Stomata morphological traits in two different genotypes of Populus nigra L.
iForest - Biogeosciences and Forestry, Volume 8, Issue 4, Pages 547-551 (2014)
doi: https://doi.org/10.3832/ifor1104-007
Published: Sep 16, 2014 - Copyright © 2014 SISEF
Short Communications
Abstract
Populus nigra L. (black poplar) possesses amphistomatic leaves, with large (giant) and normal sized stomata. The role of giant stomata in leaf development, and the consequences on stomatal density in adult leaves remains elusive. This paper describes the characteristics of ordinary and giant stomata in leaves of two black poplar genotypes (58-861 with large leaves from northern Italy, and Poli with small leaves from southern Italy). Stomatal traits in both genotypes were studied using light microscopy on mature leaf adaxial and abaxial epidermal impressions. Moreover, scanning electron microscopy was applied to study giant and normal stomata in early, young, and mature leaves. Leaf abaxial surfaces in the two genotypes revealed variable sizes and patterns of stomata related to differences in intrinsic water use efficiency (Wi). These observations provided evidence of different stomatal types in mature black poplar leaves, and new information regarding the presence and potential role of giant stomata in black poplar leaves.
Keywords
Introduction
Giant stomata (GS), as the name implies, are distinct from normal-sized stomata primarily due to size. In a treatise on the anatomy of dicotyledons, Metcalfe & Chalk ([16]) described large stomata of approximately 40 µm in relatively few families. Shiraishi et al. ([27]) reported structures in Satsuma Mandarin (Citrus unshiu Marc) similar to large-sized stomata, surrounded by a number of normal-sized stomata on juvenile leaves immediately after full leaf expansion. The giant type stomata observed in C. unshiu were consistent with GS examined and characterized by Sitholey & Pandey ([28]) and Stace ([29]) in different taxa.
Electron microscopy studies on subfossil Holocene Salix herbacea L. (Salicaceae) samples in Sweden suggested the presence of GS ([25]). Finally, Pautov ([18]) reported an eterostomatal organization in Populus tremula, with three stomatal types, i.e., paracitic, laterocitic, and intermediate anamo-paracytic.
Poplars (Populus spp.) are the fastest growing tree in temperate latitudes in Europe and North America ([15]) when raised under short rotation intensive culture conditions. Poplar is considered a model system for plant biology. P. tricocarpa is the first tree genome to be fully sequenced, and is now of high quality and relatively contiguous. Therefore, the genus offers many possibilities to study questions not easily addressed by genera considered model plant systems, e.g., Arabidopsis thaliana ([13]). Stomatal traits have been widely studied in Populus, as these traits are a useful criterion for clone discrimination in the genus. In particular, a series of clones have been explored that confirm general poplar micromorphologic features, including amphistomatic leaves, with evidence some stomatal traits change depending on environmental conditions ([1]), and in response to growing conditions ([33], [11], [8], [12]). For example, in P. trichocarpa stomata were not found on the adaxial leaf surface when grown under field conditions, however low stomatal density was observed on the adaxial surface under glasshouse conditions ([5], [21]).
Despite frequent studies on stomata in poplar ([23], [5], [1], [19], [6], [32]), reports of giant stomata in Populus remain equivocal.
Recently, interest in several poplar morphological and physiological traits has increased, with the aim of evaluating new breeding strategies for industrial applications ([14], [26]). Additional knowledge of poplar stomata might support industrial objectives, since stomatal density has been demontrated to affect biomass production in different poplar clones ([1]).
Materials and Methods
Plant material and growth conditions
Observations were conducted using homogeneous 25 cm long woody stem cuttings obtained from two different P. nigra genotypes, 58-861 and Poli. The 58-861 genotype was the maternal parent from Val Cenischia (Torino, northern Italy - 597 m a.s.l., 45° 09′ N, 07° 01′ E), and Poli was obtained from Policoro (Matera, southern Italy - 7 m a.s.l., 40° 09′ N, 16° 41′ E).
Full-sib families of both genotypes were planted in April 2008 in an open field at Azienda Didattico Sperimentale “Nello Lupori” in Viterbo (Italy, 42º 25′ N, 12º 05′ E - 309 m a.s.l.). The 0.1230 ha experimental site, considered a short rotation coppice, had alternating inter-row distances of 2 m and 0.75 m between plants in a single row. Soil was composed of sand (57.5%), lime (34.6%), and clay (7.9%), with a pH of 7.1. A randomized complete block design (five blocks) was arranged; each block included a group of 160 F1 clones, and two pairs of parental clones (two Poli and two 58-861 clones). A total number of 820 plants were cultivated in the entire plantation (frequency of 6666 plants ha-1). Due to edge effects, the two poplar rows on the outermost border were not included in the experimental design. The plantation was irrigated shortly after planting, and a third class herbicide with weed control measures was applied to obtain optimal field establishment. Average daily temperature and rainfall in the 2008 growing season were respectively 22.89 °C and 14.4 mm. Only parentals (10 Poli and 10 58-861 clones) were examined for observations.
WUE measurements
Complete leaf development was examined by observing leaves throughout the entire growing season (June - September 2008) in 30-day intervals from different leaf generations. Selected leaves marked by small ribbons around the petiole were observed daily, beginning with a young (~4.8 cm in Poli and ~5.6 in 58-861) to mature totally expanded leaf condition, following the Plastochron Index method ([10]). The same leaves were analyzed to obtain physiological measurements (assimilation and stomatal conductance) in the hours before dawn. Excised leaves were placed in water filled test tubes to avoid cavitation, protected in a portable freezer, and transported to the laboratory. A LI-6400 photosynthesis system (LICOR, Nebraska, USA) was programmed to register gas exchange at 380 ppm relative to a CO2 reference value. Intrinsic water use efficiency (Wi) was determined as the ratio between assimilation (A), expressed in µmol CO2 m-2 s-1 and stomatal conductance (gs), expressed in mol H2O m-2 s-1.
Statistical analyses
Data management and statistical analyses were performed with SYSTAT® 12 (Systat software, Chicago, IL) using 20 replicates of poplar genotypes (10 Poli and 10 58-861 clones). A preliminary GS frequency analysis revealed a Poisson-shaped, zero-bounded distribution of counting data (x), which were therefore transformed as √x.
General Linear Model (GLM) was applied to compare different generations of leaves because of their small sample size and non-normal distribution. Differences among mean values were tested by post-hoc analyses using the Bonferroni’s test (α = 0.05), which was considered the most appropriate due to the limited number of samples analyzed. Bonferroni methods for multiple comparisons are extended to sequential setting and have shown to attain an approximately 50% reduction in the expected sample size compared with earlier approaches ([7]).
Linear regression was applied to evaluate relationships between intrinsic Wi and GS pore length traits. Statistical analyses were not applied to young leaf attributes, because the sample number was considered insufficient to establish a clear relationship between intrinsic Wi and GS pore length traits.
Light and canning electron microscopy (SEM)
For light microscopy, mature leaves were used to produce impressions, prepared with clear nail polish parallel to the midrib, from the basal to apical position, and on both adaxial and abaxial surfaces. All impressions were fixed on glass slides, and examined under a Leica DM4000B light microscope connected to a Leica DFC420 camera at 200x magnification. Leica Application Suite (LAS) software was used to analyze images. Three sampling areas (apical, medium, and basal; 126 588 μm2 each) were randomly chosen from every slide sample for microscopic observations. Pore length and giant and normal stomata were studied on the abaxial surface of mature leaves. The absence of a cuticular ridge on the adaxial surface impressions resulted in unreliable data for GS statistical analyses on this surface.
To identify qualitative differences in stomata morphology during leaf development, further observations were performed on three categories of leaves: (i) early (~2.3 cm long in Poli and ~2.5 cm long in 58-861); (ii) young (~4.8 cm long in Poli and ~5.6 cm long in 58-861); and (iii) mature (~6.00 cm long in Poli and ~10.00 cm long in 58-861). Each leaf developmental stage from each genotype was used to make impressions prepared with clear nail polish parallel to the midrib for light microscopy.
For SEM, samples from early, young, and mature Poli and 58-861 leaves were preserved in 1:1 H2O:ethyl alcohol at 4 °C. Specimens were dehydrated in an ethanol series, critical-point dried in liquid CO2, and coated with 30 nm of gold. Specimens were subsequently observed under a FEI-Quantas 200 ESEM, at the CISME center of the Università degli Studi di Napoli “Federico II”. Stomatal types were classified according to Carpenter ([3]) and Metcalfe & Chalk ([16]).
Results and iscussion
Observations from light and SEM microscopy
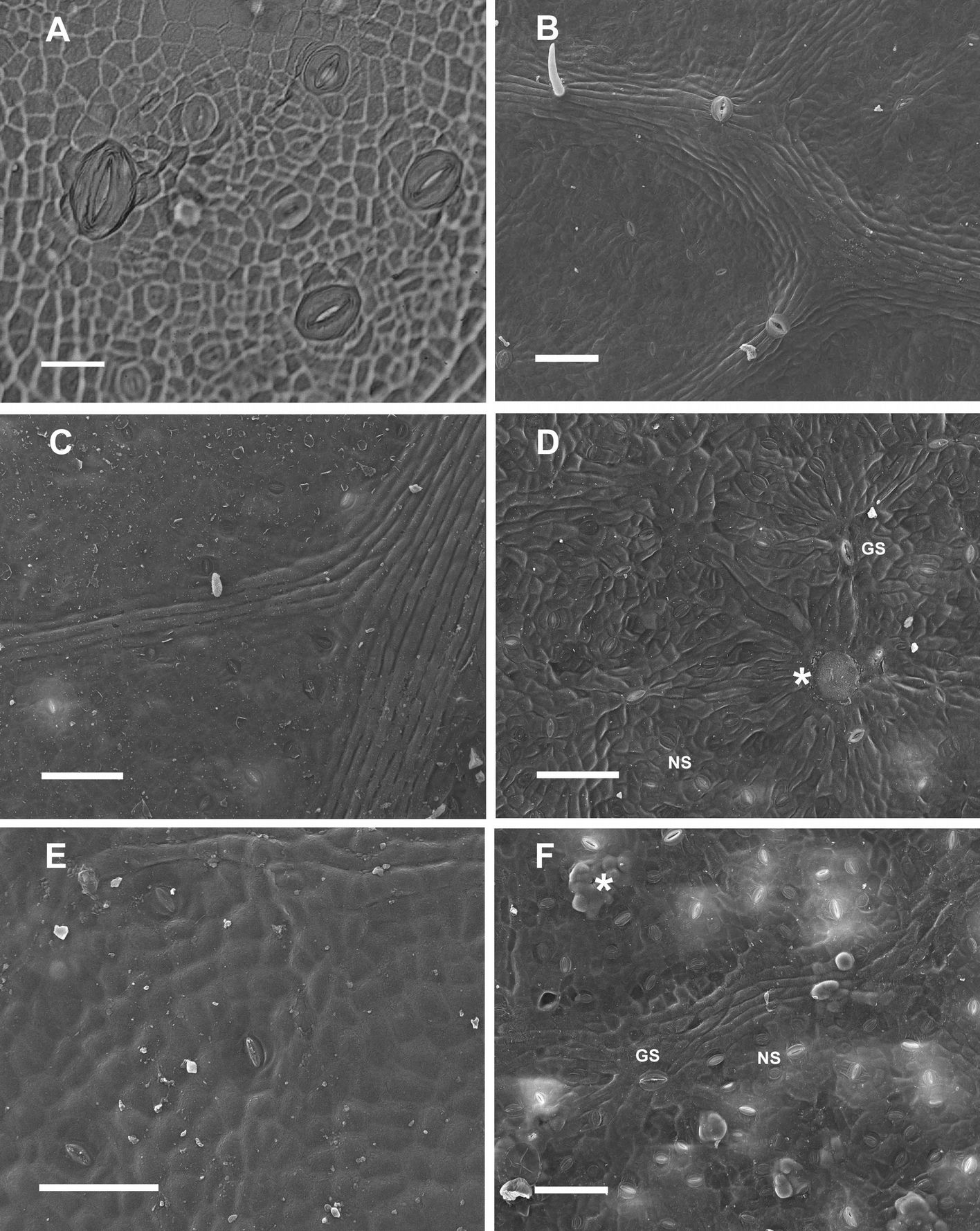
Large stomata, approximately 40 µm in length, with anomocytic type structure were surrounded by normal stomata with paracytic structure and meristemoid cells on the developing lamina in early Poli (Fig. 1A) and 58-861 leaves (data not shown). Trichomes and presumed water stomata (~50 µm long) were observed on adaxial surface venation in both genotypes; however only genotype Poli is reported here (Fig. 1B). In mature P. nigra leaves, GS with anomocytic structure were surrounded by normal stomata 20 to 30 µm long with paracytic structure (Fig. 1C, F). Initiation of normal stomata might continue on adaxial and abaxial surfaces, until the leaf has reached approximately 60% of its final size.
Fig. 1 - Populus nigra L. (A-D): genotype Poli; (E-F); genotype 58-861. (A): Light image from nail polish impression of the adaxial surface of early leaf showing developing stomata, bar = 20 µm. (B): SEM image of the adaxial surface of a young leaf showing trichomes and water stomata, bar = 100 µm. (C): SEM image of the adaxial surface of a mature leaf, bar = 100 µm. (D): SEM image of the abaxial surface of a mature leaf showing anomocytic giant stomata (GS), GS covered by wax (asterisk), paracytic normal stomata (NS), bar = 100 µm. (E): SEM image of the adaxial surface of a mature leaf, bar = 100 µm. (F): SEM image of the abaxial surface of a mature leaf showing GS frequently covered by wax plugs, bar = 100 µm.
A progressive decrease in stomata length (from ~40 µm in GS to ~20 µm in normal stomata) was observed in subsequent divisions, with a concomitant transition from anomocytic to paracytic structure. The sequence of events in stomatal development and pattern formation during the early stages of leaf development observed in P. nigra genotypes were similar to developmental stages demonstrated in A. thaliana ([30], [2], [9]), and in early divergent angiosperms ([24]). The distinct leaf stomatal pattern likely arose from GS that developed early in leaf ontogenesis, forming anomocytic stomata in primary lineages, and corresponding smaller stomata with paracytic structures in satellite lineages ([30], [4]).
In mature genotype Poli leaves, the wax cover was visible, and GS were occasionally covered by wax (Fig. 1C, D). In contrast, wax plugs frequently occluded GS in mature 58-861 leaves (Fig. 1E, F).
Mature leaves during growing season (light microscopy)
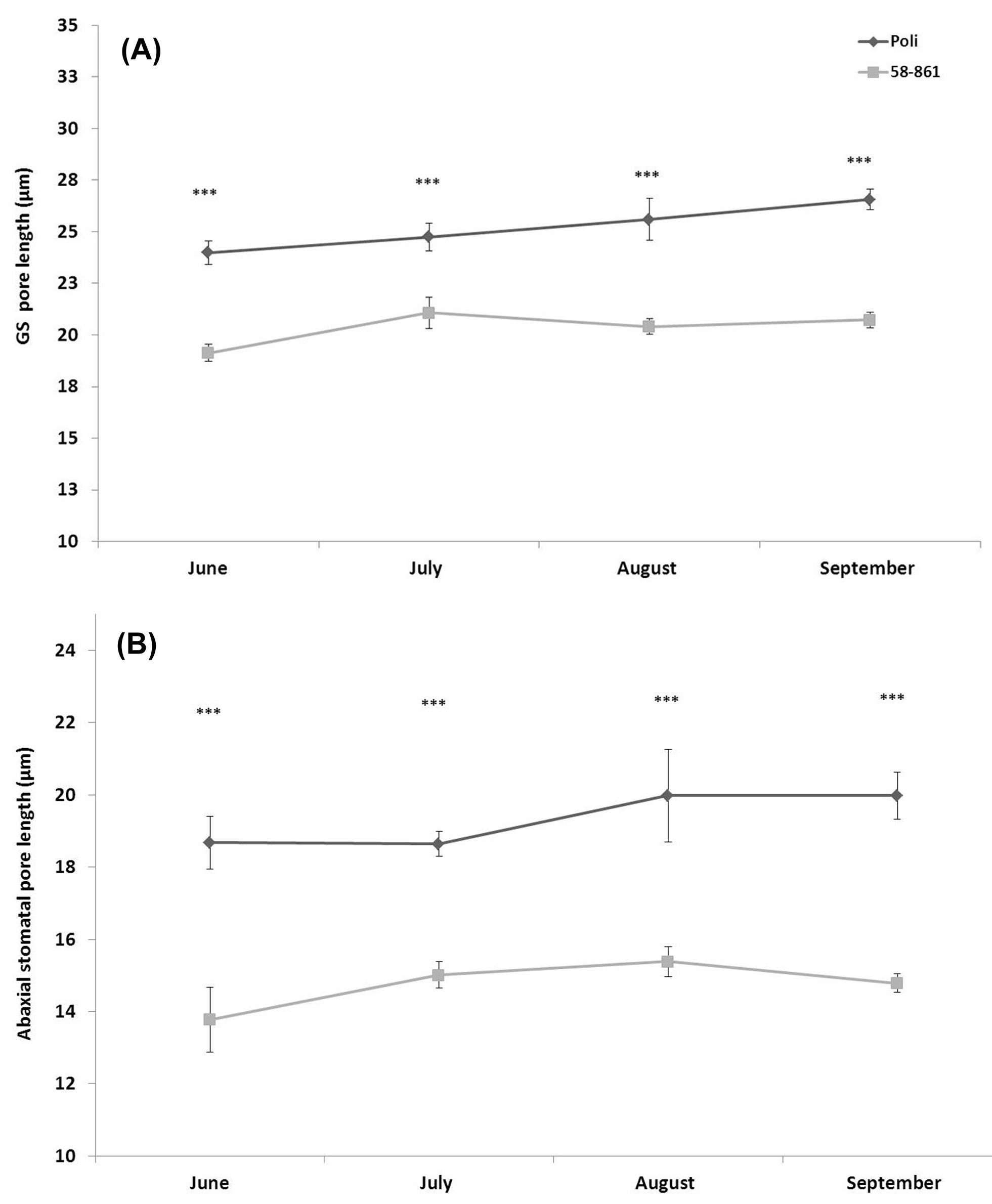
Results showed Poli compared with the genotype 58-861 generally exhibited longer GS (Fig. 2A); these length differences between genotypes were also maintained in ordinary stomata, and observed throughout the growing season (Fig. 2B).
Fig. 2 - Pore length of stomata on the abaxial surface in a mature leaf. (A): Giant stomata; (B): ordinary stomata. The number of leaves/replicates used for each poplar clone was 20. (***): P ≤ 0.001.
However, significant differences were not detected in GS number and frequency (= number of GS/total number of stomata) in the three leaf sections examined (apical, medium, basal) from both P. nigra genotypes (Tab. 1).
Tab. 1 - Number and frequency (= nGS / total number of stomata) of GS in different leaf sections and in both genotypes on the abaxial surfaces of mature leaves. The number of leaves/replicates used for each poplar clone was 20. Frequencies were transformed as √x. (n.s.): not significant.
| Genotype | Parameter | GS counts | GS frequency | ||||
|---|---|---|---|---|---|---|---|
| Apical | Medium | Basal | Apical | Medium | Basal | ||
| Poli | Mean | 1.29 | 1.00 | 1.05 | 0.08 | 0.06 | 0.07 |
| Std. Err. | 0.18 | 0.14 | 0.19 | 0.01 | 0.01 | 0.01 | |
| 58-861 | Mean | 1.45 | 1.20 | 1.10 | 0.08 | 0.06 | 0.06 |
| Std. Err. | 0.15 | 0.19 | 0.16 | 0.01 | 0.01 | 0.01 | |
| - | p-value | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
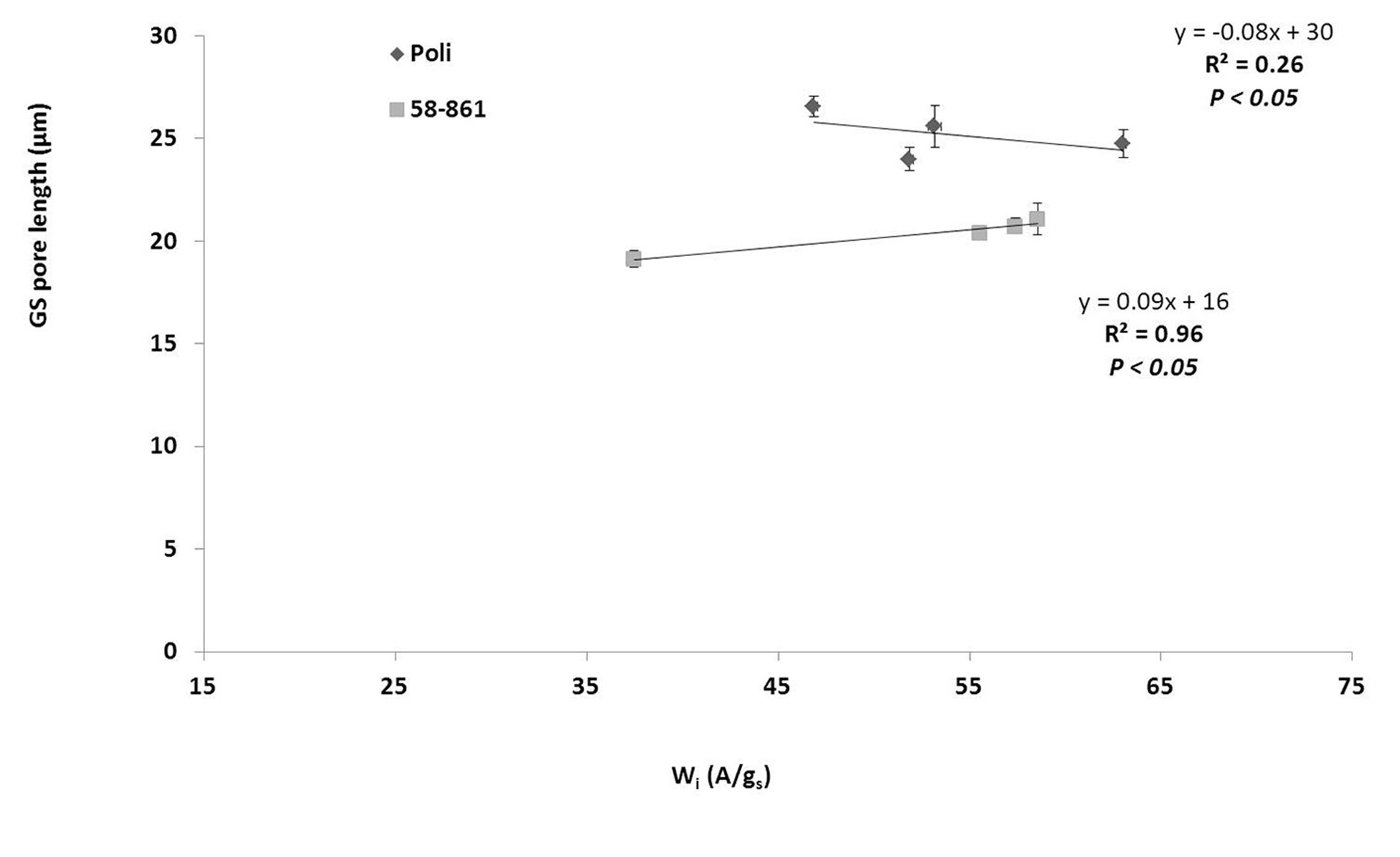
Different sized stomata (~40 to ~20 µm) were present on the adaxial and abaxial leaf sides. However, due to the absence of cuticular ridges, stomata were typically associated with GS on impressions obtained from adaxial surfaces. Therefore, statistical analyses would be unreliable if applied to GS on the adaxial surfaces. However, regression analysis performed on abaxial surfaces showed a significant relationship between GS size and Wi (assimilation / stomatal conductance), with high prediction accuracy in 58-861 (R2 = 0.96) but not in Poli (R2 = 0.26 - Fig. 3).
Fig. 3 - Relationships between mean size of giant stomata on abaxial surface and intrinsic WUE (Wi = A/gs) during growing season; mean values are referred to each month of the season. (A): assimilation (µmol CO2 m-2 s-1); (gs): stomatal conductance (mol H2O m-2 s-1).
Cocozza et al. ([6]) characterized the northern Italy genotype 58-861 as more water efficient and drought sensitive, with larger leaves compared with Poli, and the current study confirmed these data (Fig. 3). Previous studies on the same genotypes focused on stomatal densities and length, but did not examine the distinction between ordinary and large sized stomata ([22], [6]).
Regier et al. ([22]) reported a significant reduction in stomata size in genotype 58-861, but only on the abaxial leaf surface when water uptake changed, with a subsequent reduction in well-watered status. In addition, the current data suggested GS size in Poli was larger than in genotype 58-861, and might explain the increased Wi verified in the northern Italy poplar clone. Furthermore, GS size could be associated with the stomatal opening and closing mechanism (together with density), which cumulatively provides total leaf stomatal conductance, and more efficient water use in genotype 58-861 despite smaller stomata.
Observed stomatal characteristics did not follow seasonal changes, suggesting the attributes were favored by selection, and represent adaptive-genetic traits. This hypothesis emphasizes the role of GS as an adaptive and functional trait distinguishing different genotypes, and might serve to clarify certain physiological attributes, including WUE in P. nigra.
Warren & Adams ([31]) indicated leaf anatomy was the primary factor to explain WUE by measuring stable carbon isotopes. Consequently, the role of GS in leaf physiology should take a primary role in developing a greater understanding of processes, including WUE and plasticity, or seasonal acclimation ([17]). In particular, drought tolerance, as reported in the larger-sized and more water use-efficient genotype 58-861, can characterize its physiology through several adaptive traits of stomata (density, pore length, structure).
A more developed wax covering on the abaxial leaf surface of genotype 58-861 represents another distinctive trait of the northern genotype compared to the southern Poli genotype. Pearce et al. ([19]) described an additional function for wax accumulation on leaf adaxial surfaces in Populus, particularly poplars adapted to warmer regions (i.e., P. angustifolia). The abaxial epidermal wax covering in mature leaves of genotype Poli was reported by Pearce et al. ([19]). Surface roughness, caused by ridges, trichomes, or both is the chief factor limiting wetting properties of some species, such as poplar. Superficial wax plays a dominant role in affecting wetting of other taxa ([20]). The wax covering in the southern genotype Poli, and wax plugs in 58-861 might be responsible for additional differences in drought tolerance between these two genotypes, which originated from distinct environments.
Finally, stomatal analysis in the Salicaceae and closely related groups could be useful in clarifying specific features of stomatal patterns, presence of large sized stomata (GS), and the possible phylogenetic origins of normal stomata relative to GS, i.e., are GS the precursors to normal stomata. The prevailing view is that stomata arose once in evolutionary history, and subsequently radiated throughout land plants. Oversized stomata could be a significant factor in land plant evolution, with implications in physiological pathways.
An increased understanding of giant stomata can be useful to industry considering the importance of stomata in a perspective of biomass production. In fact, poplar clones with high abaxial stomatal density (where giant stomata were found) showed an in-creased biomass production ([1]).
Acknowledgements
The authors want to thank Dr. Francesco Fabbrini and all the researchers of DIBAF Department at the University of Tuscia for their helpfulness. We are grateful to two anonymous reviewers for their helpful comments on an earlier version of the manuscript. This work was partially supported by the European Project NOVELTREE (FP7-211868).
References
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Istituto di Biologia Agroambientale e Forestale (IBAF), Consiglio Nazionale delle Ricerche (CNR), Porano, TR (Italy)
Department for Innovation in Biological, Agro-food and Forest systems (DIBAF), Università della Tuscia, Viterbo (Italy)
Department of Plant and Microbial Biology, North Carolina State University, Box 7612, 27692-7612 Raleigh, NC (USA)
Dipartimento di Biologia, Orto Botanico, Università degli Studi Napoli “Federico II”, Naples (Italy)
Corresponding author
Paper Info
Citation
Russo G, De Angelis P, Mickle JE, Lumaga Barone MR (2014). Stomata morphological traits in two different genotypes of Populus nigra L.. iForest 8: 547-551. - doi: 10.3832/ifor1104-007
Academic Editor
Roberto Tognetti
Paper history
Received: Aug 13, 2013
Accepted: May 23, 2014
First online: Sep 16, 2014
Publication Date: Aug 02, 2015
Publication Time: 3.87 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2014
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 57336
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 48715
Abstract Page Views: 2824
PDF Downloads: 4298
Citation/Reference Downloads: 26
XML Downloads: 1473
Web Metrics
Days since publication: 4078
Overall contacts: 57336
Avg. contacts per week: 98.42
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2015): 5
Average cites per year: 0.45
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Energy production of poplar clones and their energy use efficiency
vol. 7, pp. 150-155 (online: 23 January 2014)
Research Articles
Soil nutrient status, nutrient return and retranslocation in poplar species and clones in northern Iran
vol. 6, pp. 336-341 (online: 29 August 2013)
Research Articles
Revealing the physiological basis of forester’s choice of poplar clones (Populus spp.)
vol. 17, pp. 156-164 (online: 14 June 2024)
Research Articles
Two Populus deltoides W.Bartram ex Marshall clones cope differentially with sodium salinity stress
vol. 18, pp. 259-266 (online: 10 October 2025)
Research Articles
Growth performance and nitrogen use efficiency of two Populus hybrid clones (P. nigra × P. maximowiczii and P. trichocarpa × P. maximowiczii) in relation to soil depth in a young plantation
vol. 9, pp. 847-854 (online: 22 September 2016)
Research Articles
Tree aging does not affect the ranking for water use efficiency recorded from δ13C in three Populus deltoides × P. nigra genotypes
vol. 12, pp. 272-278 (online: 21 May 2019)
Research Articles
Climate-wise models of biomass productivity for hybrid poplar clones in Europe
vol. 16, pp. 188-194 (online: 30 June 2023)
Research Articles
A physiological approach for pre-selection of Eucalyptus clones resistant to drought
vol. 13, pp. 16-23 (online: 15 January 2020)
Research Articles
Variations in the performance of hybrid poplars subjected to the inoculation of a microbial consortium and water restriction
vol. 16, pp. 352-360 (online: 13 December 2023)
Research Articles
Assessment of cadmium tolerance and phytoextraction ability in young Populus deltoides L. and Populus × euramericana plants through morpho-anatomical and physiological responses to growth in cadmium enriched soil
vol. 10, pp. 635-644 (online: 01 June 2017)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword