanish breeding programme are discussed.
Material and methods
Plant material
From 1990 to 2002, plant material was propagated from trees selected during surveys of adult elms in natural forests, rural areas, parks, and other urban environments in Spain (Tab. 1). The main selection criterion was good sanitary status, i.e., putative tolerance if trees had survived in a DED affected area. Trees were propagated using seeds, root cuttings and grafts (Tab. 1). In the case of seed propagation, seedlings selected for their tolerance to O. novo-ulmi were propagated by hardwood cuttings and at least six ramets per seedling were obtained. This procedure was used for the “Dehesa de Amaniel”, “Retiro”, “Toledo”, and “Fuente Umbría” clones.
Tab. 1 - Plant material specifications. (a) R: root cutting; G: graft; S: seed; (b) numbers in brackets indicate the year of inoculation.
| Clone | Origin in Spain |
Initial propagation | Inoculation test | |||||
|---|---|---|---|---|---|---|---|---|
| Typea | Year | Plot | N | Years | Location in Spain |
O. novo-ulmi ssp. americana strainb |
||
| Ademuz | Valencia 40° 4′ 52″ N, 1° 16′ 55″ W |
R | 1996 | XXIV | 10 | 2008, 2009 |
Puerta de Hierro | NA-PE (2008), CU-HU (2009) |
| Dehesa de la Villa |
Madrid 40° 27′ 29″ N, 3° 44′ W |
R | 1990 | XXV | 10 | 2009, 2010 |
Puerta de Hierro | CU-HU |
| Majadahonda | Madrid 40° 28′ 90″ N, 3° 52′ 19″ W |
G | 1993 | XXIV | 6 | 2008, 2009 |
Puerta de Hierro | NA-PE (2008), CU-HU (2009) |
| Toledo | Toledo 39° 51′ 51″ N, 4° 1′ 30″ W |
S | 1999 | XXX | 7 | 2011, 2012 |
Puerta de Hierro | Z-BU1 |
| Dehesa de Amaniel |
Madrid 40° 27′ 37″ N, 3° 43′ 17″ W |
S | 1999 | XXX | 12 | 2011, 2012 |
Puerta de Hierro | Z-BU1 |
| Retiro | Madrid 40° 24′ 56″ N, 3° 41′ 10″ W |
S | 2002 | XXX | 7 | 2011, 2012 |
Puerta de Hierro | Z-BU1 |
| Fuente Umbría | Valencia 39° 25′ 23″ N, 0° 56′ 46″ W |
S | 1995 | V and A | >10 | 2010- 2013 |
El Serranillo and Calabazanos |
CU-HU (2010), Z-BU1 (2011-2013) |
Ramets of the seven clones were planted with 157 other elm clones in five different inoculation plots in Spain (Tab. 1), under a Mediterranean phytoclimate ([1]). All clones except “Fuente Umbría” were planted in Puerta de Hierro Forest Breeding Center, Madrid (40° 27′ 24″ N; 3° 45′ 0″ W; 600 m a.s.l.). This location has an average annual rainfall of 397 mm and an average annual temperature of 14 °C. “Fuente Umbría” ramets were planted in El Serranillo Forest Breeding Center (Guadalajara - 40° 40′ 13″ N; 3° 9′ 39″ W; 685 m a.s.l.; 457 mm, 13.5 °C) and Calabazanos Forest Health Center (Palencia - 41° 57′ 7″ N; 4° 30′ 60″ W; 739 m a.s.l.; 412 mm, 11.7 °C).
Plots were designed in two blocks, with random experimental units of three to four ramets per block. Spacing in Puerta de Hierro and El Serranillo was 0.5 to 1 m between plants and 1 to 1.5 m between rows. In Calabazanos spacing was 5 × 5 m. To avoid side effects, a tree border line surrounded all plots. Plants were watered in spring and summer to ensure growth. Their main stems were fastened to supports to avoid wind shake. “Sapporo Autumn Gold”, highly tolerant to O. novo-ulmi ([30]), and UPM089, a Spanish U. minor clone classified by the Spanish elm breeding programme as very susceptible to O. novo- ulmi, were used as control clones. At least six replicates of each control were included in each inoculation plot.
Inoculations
Local strains of O. novo-ulmi were used to evaluate the tolerance level of the clones (Tab. 1). Strains NA-PE, CU-HU and Z-BU1 were isolated form DED-infected trees in Peralta (Navarra), Huelves (Cuenca) and Bubierca (Zaragoza), respectively. Strains NA-PE and CU-HU were isolated in 2002 and Z-BU1 was isolated in 2009. Inoculations were performed at the end of April or beginning of May, depending on the plant phenological stage, about 15-30 days after full leaf development. This is the time U. minor takes to reach its susceptibility peak to O. novo-ulmi in Madrid ([35]).
A bud-cell suspension of the pathogen was prepared by adding 2 × 2 mm plugs from the edge of 7-day-old cultures on malt extract agar to 50 ml Tchernoff’s liquid medium ([39]) in sterile Erlenmeyer flasks, followed by shaking in the dark for four days at room temperature. Spore suspensions were centrifuged at 50 × g for 20 min to eliminate the medium and then suspended in sterile distilled water. Pathogen inoculation was performed by inserting 0.1 ml of the spore suspension at 106 spores ml-1 into an incision made in the trunk base with a razor blade, allowing the suspension to be absorbed by the sap flow. The elms were at least four years old and 1.5 m in height, to obtain maximum disease symptoms ([36]). Disease development was evaluated by three independent observers who recorded the percentage of wilting leaves in the crown at 30, 60 and 120 days post inoculation (dpi).
Molecular characterization
In the marketing of forest reproductive material, characterization and traceability of trees are of pivotal importance. Various techniques using molecular markers are efficient tools for this purpose. Genetic characterization of the seven U. minor clones was performed at two levels. Trees were analyzed firstly with chloroplast DNA markers to determine the lineages of the individuals selected, and secondly with nuclear DNA markers to quantify genotypic diversity ([8]).
For the lineage study, two chloroplast markers were used. One corresponds to the chloroplast fragment SFm and was developed from the sequence of the SFm fragment to differentiate the U. minor lineages in Spain (Collada et al., unpublished data). The other marker corresponds to microsatellite ccmp2, which was developed for tobacco ([44]) and transferred to U. minor to enable differentiation of variants within lineages.
For the genetic description, 12 nuclear microsatellites were selected. Four of these were described in U. minor (Ulmi1-98, Ulmi1-165, Ulmi2-16 and Ulmi2-20 - [6]), three were transferred from U. laevis (Ulm2, Ulm3 and Ulm8 - [45]) and five were transferred from U. rubra (UR 123, UR 141, UR 153, UR 158 and UR 159 - [46]).
Leaves from at least two individuals for each selected clone were collected, labeled and stored in silica gel. After DNA extraction, 2.5 ng µl-1 dilutions were used in amplification reactions conducted following the literature ([44], [6], [45], [46]).
Tree morphology and phenology assessments
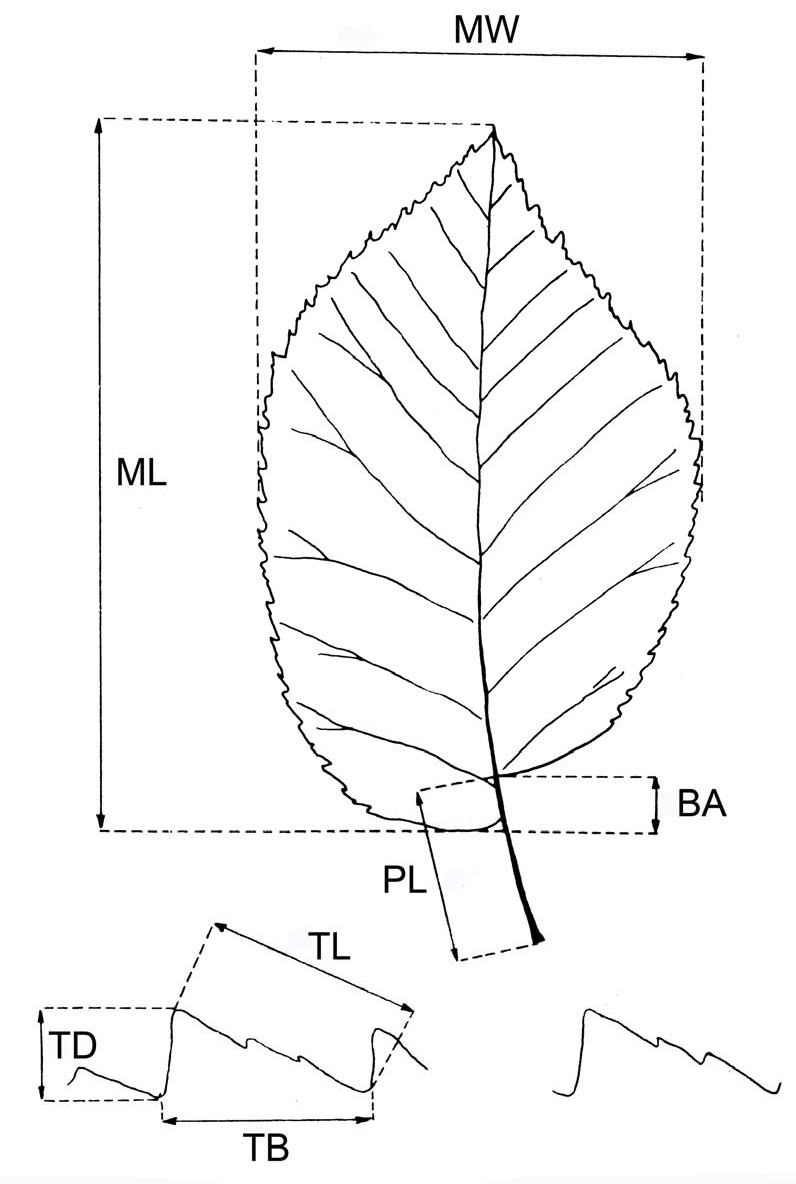
Clones were morphologically described following specific literature ([24], [13], [12]). Quantitative data on subdistal leaves of new shoots were measured on four leaves per tree. The parameters measured are shown in Fig. 1. The number of nerve pairs, total number of main teeth per leaf, and type of leaf margin serration (simple, double or triple) were also determined.
Fig. 1 - Foliar parameters measured to describe the Ulmus minor clones. (MW): maximum foliar width; (ML): maximum foliar length; (BA): basal asymmetry; (PL): petiole length; (TD): tooth depth; (TL): tooth length; (TB): tooth breadth.
Height growth of each clone was assessed in Puerta de Hierro Forest Breeding Center (Madrid), as well as ornamental qualities of trees such as growth habit and branching (erect, spreading, or pendulous), leaf density (abundance of leaves per crown volume, estimated as high, medium or low), crown shape (conical, spindle, globular or irregular) and leaf size. The ornamental value of each clone was quantified on a scale from 1 to 5, where 5 corresponded to the most frequent features of Spanish U. minor according to the clone collection (N = 363) held at Puerta de Hierro Forest Breeding Center (i.e., erect branching, globular-spindle crown, medium-high leaf density, leaf size of about 50 mm length and 30 mm width) and 1 corresponded to unusual U. minor features. The presence of corky tissue was also recorded but not considered for ornamental evaluation. After four independent observers had assessed ornamental quality, the average value was calculated.
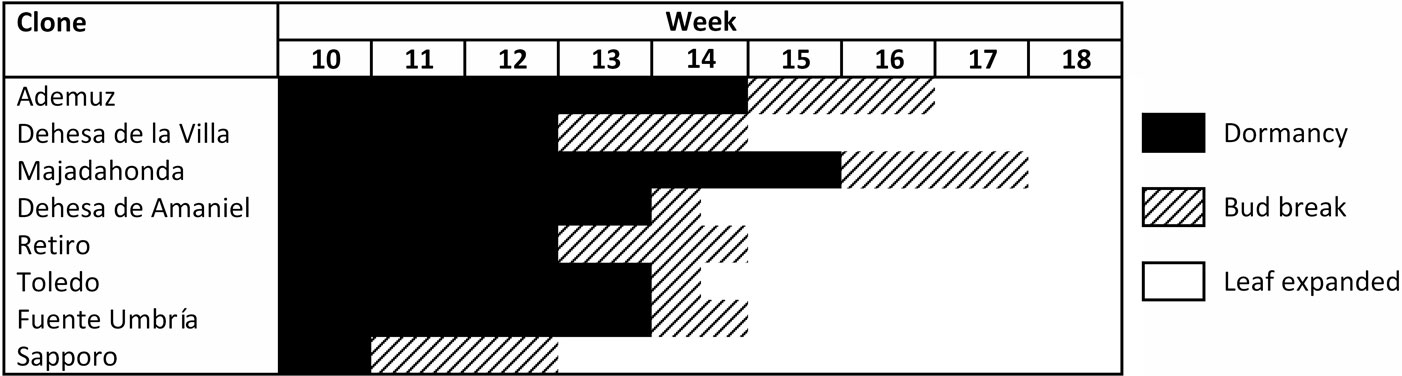
The unfolding of elm leaf buds was characterized in 2011 using the methodology described by Santini et al. ([26]). Leaf phenology is divided into five stages from bud formation to complete leaf expansion: phase 1: dormant buds; phase 2: swelling buds but with closed flakes; phase 3: flakes open and the first leaf ends are visible in the apex of the buds; phase 4: the ends of all the leaves are visible but the leaves are not expanded; Phase 5: two or more leaves are fully expanded. To compare leaf phenology between genotypes, these stages were grouped into three classes: dormancy (phases 1 and 2), bud break (phases 3 and 4), and leaf expanded (phase 5).
Statistical analysis
For each inoculation plot and year, wilting percentages at 30, 60 and 120 dpi were analyzed using repeated measures ANOVA, considering time since inoculation, block, and genotype as the main factors and tree height as a covariate. Fisher’s least significant difference (LSD) test was applied to compare average wilting values (least square means of wilting percentages at 30, 60 and 120 dpi) between clones (P < 0.05). Analyses were performed using the STATISTICA v. 7.0 package (StatSoft Inc., Tulsa, OK, USA).
Results and Discussion
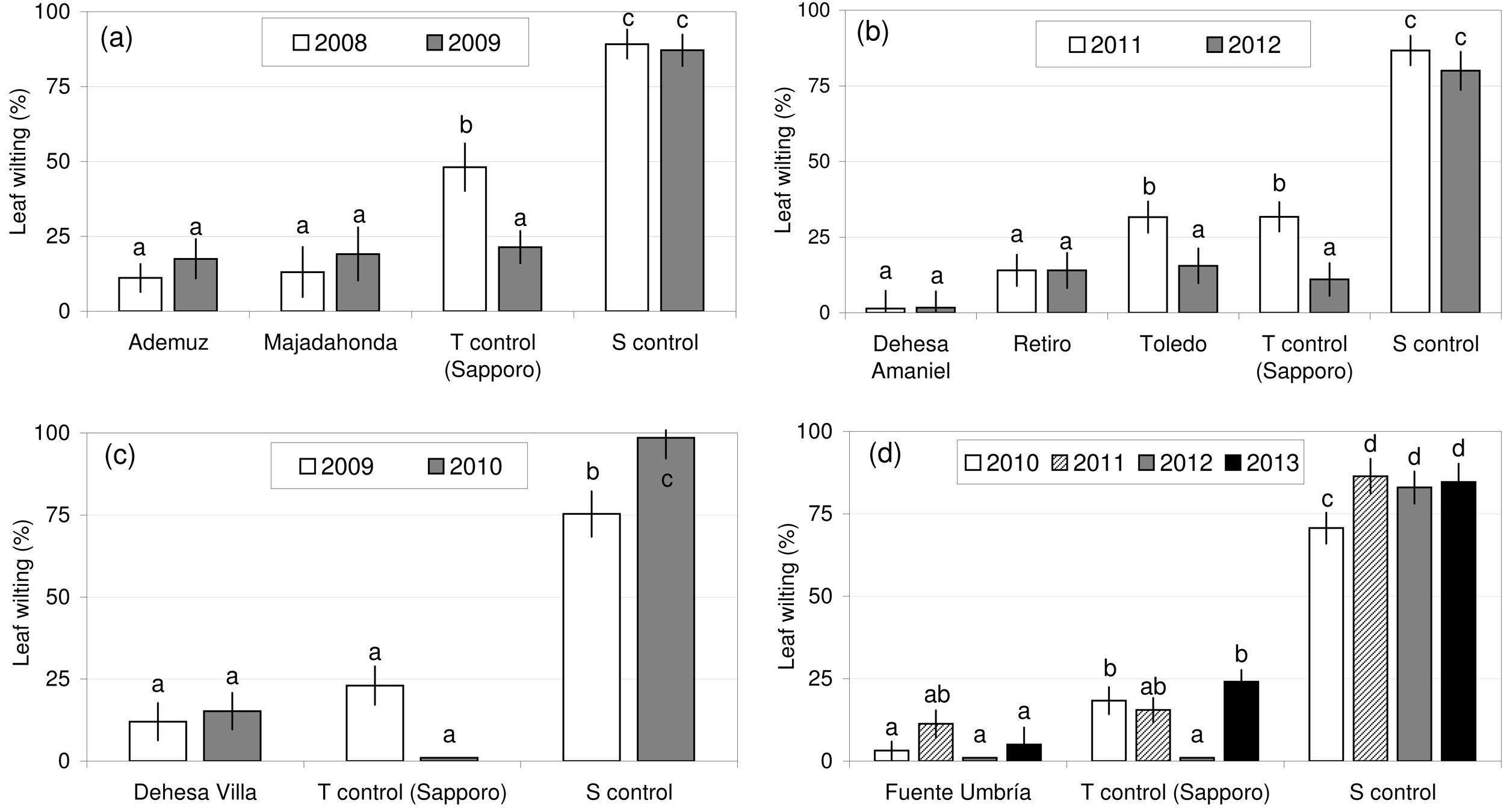
Seven U. minor clones were selected for their tolerance to O. novo-ulmi in various susceptibility tests conducted in Spain. The results of the repeated measures ANOVA of leaf wilting at 30, 60 and 120 dpi (Tab. 2) showed that genotype was a highly significant factor, block was an important source of variation in some plots (XXIV, XXV and XXX), and plant height was not a significant effect, except in 2011 in plot XXX. The effect of time since inoculation on the wilting values observed was significant in all susceptibility tests, except in 2009 in plot XXIV (Tab. 2). The highest wilting values were recorded at 60 dpi (data not shown). After pathogen inoculation, the seven clones showed leaf wilting values similar to or lower than “Sapporo Autumn Gold” (Fig. 2). In all tests, the susceptible control clone UPM089 showed wilting values above 70%, confirming the virulence of the isolates used and the correct inoculation methodology. The most tolerant clone was “Dehesa de Amaniel”, with wilting values below 5% during the two consecutive inoculation trials performed in Madrid.
Tab. 2 - Results (p-values) of repeated measures ANOVA of the wilting values shown by elm trees at 30, 60 and 120 dpi (repeated variable) considering time since inoculation, genotype, block, and genotype x block interaction as factors, and plant height as a covariate. (a): The plot had one block.
| Inoculation year | Plot | Source of variation | ||||
|---|---|---|---|---|---|---|
| Time dpi | Genotype (G) | Block (B) | G × B | Plant height | ||
| 2008 | XXIV | < 0.001 | < 0.001 | < 0.001 | 0.001 | 0.708 |
| 2009 | XXIV | 0.15 | < 0.001 | 0.029 | 0.65 | 0.928 |
| 2009 | XXV | 0.002 | < 0.001 | 0.032 | < 0.001 | 0.078 |
| 2010 | XXV | < 0.001 | < 0.001 | 0.064 | < 0.001 | 0.513 |
| 2010 | V | < 0.001 | < 0.001 | 0.163 | 0.751 | 0.951 |
| 2011 | V | < 0.001 | < 0.001 | 0.185 | 0.572 | 0.839 |
| 2011 | XXX | < 0.001 | < 0.001 | 0.001 | < 0.001 | < 0.001 |
| 2012 | XXX | < 0.001 | < 0.001 | 0.068 | 0.01 | 0.91 |
| 2012 | Aa | < 0.001 | < 0.001 | - | - | 0.623 |
| 2013 | Aa | 0.001 | < 0.001 | - | - | 0.086 |
Fig. 2 - Susceptibility of the seven Ulmus minor clones (least squares means of wilting percentages at 30, 60 and 120 dpi) after tests performed at various experimental plots in Spain. (a) “Ademuz” and “Majadahonda” clones, tested in Puerta de Hierro Forest Breeding Center, Madrid; (b) “Dehesa de Amaniel”, “Retiro” and “Toledo” clones, tested in Puerta de Hierro; (c) “Dehesa de la Villa” clone, tested in Puerta de Hierro; (d) “Fuente Umbría” clone, tested in 2010 and 2011 in El Serranillo Forest Breeding Center, Guadalajara, and in 2012 and 2013 in Calabazanos Forest Health Centre, Palencia. (T): tolerant; (S): susceptible.
Six clones were tested at one location (Madrid), but the “Fuente Umbría” clone was tested over four consecutive years in Guadalajara and Palencia (Fig. 2d). These two locations are 280 km apart and have different climate conditions. “Fuente Umbría” should therefore be regarded as the Spanish clone with the most reliable tolerance level to O. novo-ulmi. The six other clones will need to pass a second inoculation test under a different environment from Madrid before they can be registered as controlled material. Given the availability of ramets from this material, the second test will be performed in 2016 for the “Ademuz”, “Dehesa de la Villa”, “Dehesa de Amaniel”, “Retiro” and “Toledo” clones, and in 2018 for the “Majadahonda” clone.
Environmental conditions can strongly influence elm susceptibility to O. novo-ulmi ([28], [38], [31], [19]). Use of registered material in areas with a similar environment to the area of the susceptibility test is therefore highly recommended. The seven clones performed well during the inoculation years under the environmental conditions described, but their long-term tolerance to possible emerging races of the pathogen under different climate conditions need to be assessed. Before the clones are used in other areas, it would be advisable to establish adaptation trials to allow quantification of their susceptibility to drought, frosts, flooding and pests such as bark beetles, Hemiptera and the elm leaf-beetle, Galerucella luteola.
DED fungi have caused mortality not only of large elm groves of U. minor in Spain, but also of many centuries-old elms that had decorated parks, gardens and town and city squares. Therefore, although registration of the seven clones was focused on forest use, recovery of U. minor for urban use was also a key objective of the programme. To this end, distinct morphological features and appreciation of the ornamental value of the seven clones are shown in Tab. 3, Fig. 3 and Fig. 4. Three of the clones (“Ademuz”, “Toledo” and “Dehesa de Amaniel”) showed growth rates very similar to “Sapporo Autumn Gold”, which grows 94.5 cm in height per year in Puerta de Hierro. Foliar density of the seven clones was medium or high compared to “Sapporo Autumn Gold”, whi- ch shows low or medium-low density in Madrid. The “Ademuz” and “Majadahonda” clones have the highest ornamental scores and are promising trees for use in urban environments and tree breeding for ornamental quality. These two genotypes showed a later bud burst phenology than the other U. minor clones (Fig. 5), which demonstrates probable suitability to areas with late frost events. The earlier phenology in “Sapporo Autumn Gold” (U. pumila × U. davidiana var. japonica hybrid) than in the Spanish elms is due to its Asian background. Asian elms exhibit earlier bud burst phenology than European elms ([7]).
Tab. 3 - Morphological description of the seven Ulmus minor clones. Numbers in brackets indicate range values. (a): on a scale from 1 to 5, where 5 = most attractive (typical U. minor traits), 1 = least attractive (unusual U. minor traits).
| Feature | Clone | ||||||
|---|---|---|---|---|---|---|---|
| Ademuz | Dehesa de la Villa | Majadahonda | Toledo | Dehesa de Amaniel | Retiro | Fuente Umbría | |
| Height growth in Puerta de Hierro, Madrid (cm year-1) |
100.0 | 63.0 | 60.8 | 89.3 | 90.0 | 70.5 | 51.7 |
| Petiole length (mm) | 5.2 (4.2-6.2) |
6.3 (3.5-10.0) |
11.0 (10-12.7) |
5.8 (4.6-7.4) |
2.6 (1.9-3.4) |
7.3 (6.1-8.0) |
10.2 (8.3-12.2) |
| Leaf basal asymmetry (mm) | 1.6 (1.1-1.9) |
2.9 (2.0-3.8) |
3.8 (2.0-4.8) |
1.3 (0.7-2.4) |
1.3 (0.8-1.8) |
1.2 (0.3-1.7) |
3.1 (2.2-4.2) |
| Maximum foliar length (mm) | 53.7 (43.5-65.1) |
55.4 (44.0-70.0) |
50.4 (46.7-53.6) |
47.0 (35.6-71.5) |
38.5 (36.2-39.6) |
71.4 (63.9-79.4) |
75.9 (69.8-85.9) |
| Maximum foliar width (mm) | 33.8 (30.0-38.6) |
35.6 (28.0-45.0) |
28.8 (25.5-30.6) |
26.6 (19.6-35.2) |
29.7 (27.3-33.0) |
42.2 (36.4-48.9) |
44.9 (39.3-48.7) |
| Tooth breadth (mm) | 2.2 (1.9-2.6) |
3.5 (3.0-4.9) |
1.1 (0.8-1.3) |
4.1 (3.6-4.7) |
2.2 (1.2-2.6) |
2.8 (2.5-3.7) |
2.8 (1.9-3.8) |
| Tooth depth (mm) | 3.4 (2.8-4.0) |
3.7 (2.4-5.0) |
1.8 (1.4-2.3) |
3.2 (2.6-4.4) |
2.7 (2.1-3.5) |
2.2 (1.4-2.8) |
2.3 (2.1-2.6) |
| Tooth length (mm) | 4.2 (3.8-4.4) |
4.6 (4.0-5.1) |
2.3 (1.9-2.9) |
5.4 (4.8-5.9) |
3.5 (2.8-4.2) |
2.9 (2.1-3.8) |
3.0 (2.2-4.1) |
| Teeth per leaf (N) | 38 (35-42) |
44 (30-64) |
54 (52-57) |
30.0 (28-33) |
33 (31-35) |
49 (45-53) |
32 (28-34) |
| Pairs of secondary nerves (N) | 10 (9-11) |
10 (9-12) |
12 (11-12) |
8.8 (8-10) |
9.3 (8-10) |
13 (12-15) |
12 (11-13) |
| Leaf serration | Double | Double | Simple | Double | Triple | Double | Double |
| Presence of corky tissue | No | No | No | No | Yes | No | Yes |
| Foliar density | Medium | High | High | Medium | High | High | Medium |
| Branching | Erect | Erect | Erect | Erect | Spreading | Erect | Erect |
| Crown shape | Spindle | Spindle | Globular | Irregular | Irregular | Globular | Irregular |
| Ornamental valuea | 4.5 | 4.1 | 4.3 | 2.9 | 3.0 | 4.0 | 3 |
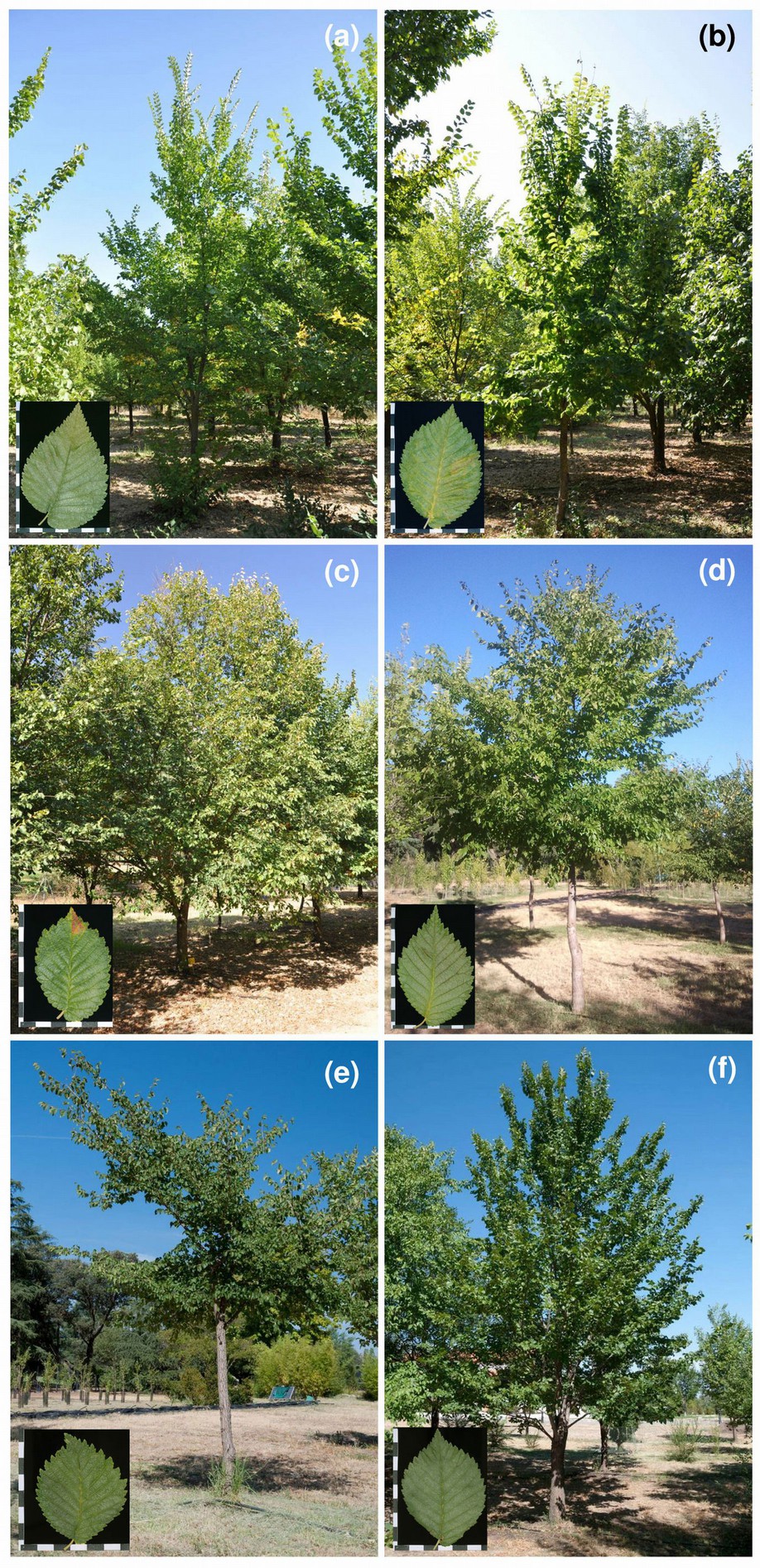
Fig. 3 - Registered Ulmus minor clones grown in the clonal bank of Puerta de Hierro Forest Breeding Center, Madrid. (a) “Ademuz”: (b) “Dehesa de la Villa”; (c) “Majadahonda”; (d) “Toledo”; (e) “Dehesa de Amaniel”; and (f) “Retiro” clones met the requirements to be registered as “qualified forest reproductive material” in Spain. Scale bars in leaf close-ups = 1 cm.
Fig. 4 - Registered “Fuente Umbría” clone grown in the clonal bank of Puerta de Hierro Forest Breeding Center (Madrid). This Ulmus minor clone met the requirements to be registered as “controlled forest reproductive material” in Spain. Scale bars in leaf close-up = 1 cm.
Fig. 5 - Leaf phenology in 2011 of the seven registered Ulmus minor clones and the “Sapporo Autumn Gold” control clone in Puerta de Hierro Forest Breeding Centre, Madrid.
The results of the genetic characterization of the seven clones are shown in Tab. 4. This description is intended to guarantee the traceability of the plant material during use, especially if clones are commercialized in the near future. From the experience of the Spanish elm breeding programme, the high level of polymorphism obtained with the microsatellites selected allows rigorous identification of each clone.
Tab. 4 - Genetic characterisation of the seven Ulmus minor clones, showing alleles for the two chloroplast and 12 nuclear microsatellites used. (a): not amplified.
| Organelle | DNA marker |
Clone | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ademuz | Dehesa de la Villa |
Majadahonda | Toledo | Dehesa de Amaniel | Retiro | Fuente Umbría |
|||||||||
| Chloro- plast |
ccmp2 | 237 | 236 | 236 | 215 | 236 | 216 | 215 | |||||||
| SFm | 278 | 278 | 278 | 297 | 278 | 297 | 297 | ||||||||
| Nuclear | Ulm 2 | 102 | 108 | 102 | 102 | 106 | 108 | 102 | 102 | 106 | 108 | 108 | 108 | 102 | 102 |
| Ulm 3 | 161 | 176 | 176 | 176 | 176 | 180 | 161 | 180 | 176 | 176 | 176 | 180 | 161 | 161 | |
| Ulm 8 | 196 | 196 | 196 | 196 | 194 | 196 | 194 | 198 | 196 | 196 | 196 | 196 | 194 | 194 | |
| UR 123 | 250 | 250 | 252 | 254 | 250 | 254 | 242 | 250 | 250 | 254 | 252 | 254 | 255 | 259 | |
| UR 141 | 150 | 152 | 152 | 160 | 152 | 158 | 152 | 152 | 158 | 158 | 158 | 158 | 152 | 158 | |
| UR 153 | 178 | 190 | 184 | 188 | 188 | 188 | 188 | 188 | 178 | 188 | 186 | 188 | 178 | 190 | |
| UR 158 | 195 | 195 | 179 | 195 | 195 | 195 | 179 | 179 | 179 | 199 | 179 | 179 | 179 | 179 | |
| UR 159 | 258 | 260 | 260 | 278 | 278 | 278 | 260 | 278 | 278 | 280 | 278 | 278 | 258 | 258 | |
| Ulm 1-98 | 151 | 151 | 151 | 151 | 151 | 151 | 151 | 151 | 151 | 151 | 151 | 151 | 151 | 154 | |
| Ulm 1-165 | 204 | 204 | 146 | 146 | 164 | 164 | 130 | 148 | 204 | 204 | 156 | 156 | 160 | 160 | |
| Ulm 2-16 | 90 | 90 | 90 | 90 | 90 | 90 | - | - | 90 | 90 | 82 | 94 | 90 | 90 | |
| Ulm 2-20 | -a | - | 184 | 202 | 172 | 206 | 206 | 220 | 186 | 202 | 180 | 184 | 220 | 220 | |
The Spanish programme aimed to directly control DED ([32], [20], [21], [42]) and gain a better understanding of tolerance through new techniques ([14], [15], [16]). One of its medium-term priorities is to increase the genetic diversity of tolerant native elms. Elm tolerance to O. novo-ulmi has been shown to be inheritable ([40], [9], [37], [41]) and polygenic (quantitative) in nature ([2]). It also depends on constitutive and inducible mechanisms of defence (e.g., [15], [17], [22]). In addition, the defense mechanisms of elms to O. novo-ulmi seem to differ between genotypes. This is the case of some anatomical features of the xylem associated with pathogen dispersal, such as pit and vessel size ([18], [22]). When more resistance mechanisms are gathered in the same genotype, the chances of overcoming an infection are likely to increase. Thus it would be desirable to perform controlled crossings between genotypes that express different, and preferably complementary, defense mechanisms. If multiple resistance layers act jointly and in a complementary fashion, the influence of environmental factors in tree tolerance to O. novo-ulmi would probably be lower. The possibility of any emerging variant or pathogen mutation overcoming the resistance mechanisms would also decrease. Understanding the genetic basis of elm tolerance to O. novo-ulmi is our second main research challenge.
To broaden the genetic base of tolerant native elms, the Spanish programme has grown 1400 seedlings from controlled F1 crossings between the seven U. minor clones. In 2016, when seedlings are four years old, they will be inoculated with O. novo-ulmi. The tolerance of new genotypes from different provenances in Spain to O. novo-ulmi will be assessed through clonal replicates (N ≥ 6) in the near future. Most of these genotypes have shown high tolerance levels when individually tested as seedlings. With this material, the Spanish programme expects to substantially increase the number of U. minor clones tolerant to O. novo-ulmi.
Conclusions
Although tree selection, trial establishment and breeding cycles require a major investment in time and effort, breeding programmes are the most reliable option for recovery of native elm populations. Results reported here show that selection of tolerant native U. minor genotypes is possible. The seven clones registered as forest reproductive material have shown high tolerance to DED in Spain. Their form and foliage are attractive and they are fast-growing trees. Longitudinal monitoring of the performance of the selected clones under different environments will make it possible to determine the suitable environmental range of each clone. New elm varieties likely to show low wilting values after O. novo-ulmi inoculation will be obtained in the next few years.
Acknowledgements
This paper is dedicated to Margarita Burón, who spent the last ten years of her life working on the conservation and breeding of Spanish elms.
The authors are grateful to everyone who has participated in the Spanish elm breeding programme during its 27 years of existence. We would also like to express our gratitude to the Dutch and Italian elm breeding programmes and the participants in the EU Project RESGEN CT96-78 for sharing their knowledge and materials. The programme is funded by the Spanish Directorate-General of Rural Development and Forestry Policy (Dirección General de Desarrollo Rural y Política Forestal) and the Spanish Ministry of Agriculture, Food and Environmental Affairs (Ministerio de Agricultura, Alimentación y Medio Ambiente).
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Martin Venturas
Carmen Collada
Jorge Domínguez
Eva Miranda
Margarita Burón
Luis Gil
ETSI Montes, Universidad Politécnica de Madrid, Ciudad Universitaria s/n, E-28040 Madrid (Spain)
Ingeniería Forestal y del Medio Natural, Universidad de Extremadura, Avenida Virgen del Puerto 2, E-10600 Plasencia (Spain)
Institute of Evolutionary Biology, The University of Edinburgh, West Mains Rd., Edinburgh EH9 9JT (United Kingdom)
Dirección General de Desarrollo Rural y Política Forestal, Ministerio de Medio Ambiente y Medio Rural y Marino, c/ Ríos Rosas 24, E-28003 Madrid (Spain)
Corresponding author
Paper Info
Citation
Martín JA, Solla A, Venturas M, Collada C, Domínguez J, Miranda E, Fuentes P, Burón M, Iglesias S, Gil L (2015). Seven Ulmus minor clones tolerant to Ophiostoma novo-ulmi registered as forest reproductive material in Spain. iForest 8: 172-180. - doi: 10.3832/ifor1224-008
Academic Editor
Alberto Santini
Paper history
Received: Dec 30, 2013
Accepted: May 21, 2014
First online: Aug 13, 2014
Publication Date: Apr 01, 2015
Publication Time: 2.80 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2015
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 66018
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 52069
Abstract Page Views: 6500
PDF Downloads: 5887
Citation/Reference Downloads: 39
XML Downloads: 1523
Web Metrics
Days since publication: 4169
Overall contacts: 66018
Avg. contacts per week: 110.85
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2015): 42
Average cites per year: 3.82
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Review Papers
Dutch elm disease and elm bark beetles: a century of association
vol. 8, pp. 126-134 (online: 07 August 2014)
Review Papers
Genomics of the Dutch elm disease pathosystem: are we there yet?
vol. 8, pp. 149-157 (online: 07 August 2014)
Research Articles
Comparison of commercial elm cultivars and promising unreleased Dutch clones for resistance to Ophiostoma novo-ulmi
vol. 8, pp. 158-164 (online: 07 August 2014)
Review Papers
Avoidance by early flushing: a new perspective on Dutch elm disease research
vol. 2, pp. 143-153 (online: 30 July 2009)
Research Articles
Monitoring of the incidence of Dutch Elm Disease and mortality in experimental plantations of French Ulmus minor clones
vol. 15, pp. 289-298 (online: 29 July 2022)
Research Articles
Adaptive response of Pinus monticola driven by positive selection upon resistance gene analogs (RGAs) of the TIR-NBS-LRR subfamily
vol. 10, pp. 237-241 (online: 02 February 2017)
Research Articles
Outcome of Ceratocystis platani inoculations in Platanus × acerifolia in relation to season and inoculum dose
vol. 9, pp. 608-617 (online: 17 March 2016)
Research Articles
Natural spread of Verticillium wilt as effective constraint on Ailanthus altissima invasion
vol. 18, pp. 391-398 (online: 22 December 2025)
Research Articles
Genetic variation of Fraxinus excelsior half-sib families in response to ash dieback disease following simulated spring frost and summer drought treatments
vol. 9, pp. 12-22 (online: 08 September 2015)
Research Articles
Variation in resistance to the rust fungus Melampsora larici-populina Kleb. in Populus nigra L. in the Czech Republic
vol. 9, pp. 146-153 (online: 26 October 2015)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword