De novo adventitious root formations in mini-cuttings of Azadirachta indica in response to different rooting media and auxin treatments
iForest - Biogeosciences and Forestry, Volume 8, Issue 4, Pages 558-564 (2014)
doi: https://doi.org/10.3832/ifor1189-007
Published: Dec 09, 2014 - Copyright © 2014 SISEF
Technical Reports
Abstract
Neem (Azadirachta indica A. Juss) is a multipurpose Indian tree important to local economy. Conservation of the genetic resources of neem is essential for the adaptability of this tree species to projected climate change impacts. Here, the effect of type and concentration of auxins in different rooting media on adventitious root formation (ARF) in mini-cuttings of Azadirachta indica is depicted. Three different rooting media (i.e., sand, vermiculite and soil) were used, and the experiment was established using three types of auxin (IBA, IAA and NAA) and 6 concentration treatment combinations (100, 250, 500, 750, 1000 and 1500 mg l-1), in a complete randomized block design (CRBD). Significant effects of different auxin types, concentration treatments and rooting media on adventitious root formation of neem mini-cuttings were observed. Mini-cuttings were assessed for rooting percentage, number of roots, root length and number of leaves. IBA resulted in higher rooting percentage (90%), number of roots (149.56), root length (14.83 cm) and number of leaves per rooted mini-cuttings (12.78), when growing in sand. The determination of proper rooting protocols and the use of mini-cuttings were proved important for improving mass propagation of A. indica.
Keywords
Mini-cuttings, Adventitious Root Formation (ARF), Azadirachta Indica, Auxin
Introduction
Azadirachta indica A. Juss, a member of Meliaceae family commonly known as neem, is a multipurpose evergreen tropical tree. It has been recognized as a versatile tree agroforestry purposes. Neem contains potent bio-degradable and economically safe bio-pesticide compounds, such as azadirachtin ([26]). When considered together, the various uses of neem can be very attractive to rural people that can easily get economic benefits through production of seeds and leaves within a short period of time.
Vegetative propagation ([25]) and breeding strategies for multipurpose traits including timber and non-timber products ([21]) are desirable for this species. However, when propagated through cuttings, neem shows difficult rooting. Goel & Behl ([9]) suggested the use of juvenile top cuttings for rooting induction in hard wood species such as neem, because physiologically mature tissues have lower rooting potential, take a long time to initiate roots and develop fewer roots.
Factors that influence the successful rooting of cuttings are reported by Garner & Chaudri ([8]) and Hartman et al. ([12]): age of mother plant, season of cutting, type and height of cutting, presence or absence vegetative buds, number of leaves on the cutting, water content of stock plant and cutting. In addition, the formation of adventitious roots is a process induced and regulated by environmental and endogenous factors such as temperature, light, hormones (especially auxins), sugars, mineral salts and other molecules ([38]).
Auxin is one of the major endogenous hormones involved in the process of adventitious rooting ([37]). Physiological stages of rooting are correlated with changes in endogenous auxin concentrations ([15]). Moreover, the use of exogenous plant growth regulators (PGRs) plays a vital role in influencing the sprouting and rooting of stem cuttings. The widely used sources of growth hormones for cuttings rooting are IBA (Indole-3-butyric acid), NAA (α-naphthalene acetic acid), and IAA (Indole-3-Acetic Acid) and commercialized root promoters (root-growing powders - [37], [14]).
Use of mini-cuttings for propagation of different perennial plant species emerged as a popular technique recently ([32]) and has been proven to be effective and reliable for mass propagation of desired plants. Mini-cuttings allow genetic characteristics to be maintained ([31], [36], [27], [35], [24]). The adventitious root ability in mini-cuttings has been extensively tested in Eucalyptus cloeziana ([1]), E. benthamii Maiden & Cambage × E. dunnii Maiden ([2]), E. globulus × E. maidennii ([27]) clones of E. benthamii and E. dunnii ([3]), E. benthamii ([4]) and Pinus pinaster ([24]) with positive results.
The de novo rooting is the most common form of rooting from cuttings. In the de novo Adventitious Root Formation (ARF), a new area of meristematic tissue is formed among the parenchyma cells at the anti-clinal division in the phloem ray parenchyma. The formation of adventitious roots is an essential step in vegetative propagation ([23]). Adventitious roots can arise naturally from stem tissue under stressful environmental conditions, or may be induced by mechanical damage or following tissue culture regeneration of shoots ([22]).
The present study was aimed to develop a cost-effective novel approach for clonal propagation of Azadirachta indica. We took advantage of controlled nursery conditions to apply ARF techniques for rapid and uniform production of high quality stocks within a short period, for plantation programs at mass level.
Materials and ethods
Selection of planting material
The study was carried out at the Arid Forest Research Institute, Jodhpur, Rajasthan, India (24° 40′ N, 71° 15′ E) during July-September (Monsoon season) of 2011. The climate of this region is hot and semi-arid, but influenced by monsoons.
Preparation of stem cuttings
The selection of Candidate Plus Tree (CPT) was done based on vegetative characters (i.e., growth potential; diameter at breast height; plant height; stem form; crown projection) and reproductive characters (i.e., regeneration ability; initiation of leaf fall; initiation of new leaves; initiation of flowering; number of flowers; initiation of fruiting; number of fruits/bunch; fruiting period), and seed traits (100 seeds weight, g; oil percentage; seed viability; Azadirachtin percentage). The mini-cuttings were collected from selected CPT naturally growing at the Forest Genetics and Tree Breeding experimental field, in Jodhpur. The mini-stumps were propagated by conventional cutting methods ([31], [27], [35]) from trees 30 months old. The newly emerging juvenile apical shoots (mini-cuttings) were harvested using sterile pruning scissors in the morning. After harvesting, these were first screened for the desired length (30-35 cm) and diameter (<0.5cm) by scale and calibrated Vernier caliper. Then, they were kept in wet cloth (for prevention from damage) for transportation to the laboratory.
The mini-cuttings were defoliated and then treated with aqueous solution of 0.1% Carbendazim (Bavistin 50% WP, Systemic fungicide, BASF India Limited, Bombay) for 1 minute, and subsequently washed with distilled water to remove the excess of fungicide. The selected cuttings were treated with freshly prepared aqueous solution of root promoting auxins as described by Kroin ([18], [19], [20]). The cuttings dipped in distilled water were considered as control or treated with IBA (Indole-3-Butyric Acid, Duchefa Biochemi, Postbus, Netherland), IAA (Indole-3-Acetic Acid, Duchefa Biochemi, Postbus, Netherland) and NAA (α-naphthalene acetic acid, Duchefa Biochemi, Postbus, Netherland), at different concentration of 100, 250, 500, 750, 1000 and 1500 mg l-1.
Twenty cuttings of each treatment were stuck in root trainer (250 cc) containing three nursery grade rooting media: vermiculite, sand and soil.
The experiment was laid out in a Complete Randomized Block Design (CRBD). To prevent any form of damage to the cambium of cuttings during insertion into rooting medium, holes were made by a glass rod into the root trainer. The cuttings were kept under intermittent mist (misting flow for 60 seconds every 30 minutes), maintained at 60-80% relative humidity and 25-30 °C / 15-20 °C day/night temperature. The cuttings were regularly watered and treated with 0.1% Bavistin to avoid desiccation damage and attack of pathogens respectively, at 15-day interval.
The rooting experiments were run for 60 days, then rooted cuttings were transferred to polythene bags (16 × 28 cm) containing soil: FYM (field yard manure) (5:1) and kept in poly house for 15 to 20 days. The polythene bags were moved daily in order to minimize misting variation. After this, polythene bags containing rooted mini-cutting were transferred to agro-shade house for hardening. In agro-shade house, plants were manually irrigated by tap water once a day. After 35-45 days of hardening, plants were planted in the field and were manually irrigated by tap water once a week.
Analysis of rooting in mini-cuttings
After completion of experiment, all the cuttings were uprooted carefully with the help of running water. The number of leaves were recorded just before uprooting of a treatment lot, whereas the rooting success, numbers of primary roots and length of roots (cm) were recorded by observing all mini-cuttings of each replication.
Data collection and statistical analyses
The recorded data were analyzed by general linear models using the software package SPSS® 8.0 (SPSS Inc., Chicago, Illinois, USA). Data were subjected to ANOVA. Data in percentage was subjected to arcsine (√X) transformation ([30]) before statistical analysis.
Results and iscussion
A highly significant effect (p<0.001) of the three different rooting media on cuttings’ performance was observed. The interaction of auxin, their concentration and rooting media had a significant effect (p<0.001) on the number of roots, root length and number of leaves (Tab. 1). The highest rooting percentage, numbers of roots, root length and numbers of leaves were 46.72%, 53.31, 8.79 and 6.54, respectively, in sand rooting media, and lowest in soil rooting media with 26.73%, 20.37, 4.88 cm and 3.16, respectively (Tab. 2). The high root formation in sand could probably be attributed to a combination of water holding capacity and good aeration of the substrate. Indeed, aeration plays a significant role in root initiation as well as on root elongation ([17]). This could explain the observed low numbers of root and length in soil rooting media. In fact, the aeration in soil is usually low, while the water holding capacity is good.
Tab. 1 - Analysis of variance for the effect of auxins (IBA, IAA and NAA), their concentration and media on rooting percentage, number of root, root length (cm) and number of leaves of A. indica mini-cuttings. (df): degrees of freedom.
| Source of Variance |
df | Rooting % | Number of roots | Root length | Number of leaves | ||||
|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | ||
| Auxin | 2 | 19.71 | 0.00 | 66.27 | 0.00 | 1.01 | 0.36 | 20.31 | 0.00 |
| Media | 2 | 52.99 | 0.00 | 47.15 | 0.00 | 77.89 | 0.00 | 158.69 | 0.00 |
| Concentration | 5 | 6.21 | 0.00 | 42.35 | 0.00 | 25.22 | 0.00 | 74.93 | 0.00 |
| Auxin×Media | 4 | 1.48 | 0.20 | 9.45 | 0.00 | 0.03 | 0.99 | 36.39 | 0.00 |
| Auxin×Concentration | 10 | 2.99 | 0.00 | 17.44 | 0.00 | 19.91 | 0.00 | 56.53 | 0.00 |
| Concentration×Media | 10 | 1.38 | 0.19 | 6.92 | 0.00 | 2.48 | 0.00 | 7.68 | 0.00 |
| Auxin×Concentration× Media |
20 | 0.99 | 0.46 | 4.29 | 0.00 | 7.34 | 0.00 | 19.83 | 0.00 |
Tab. 2 - Mean values of the parameters analyzed (rooting percentage, number of root, root length in cm, and number of leave) in mini cuttings of A.indica supplied with three different auxins and grown on different rooting media.
| Parameter | Treatment | Sand | Vermiculite | Soil | Grand Mean |
|---|---|---|---|---|---|
| Rooting % | Control | 29.73 | 38.95 | 26 | 31.56 |
| IBA | 59.07 | 54.44 | 30.78 | 48.09 | |
| IAA | 46.07 | 40.39 | 29.2 | 38.55 | |
| NAA | 37.85 | 37.94 | 20.24 | 32.01 | |
| Grand Mean | 46.72 | 44.15 | 26.73 | - | |
| Number of root |
Control | 8 | 7.5 | 5 | 6.83 |
| IBA | 76.24 | 53.05 | 33.81 | 54.36 | |
| IAA | 26.67 | 21.82 | 12.31 | 20.26 | |
| NAA | 53.13 | 43.91 | 12.89 | 36.94 | |
| Grand Mean | 53.31 | 42.98 | 20.37 | - | |
| Root length (cm) | Control | 2.6 | 2 | 2 | 2.2 |
| IBA | 8.94 | 7.9 | 5.09 | 7.31 | |
| IAA | 8.83 | 6.8 | 5.17 | 6.93 | |
| NAA | 9.13 | 6.66 | 4.67 | 6.82 | |
| Grand Mean | 8.79 | 7.06 | 4.88 | - | |
| Numbers of leaves |
Control | 3.4 | 2.75 | 2.5 | 2.88 |
| IBA | 9.19 | 6.2 | 3.66 | 6.35 | |
| IAA | 4.07 | 3.25 | 2.72 | 3.34 | |
| NAA | 5.54 | 3.95 | 3.11 | 4.2 | |
| Grand Mean | 6.54 | 4.76 | 3.16 | - |
El-Naggar & El-Nasharty ([6]) reported that rooting (pot size) and nutritional requirements are the most important factors affecting growth of ornamental plants. Khayyat et al. ([17]) observed that the type of rooting media and their characteristics are of utmost importance for the quality of rooted cuttings, and attributed the improved root formation and growth of Epipremnum aureum cuttings to substrates containing mixtures of leaf mold and sand, due to the better aeration, drainage and water holding capacity.
In this investigation, the highest number of leaves were formed in sand treatment, which can be attributed to the coordination between leaf and root development. According to Govinden-Soulange et al. ([10]), the number of leaves produced per cutting is determined by the type of cutting used, plant growth regulators supplied and health status of the mother plant. Since all cuttings used in this investigation were uniform, the highest number of leaves per cutting observed for cuttings grown in sand could be attributed to intrinsic characteristics of stem cuttings, like root number and length.
A strong effect (p<0.001) of all the three different auxins on the studied parameters was also observed. Results indicated that the maximum rooting percentage, numbers of roots, root length and numbers of leaves were 48.09%, 54.36, 7.3 cm and 6.35, respectively, for the treatment with IBA; and the minimum values were 31.56%, 6.83, 2.20 cm and 2.88, respectively, in control (Tab. 2). Clearly, hormones of the auxin group promoted root formation. A higher percentage of rooting and number of roots per cutting were recorded with IBA than IAA (38.55% and 26.94) and NAA (32.01% and 36.94 - Fig. 1). Gehlot et al. ([7]) also found that the higher the rooting percent, the higher is the number of roots and length of roots in cuttings treated with IBA. This suggested that the use of IBA is more efficient than IAA and NAA in the present conditions (summer season).
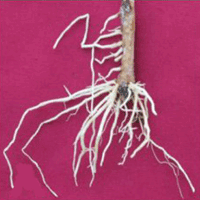
Fig. 1 - De novo adventitious root formation in mini-cutting of A. indica. (A, B, C, D): Adventitious root formation in mini-cutting; (E, F, G, H): high rate of rooting in mini-cuttings treated with IBA 250 ppm; (I): field plantation of plant produced from mini-cutting; (J): plant produced from mini-cuttings in poly bags under poly house conditions.
The regeneration of adventitious root from softwood cuttings was also examined by Singh & Chander ([29]) who found higher rooting percentage and root numbers in cuttings treated with IBA (33.30% and 11.63) followed by IAA (23.30% and 6.67). Kesari et al. ([16]) found relatively poor rooting with IAA treated stem cuttings of Pongamia pinnata in comparison to IBA. Tomar ([33]) found that cuttings rooted effectively with IBA, and those from coppice shoots gave better results than cuttings from the main stem. The rooting capacity found in the present study indicates that the induction treatments imposed significantly enhanced the rooting ability of Azadirachta indica mini-cuttings, in the following order: IBA > IAA > NAA (Fig. 2, Fig. 3, Fig. 4).
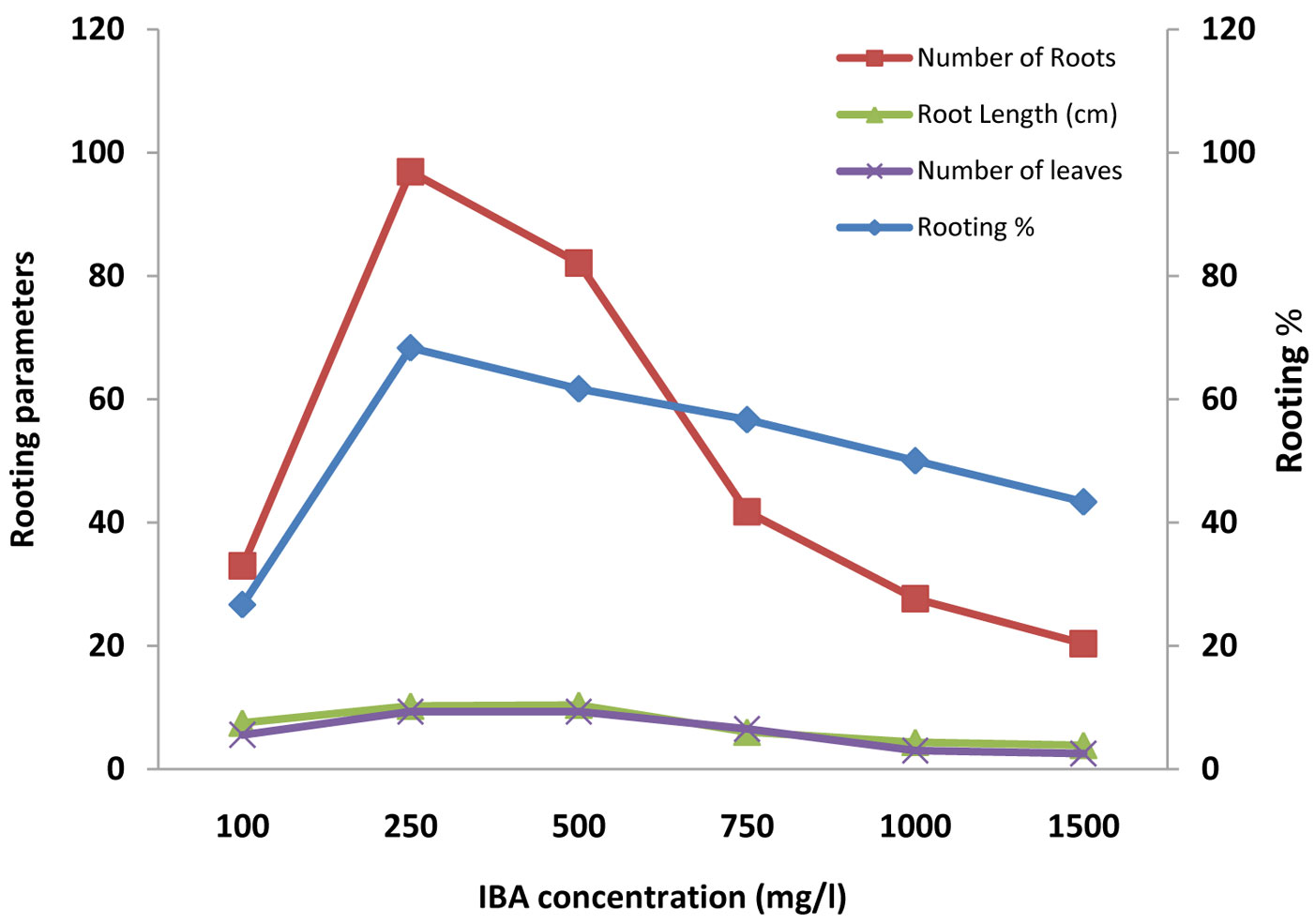
Fig. 2 - Effect of IBA and rooting media (sand, vermiculite and soil) on rooting percentage, number of root, root length (cm) and number of leaves in mini-cuttings of A.indica.
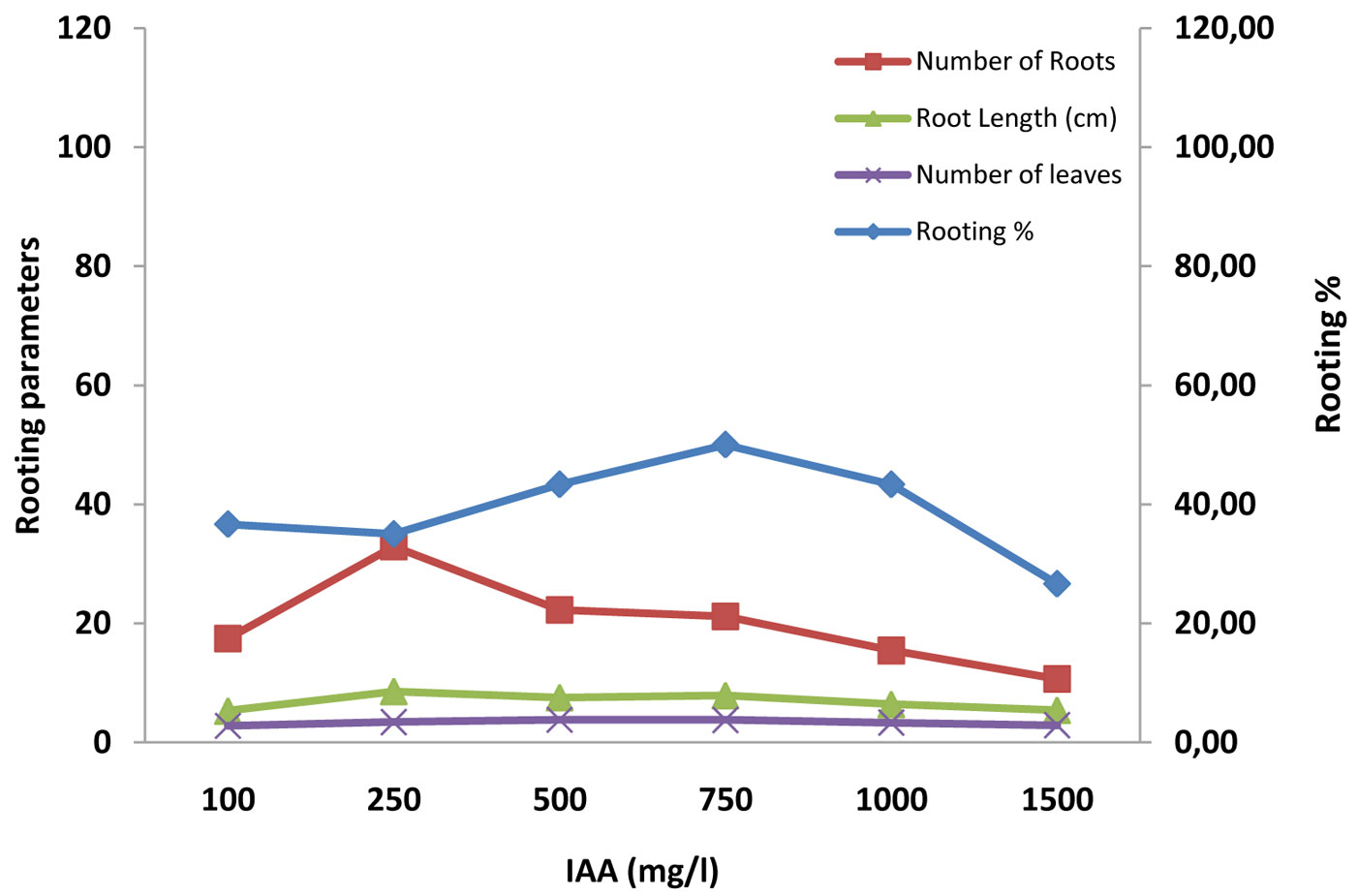
Fig. 3 - Effect of IAA and rooting media (sand, vermiculite and soil) on rooting percentage, number of root, root length (cm) and number of leaves in mini-cuttings of A.indica.
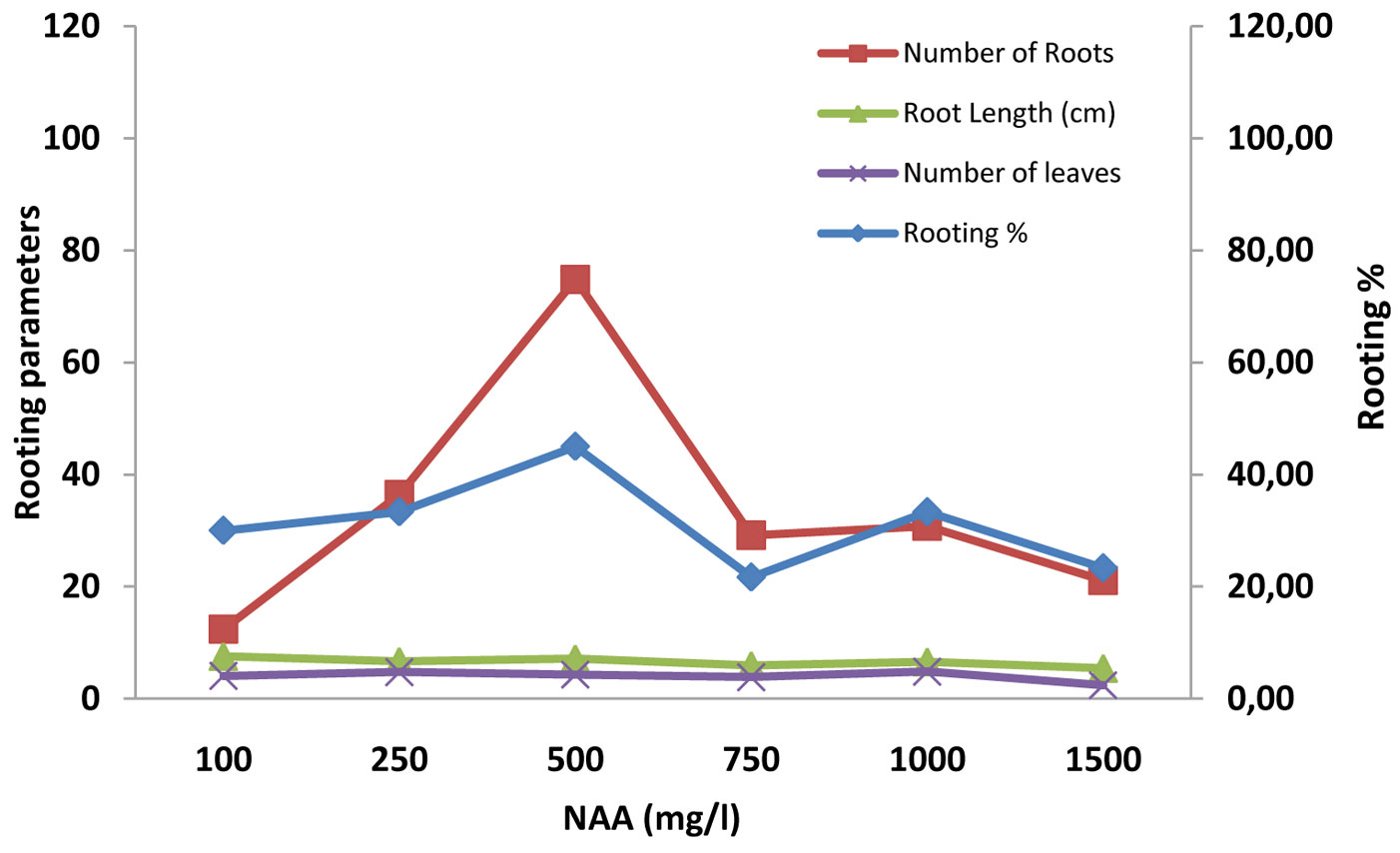
Fig. 4 - Effect of NAA and rooting media (sand, vermiculite and soil) on rooting percentage, number of root, root length (cm) and number of leaves in mini-cuttings of A.indica.
A significant relationship (p<0.001) was also found between the rooting percentage and the concentration of auxins, in association with different rooting media. The overall rooting percentage varied greatly (Tab. 2). It was the highest (90%) in treatment with 250 mg l-1 IBA in sand rooting media, followed by (80%) 500 mg l-1 IBA in sand media; the lowest value was 10% (Tab. 3). Cuttings treated with 250 mg l-1 IBA in all the three potting media (sand, vermiculite and soil) gave the maximum success rate, in comparison with similar concentration of IAA and NAA; sand (90%) > vermiculite (80%) > soil (35%). We hypothesize an interaction between physical properties of the rooting media and the effective auxin concentration (Fig. 2, Fig. 3, Fig. 4).
Tab. 3 - Interactive effect of auxin (IBA, IAA & NAA), their different concentration and rooting media (sand, vermiculite and soil) on rooting percentage and number of root in mini-cuttings of A.indica. (*): Arcsine values in parentheses.
| Treat-ment | Conc. (mg l-1) |
Rooting percentage (%) |
Mean | Number of root (Mean ± SE) |
Mean | ||||
|---|---|---|---|---|---|---|---|---|---|
| Sand | Vermi-culite | Soil | Sand | Vermi-culite | Soil | ||||
| Control | 0 | 25 (29.73)* |
40 (38.95)* |
20 (26.56)* |
28.33 (31.74) |
8.00 ± 1.22 | 7.50 ± 0.57 | 5.00 ± 0.57 | 6.83 |
| IBA | 100 | 30 (32.90) |
30 (32.90) |
20 (26.56) |
26.66 (30.79) |
36.67 ± 1.67 | 36.00 ± 0.82 | 26.25 ± 2.39 | 32.97 |
| 250 | 90 (76.08) |
80 (66.59) |
35 (36.06) |
68.33 (59.58) |
149.56 ± 10.76 | 95.45 ± 3.12 | 45.71 ± 1.30 | 96.91 | |
| 500 | 80 (69.75) |
70 (60.54) |
35 (36.06) |
61.66 (55.45) |
105.69 ± 17.00 | 93.64 ± 1.52 | 47.00 ± 6.62 | 82.11 | |
| 750 | 70 (63.98) |
70 (63.98) |
30 (32.90) |
56.66 (53.62) |
51.86 ± 1.77 | 40.00 ± 1.34 | 33.33 ± 2.11 | 41.73 | |
| 1000 | 70 (63.98) |
60 (57.64) |
20 (26.56) |
50.00 (49.39) |
32.14 ± 1.79 | 30.83 ± 0.56 | 20.00 ± 5.77 | 27.66 | |
| 1500 | 60 (54.49) |
50 (45) |
20 (26.56) |
43.33 (42.02) |
26.67 ± 0.71 | 22.40 ± 2.24 | 12.00 ± 0.98 | 20.36 | |
| IAA | 100 | 50 (45) |
30 (29.46) |
30 (32.90) |
36.66 (35.79) |
24.00 ± 0.67 | 18.33 ± 1.05 | 10.00 ± 0.90 | 17.44 |
| 250 | 40 (38.95) |
40 (38.95) |
25 (29.73) |
35.00 (35.88) |
42.50 ± 0.94 | 31.25 ± 0.82 | 25.00 ± 0.85 | 32.92 | |
| 500 | 60 (51.34) |
50 (45) |
20 (26.56) |
43.33 (40.97) |
30.83 ± 1.35 | 26.00 ± 1.25 | 10.00 ± 0.76 | 22.28 | |
| 750 | 70 (63.98) |
60 (51.05) |
20 (26.56) |
50.00 (47.20) |
27.86 ± 0.69 | 26.67 ± 0.71 | 9.00 ± 0.58 | 21.18 | |
| 1000 | 50 (45) |
50 (45) |
30 (32.90) |
43.33 (40.97) |
18.00 ± 0.82 | 17.00 ± 0.82 | 11.33 ± 0.42 | 15.44 | |
| 1500 | 30 (32.90) |
30 (32.90) |
20 (26.56) |
26.66 (30.79) |
13.33 ± 1.05 | 11.67 ± 1.05 | 7.00 ± 0.58 | 10.67 | |
| NAA | 100 | 50 (45) |
30 (32.90) |
10 (13.92) |
30.00 (30.61) |
18.00 ± 0.82 | 13.33 ± 1.05 | 6.00 ± 0.98 | 12.44 |
| 250 | 40 (38.95) |
40 (38.95) |
20 (26.56) |
33.33 (34.82) |
37.50 ± 2.11 | 61.71 ± 3.24 | 10.00 ± 0.57 | 36.40 | |
| 500 | 60 (51.34) |
55 (48.17) |
20 (26.56) |
45.00 (42.02) |
115.00 ± 7.49 | 89.50 ± 9.94 | 20.00 ± 0.57 | 74.83 | |
| 750 | 10 (13.92) |
45 (41.83) |
10 (13.92) |
21.66 (23.22) |
30.00 ± 1.07 | 37.50 ± 2.11 | 20.00 ± 0.37 | 29.17 | |
| 1000 | 50 (45) |
40 (39.23) |
10 (13.92) |
33.33 (32.72) |
46.00 ± 4.99 | 36.43 ± 1.92 | 10.00 ± 0.57 | 30.81 | |
| 1500 | 30 (32.90) |
20 (26.56) |
20 (26.56) |
23.33 (28.67) |
28.33 ± 1.05 | 25.00 ± 0.92 | 10.00 ± 0.57 | 21.11 | |
| Total Mean | - | 50.79 (46.72) |
47.11 (44.15) |
21.84 (26.73) |
- | 53.31 | 42.98 | 20.37 | - |
IBA was found more effective than IAA and NAA, in that order, in root induction in mini-cuttings of Neem and Commiphora wightii ([7], [34]). Kesari et al. ([16]) found relatively poor rooting with IAA treated stem cutting of Pongamia pinnata in comparison to IBA. Our study revealed that the rate of rooting success at various auxin concentrations increased until a threshold is reached, after which the rooting potential dropped, differentially for the auxins tested. In the case of IBA, the maximum success was reached with 250 mg l-1 (59.58%), in IAA with 750 mg l-1 (47.20%) and in NAA with 500 mg l-1 (42.03% - Tab. 3). Differences might be due to the auxin-specific toxic concentration ([11]). Toxicity is followed by accelerated foliar senescence, chloroplast damage, destruction of membrane and vascular system integrity, necrosis and plant death. A high level of auxins stimulates biosynthesis of ethylene, which in turn triggers abscissic acid (ABA) production. The ABA is translocated through the plant, triggering stomatal closure and, together with ethylene, promotes leaf senescence and ultimately death ([11]). Gehlot et al. ([7]) also found different thresholds of auxin toxicity, in summer season, with values ranging from 57.46% (with IBA 500 mg l-1), to 45.09% (with IAA 1000 mg l-1) and 49.31% (with NAA 750 mg l-1).
Statistically significant (p<0.001) effect of different doses of auxins and rooting media on the number and length of roots were observed. Overall the number and length of roots ranged from 5.00 and 2.60 cm (lower in control with soil and control with vermiculite) to 149 and 14.83 cm (highest in 250 mg l-1 of IBA with sand), respectively (Tab. 3 Tab. 4). It is important to consider that growth regulators not only influence the percentage of rooting, rather they may also accelerate the onset of the rooting process and increase the number and quality (in terms of length) of roots. The maximum number of roots was recorded in the treatment with 250 mg l-1 of IBA in all the rooting media. The control treatment resulted in thin roots, rather than in the inhibition of rooting, which indicates that endogenous auxin along with some root inducing factors might occur naturally within the mini cuttings that may boost root primordia initiation. Differences in the threshold of toxicity among the studied auxins was also an indication for specific endogenous levels ([5]). The proper type and concentration of auxins in association with a proper rooting media is necessary to maximize the success of this propagation approach ([28]).
Tab. 4 - Interactive effect of auxin (IBA, IAA and NAA), their different concentration and rooting media (sand, vermiculite and soil) on root length (cm) and number of leaves in mini-cuttings of A.indica.
| Treatment | Conc. (mg l-1) |
Root Length (Mean ± SE, cm) |
Mean | Number of leaves (Mean ± SE) |
Mean | ||||
|---|---|---|---|---|---|---|---|---|---|
| Sand | Vermiculite | Soil | Sand | Vermiculite | Soil | ||||
| Control | 0 | 2.60 ± 0.24 | 2.00 ± 0.38 | 2.00 ± 0.11 | 2.20 | 3.40 ± 0.40 | 2.75 ± 0.31 | 2.50 ± 0.29 | 2.88 |
| IBA | 100 | 8.00 ± 0.26 | 7.50 ± 0.50 | 7.00 ± 0.41 | 7.50 | 6.00 ± 0.26 | 5.00 ± 0.45 | 5.75 ± 0.25 | 5.58 |
| 250 | 14.83 ± 1.11 | 12.94 ± 1.10 | 3.00 ± 0.25 | 10.25 | 12.78 ± 0.57 | 11.29 ± 0.66 | 4.00 ± 0.38 | 9.35 | |
| 500 | 11.25 ± 1.20 | 10.00 ± 0.21 | 10.00 ± 0.44 | 10.41 | 13.19 ± 0.43 | 9.75 ± 0.19 | 5.14 ± 0.55 | 9.36 | |
| 750 | 6.57 ± 0.14 | 8.50 ± 0.33 | 3.00 ± 0.32 | 6.02 | 11.71 ± 0.49 | 5.50 ± 0.19 | 2.33 ± 0.21 | 6.51 | |
| 1000 | 5.43 ± 0.14 | 4.67 ± 0.14 | 3.00 ± 0.41 | 4.36 | 4.43 ± 0.14 | 2.67 ± 0.14 | 2.00 ± 0.14 | 3.03 | |
| 1500 | 4.33 ± 0.14 | 3.80 ± 0.13 | 3.50 ± 0.29 | 3.87 | 2.67 ± 0.14 | 3.00 ± 0.21 | 2.00 ± 0.11 | 2.55 | |
| IAA | 100 | 6.60 ± 0.16 | 5.00 ± 0.48 | 4.33 ± 0.21 | 5.31 | 3.00 ± 0.11 | 3.00 ± 0.23 | 2.33 ± 0.21 | 2.77 |
| 250 | 11.25 ± 0.82 | 8.50 ± 0.33 | 6.00 ± 0.75 | 8.58 | 4.50 ± 0.33 | 2.75 ± 0.16 | 3.00 ± 0.21 | 3.41 | |
| 500 | 9.83 ± 0.82 | 6.80 ± 0.25 | 6.00 ± 0.69 | 7.54 | 5.17 ± 0.11 | 3.20 ± 0.39 | 3.00 ± 0.37 | 3.79 | |
| 750 | 10.00 ± 0.30 | 8.67 ± 0.28 | 5.00 ± 0.56 | 7.89 | 4.00 ± 0.33 | 4.33 ± 0.14 | 3.00 ± 0.45 | 3.77 | |
| 1000 | 7.40 ± 0.27 | 5.50 ± 0.33 | 6.33 ± 0.21 | 6.41 | 3.80 ± 0.13 | 3.20 ± 0.13 | 3.00 ± 0.37 | 3.33 | |
| 1500 | 7.00 ± 0.37 | 6.33 ± 0.21 | 3.00 ± 0.34 | 5.44 | 3.67 ± 0.21 | 3.00 ± 0.33 | 2.00 ± 0.11 | 2.89 | |
| NAA | 100 | 15.20 ± 0.13 | 4.57 ± 0.14 | 3.00 ± 0.41 | 7.59 | 6.00 ± 0.21 | 4.27 ± 0.14 | 2.00 ± 0.21 | 4.09 |
| 250 | 5.50 ± 0.33 | 8.57 ± 0.98 | 6.00 ± 0.54 | 6.69 | 4.75 ± 0.16 | 5.00 ± 0.67 | 4.50 ± 0.29 | 4.75 | |
| 500 | 7.67 ± 0.41 | 8.80 ± 0.33 | 5.00 ± 0.48 | 7.15 | 5.17 ± 0.11 | 4.75 ± 0.16 | 3.00 ± 0.37 | 4.30 | |
| 750 | 5.00 ± 0.21 | 6.82 ± 0.23 | 6.00 ± 0.37 | 5.94 | 3.00 ± 0.00 | 3.55 ± 0.21 | 5.00 ± 0.67 | 3.85 | |
| 1000 | 9.60 ± 0.16 | 5.18 ± 0.18 | 5.00 ± 0.32 | 6.59 | 8.40 ± 0.58 | 3.14 ± 0.27 | 3.00 ± 0.35 | 4.84 | |
| 1500 | 7.33 ± 0.84 | 6.00 ± 0.21 | 3.00 ± 0.21 | 5.44 | 2.67 ± 0.21 | 3.00 ± 0.68 | 1.50 ± 0.29 | 2.39 | |
| Total Mean | - | 8.79 | 7.06 | 4.88 | - | 6.54 | 4.76 | 3.16 | - |
In most tree species, the rooting ability of cuttings has been reported to increase from the apical to the basal portion of the shoot, which has been attributed to accumulation of carbohydrates ([13]). In our case, the number of leaves was significantly (p<0.001) affected by rooting media and concentration. The maximum number of leaves was observed in 500 mg l-1 IBA in association with sand (13.19), and the minimum (2.00) in soil with low or high concentrations of auxin (Tab. 3, Tab. 4). Cuttings treated with IBA in all the media exhibited a significant enhancement in the number of leaves compared to other auxins ([7]). The similarity in terms of rooting percentage, number and length of roots and numbers of leaves (Fig. 2, Fig. 3, Fig. 4) suggests that there was a physiological correlation between roots and the shoot, and opens new ground for studying vegetative propagation performance in neem.
Conclusion
In conclusion, sand was found to be superior in the propagation of Azadirachta indica cuttings when compared to the other media, for most of the root parameters determined. In mass propagation of Azadirachta indica for agroforestry purposes there is a need to maximize effective rooting, which may be obtained using mini-cuttings. Vegetative propagation through mini-cuttings was found to be a very promising method, which may replace the existing stem cuttings. The rooting media and plant growth regulator play a key role in this process. The formation of healthy plants after hardening in outdoor conditions further showed that Azadirachta indica could be successfully propagated by using mini cutting techniques. This new method of propagation provided the extension of propagation season (exploiting the months free of monsoon), higher yields of rooted cuttings per stock plant (using mini-cuttings) and a high rooting percentage (through rooting hormones).
Acknowledgements
Authors are thankful to the Director of AFRI, Jodhpur, Rajasthan, India for providing necessary facilities in the course of work. AG is also grateful to Department of Science and Technology, Rajasthan, India sanctioned student project for the year 2012-2013, and to Gilvano Ebling Brondani, Federal University of Mato Grosso / UFMT, Cuiabá - MT, Brazil for his valuable suggestions on an earlier version of the manuscript.
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Rajesh Kumar Gupta
Inder Dev Arya
Sarita Arya
Forest Genetics and Tree Breeding Division, Arid Forest Research Institute, Jodhpur 342001, Rajasthan (India)
Regional Pesticides Testing Laboratory, Department of Agriculture and Co-operation, Chandigarh (India)
Corresponding author
Paper Info
Citation
Gehlot A, Gupta RK, Arya ID, Arya S, Tripathi A (2014). De novo adventitious root formations in mini-cuttings of Azadirachta indica in response to different rooting media and auxin treatments. iForest 8: 558-564. - doi: 10.3832/ifor1189-007
Academic Editor
Gianfranco Minotta
Paper history
Received: Nov 28, 2013
Accepted: Nov 13, 2014
First online: Dec 09, 2014
Publication Date: Aug 02, 2015
Publication Time: 0.87 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2014
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 59424
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 48864
Abstract Page Views: 3304
PDF Downloads: 5535
Citation/Reference Downloads: 23
XML Downloads: 1698
Web Metrics
Days since publication: 3996
Overall contacts: 59424
Avg. contacts per week: 104.10
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2015): 6
Average cites per year: 0.55
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Conservation of Betula oycoviensis, an endangered rare taxon, using vegetative propagation methods
vol. 13, pp. 107-113 (online: 23 March 2020)
Research Articles
Mini-tunnel and season influence in clonal garden on the production of clonal seedlings for two subtropical clones: Eucalyptus saligna and Corymbia torelliana × Corymbia citriodora
vol. 18, pp. 154-162 (online: 09 June 2025)
Research Articles
Effect of family, crown position, number of winter buds, fresh weight and the length of needle on rooting ability of Pinus thunbergii Parl. cuttings
vol. 9, pp. 370-374 (online: 11 January 2016)
Research Articles
Use of alternative containers for promoting deep rooting of native forest species used for dryland restoration: the case of Acacia caven
vol. 10, pp. 776-782 (online: 02 September 2017)
Research Articles
Gas exchange characteristics of the hybrid Azadirachta indica × Melia azedarach
vol. 8, pp. 431-437 (online: 17 December 2014)
Research Articles
Forest litter as the mulch improving growth and ectomycorrhizal diversity of bare-root Scots pine (Pinus sylvestris) seedlings
vol. 8, pp. 394-400 (online: 20 August 2014)
Technical Reports
Effects of different mechanical treatments on Quercus variabilis, Q. wutaishanica and Q. robur acorn germination
vol. 8, pp. 728-734 (online: 05 May 2015)
Research Articles
Linking nursery nutritional status and water availability post-planting under intense summer drought: the case of a South American Mediterranean tree species
vol. 9, pp. 758-765 (online: 03 June 2016)
Research Articles
Fertilisation of Quercus seedlings inoculated with Tuber melanosporum: effects on growth and mycorrhization of two host species and two inoculation methods
vol. 10, pp. 267-272 (online: 13 December 2016)
Research Articles
Controlled-release fertilizers combined with Pseudomonas fluorescens rhizobacteria inoculum improve growth in Pinus halepensis seedlings
vol. 8, pp. 12-18 (online: 12 May 2014)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword