Growth performance and nitrogen use efficiency of two Populus hybrid clones (P. nigra × P. maximowiczii and P. trichocarpa × P. maximowiczii) in relation to soil depth in a young plantation
iForest - Biogeosciences and Forestry, Volume 9, Issue 6, Pages 847-854 (2016)
doi: https://doi.org/10.3832/ifor2016-009
Published: Sep 22, 2016 - Copyright © 2016 SISEF
Research Articles
Abstract
It is a challenge to produce woody crops on marginal land. The goal of this study was to examine growth responses and nitrogen use efficiency of different poplar species on shallow soil. Typical biomass poplar clones of Max1 (P. nigra × P. maximowiczii) and H275 (P. trichocarpa × P. maximowiczii) were planted on a marginal site where a gradient in soil depth was present. The growth, biomass production, and nitrogen uptake rate as well as nitrogen use efficiency of Max1 and H275 were determined for three consecutive years. Both poplar clones showed decreased growth and biomass production in the shallow soil. Max1 showed better adaptation to shallow soil with higher survival rate and more biomass production than H275. Max1 had lower nitrogen use efficiency on shallow soil than H275. The results suggest that higher nitrogen uptake of poplar species might be an important adaptation to maintain productivity under unfavorable soil conditions.
Keywords
Introduction
Woody biomass is considered as a sustainable alternative source of energy for fossil fuels ([34]). Short rotation woody crops such as poplar trees (Populus spp.) are well suited for woody biomass production because they are highly productive and can be managed using agronomic techniques ([18]). To avoid competition for fertile land with food production, poplar plantations are expected to be established on marginal land which is less suitable for agriculture because of limited water and nutrient availability ([41]). Since the productivity of a poplar plantation depends on the proper selection of genotypes ([48]), research is needed to identify poplar cultivars with high survival rates, high biomass productivity and high disease resistance for cultivation on marginal soil ([35]).
Shallow soil with limited rooting depth, low drainage and high stone content are typical features of marginal land ([17]). Rooting depth is an important determinant for the successful establishment and production of woody biomass ([7]). N storage and cycling are vital processes for growth, adaptation and productivity of poplar trees ([29]). N storage and remobilization are particularly important to meet the N demand of forest trees for continuous growth ([38]). Poplar species differ in the way they take up and assimilate N ([25], [14]), which may impact their competitive ability for soil nutrients and resistance to environmental stresses ([8], [26], [31]). Several studies have focused on identifying and quantifying the best management practices to successfully establish hybrid poplar plantations under different nitrogen regimes ([21], [11]). However, there is little information on how different poplar species cope with shallow soil and whether shallow soil affects nitrogen use efficiency.
In this study, two different poplar clones, Max1 (P. nigra × P. maximowiczii) and H275 (P. trichocarpa × P. maximowiczii), were selected to assess the productivity and nitrogen use efficiency on shallow soil. Max1 and H275 are both commercial poplar clones often used in poplar plantations ([6], [32]). Here we expected that both poplar clones showed decreased growth and biomass production on shallow than on deep soil. Since Max1 was found to be well growing under drought conditions ([40]), we hypothesized that (i) clonal differences in adaptation to shallow soil do exist, and that (ii) the clone with higher nitrogen uptake has a greater productivity on shallow soil compared to the clone with lower nitrogen uptake.
To test these hypotheses, a plantation with Max1 and H275 was established on shallow and deep soil, and the performance of both clones was studied for 3 years.
Material and methods
Plant material and site description
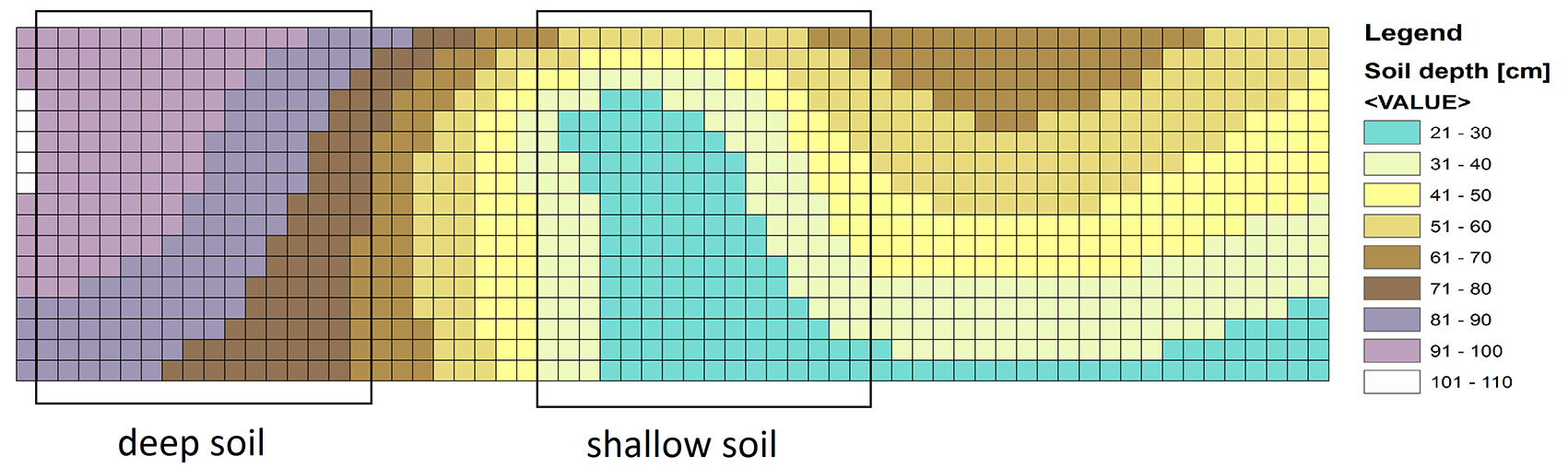
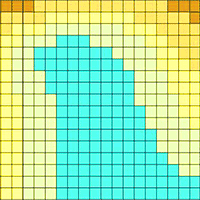
Cuttings of fast growing hybrid poplars Max 1 (P. nigra × P. maximowiczii) and H275 (P. trichocarpa × P. maximowiczii) 21cm in length, were planted at the end of March 2011 in a block design (see Fig. S1 in Supplementary material) on a previously unmanaged grassland (51.560 N, 9.956 E) in Göttingen, Germany. Cuttings were planted by inserting the whole length of the cutting into the soil (1 cm underneath the ground surface) with 0.75 × 0.75 m spacing. The plantation was watered just after planting and in August of 2011. Weed control was done by cutting weeds at the end of August in 2011. A soil survey was carried out by drilling a Pürckhauer soil driller (Eijkelkamp, Giesbeek, Netherlands) in a regular grid of 5 × 5 m into the soil and classifying each soil parameter at each point according to the German soil classification system ([1]). Especially, the height of the Cv-horizon, i.e., the transition zone between the soil solum and the weathered rock with high content of coarse rock fragments was addressed. Soil depth data were digitized and interpolated in a GIS-system (Arc-Info® version 5.01, Environmental Systems Research Institute, Redlands, California - Fig. 1). The N concentrations in the upper layer (0-60 cm) of the deep and shallow soils were similar (0.15 ± 0.04 % g N g-1 dry soil).
Fig. 1 - Map of soil depth in the poplar plantation, determined according to the Ad-Hoc-Arbeitsgruppe ([1]), and digitized and interpolated in an ARC/INFO GIS system.
During the years 2011 to 2014, the mean temperature was 14.9 ± 0.3 °C in Göttingen, total precipitation was 295.6 ± 35.6 mm and sunshine duration was 968 ± 61.40 h in the growth period from April to August ([47]). The precipitation in spring (April to Mai) during the time of bud break and the start of the main growth phase were 69 mm in 2012, 160 mm in 2013, and 109 mm in 2014.
Growth and biomass determination
The heights and basal diameters of the shoots were measured at end of the growing season in the year of 2011 and 2012 and before the growing season in 2014 using a meter stick and a digital caliper, respectively.
Seven plants of each species in the deep and shallow soil of the plantation (Fig. 1) were randomly selected for each harvest. Harvests were carried out when the terminal buds of main shoots of poplars had been formed in the years 2012, 2013 and 2014. At harvest, fresh biomass of plant tissues (roots, stems and leaves) was measured. Stems, leaves and roots were oven-dried at 60 °C for 3 weeks and weighed to determine dry biomass.
Leaf area determination
Three young and three old leaves were selected for each plant at each harvest to determine the leaf area. The leaves were weighed and scanned together with a ruler, and used to determine the leaf area with the image analysis software Image J® (NIH, Bethesda, Maryland, USA). The total leaf area was calculated as: total leaf area = leaf area of 6 selected leaves × fresh weight of total leaves / fresh weight of 6 selected leaves. Specific leaf area (SLA) was calculated as: leaf area / leaf dry mass (m2 g-1).
Nitrogen analysis
The leaves used for leaf area determination were pooled and milled. The whole stem (2012, 2013) was cut into small pieces and milled. Stem segments from the bottom of the main stem were milled for plants in 2014. One root including fine roots from each plant was milled. The plant tissues and soil samples were milled to fine powders (MM2 Retsch, Hannover, Germany). About 1 mg (0.7-0.9 mg) of the milled samples were weighed (Sartorius Supermicro S4®, Göttingen, Germany) into tin capsules (Hekatech, Wegberg, Germany). Nitrogen concentrations of the samples were determined using the Elemental Analyzer EA1108® (Carlo Erba Strumentazione, Rodano, Italy). Acetanilide (10.36% N - Carlo Erba Strumentazione) was used as the standard.
Nitrogen uptake and nitrogen use efficiency
Because leaves are shed in autumn, the annual N uptake rate was calculated without considering the N content in leaves from the previous year (eqn. 1):
Nitrogen use efficiency (NUE) has been defined as the amount of biomass produced per unit of N taken up from the soil. The amount of stem biomass produced per unit of N taken up from the soil is of interest for the calculation of wood nitrogen use efficiency (WNUE).
To determine the annual changes of nitrogen use efficiency for wood biomass production, wood nitrogen use efficiency was calculated as (eqn. 2):
To determine the annual changes of nitrogen use efficiency for the whole plant biomass production, NUE was calculated as (eqn. 3):
with (eqn. 4):
and (eqn. 5):
Statistical analyses
Data are means ± SE of 5 or 7 individual plants. To determine differences between treatments, the Student’s t-test (α = 0.05) was used. To determine poplar species and soil depth effects, two-way ANOVA was performed and the differences between means were tested using the post-hoc Tukey’s HSD test. All analyses were carried out using the software package Origin Pro® ver. 8 (OriginLab Corporation, Northampton, USA).
Results
Survival, growth and biomass production
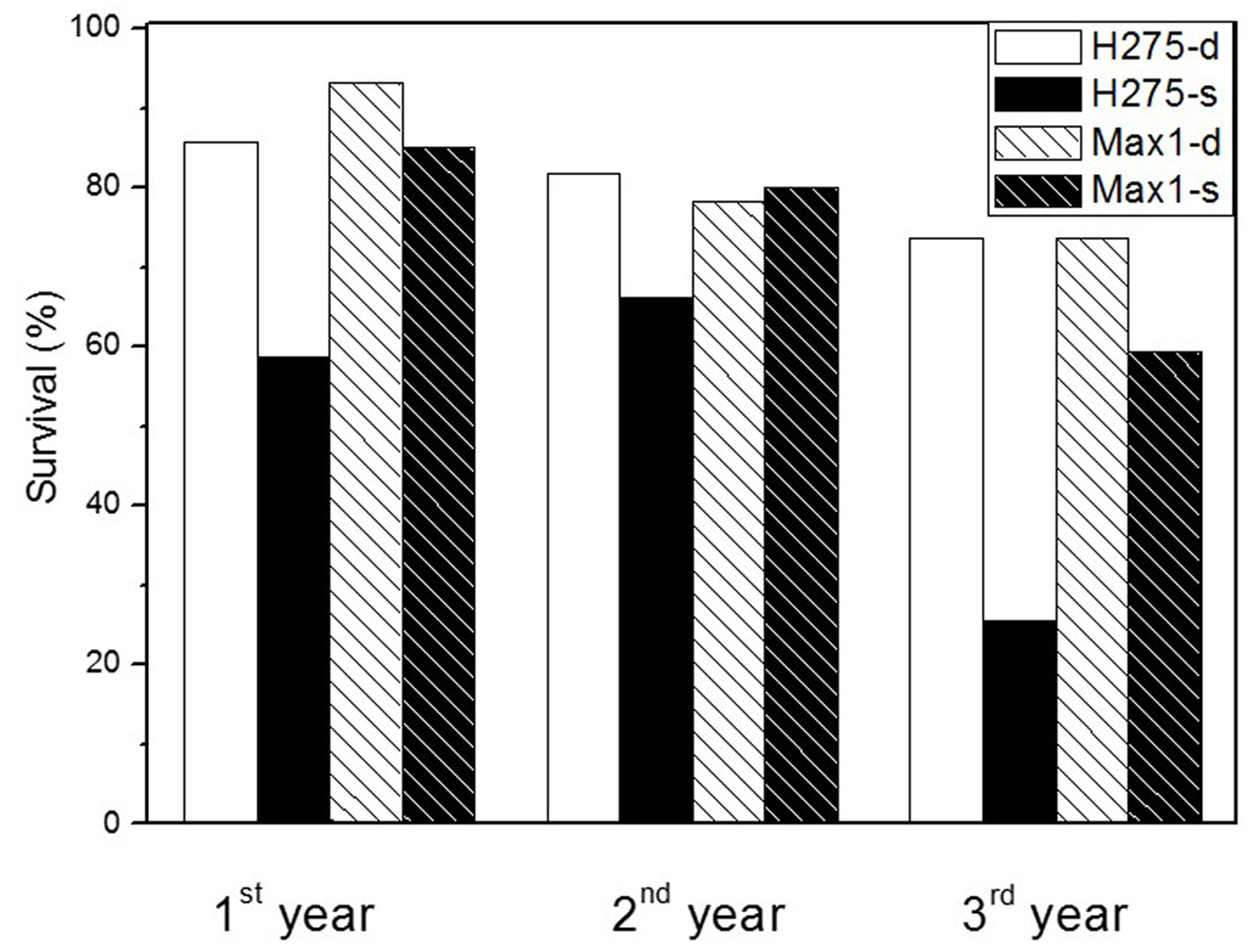
The soil depths were 70-100 cm for the deep and 20-50 cm for the shallow soil conditions, respectively (Fig. 1). Lower survival of H275 and Max1 was found in the plantation on shallow soil than that on deep soil (Fig. 2). H275 showed a lower survival rate than Max1 in all three years on shallow soil (Fig. 2). After 3 years of planting, the same survival (about 74%) was found in the deep soil for both poplar clones, whereas the survival of H275 (26 %) was lower than that of Max1 (60 %) in the shallow soil (Fig. 2).
Fig. 2 - Survival of Max1 and H275 in the deep soil (-d) and shallow soil (-s). Survival (%) was calculated as: number of existing plants / total planted plants × 100 (%), n = 160.
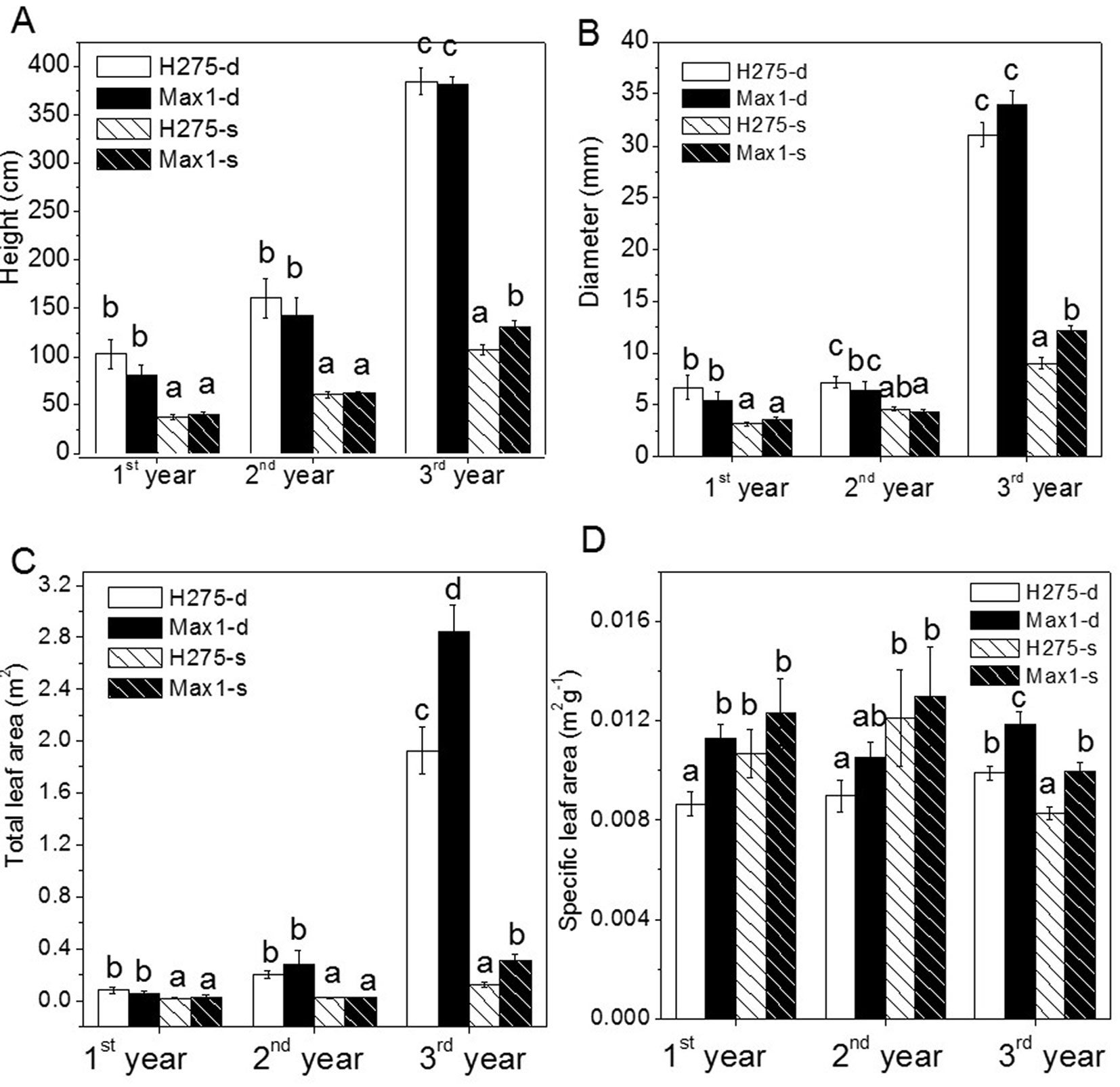
H275 and Max1 showed significant growth decline on shallow soil compared with deep soil (Fig. 3A, Fig. 3B, Fig. 3C). Height and diameter growth of Max1 was similar to that of H275 in first two years (Fig. 3A, Fig. 3B). There were no significant differences in height and diameter of H275 and Max1 on deep soil in year 3. However, the leaf area of Max1 was higher than that of H275 (Fig. 3C). Max1 was significantly taller and thicker in stem on shallow soil as compared with H275 (Fig. 3A, Fig. 3B) in the third year.
Fig. 3 - Height, root collar diameter, total leaf area and specific leaf area of Max1 and H275 on the deep soil (-d) and shallow soil (-s). Data (n=7) are mean ± SE. Different letters above the bars indicate significant differences after Student’s t-test (p < 0.05).
SLA of three-year-old poplar Max1 and H275 was significantly lower for plants on shallow soil than for plants on deep soil (p < 0.01 - Fig. 3D). SLA of H275 was significantly lower than that of Max1 after 3 years of growth (Fig. 3D).
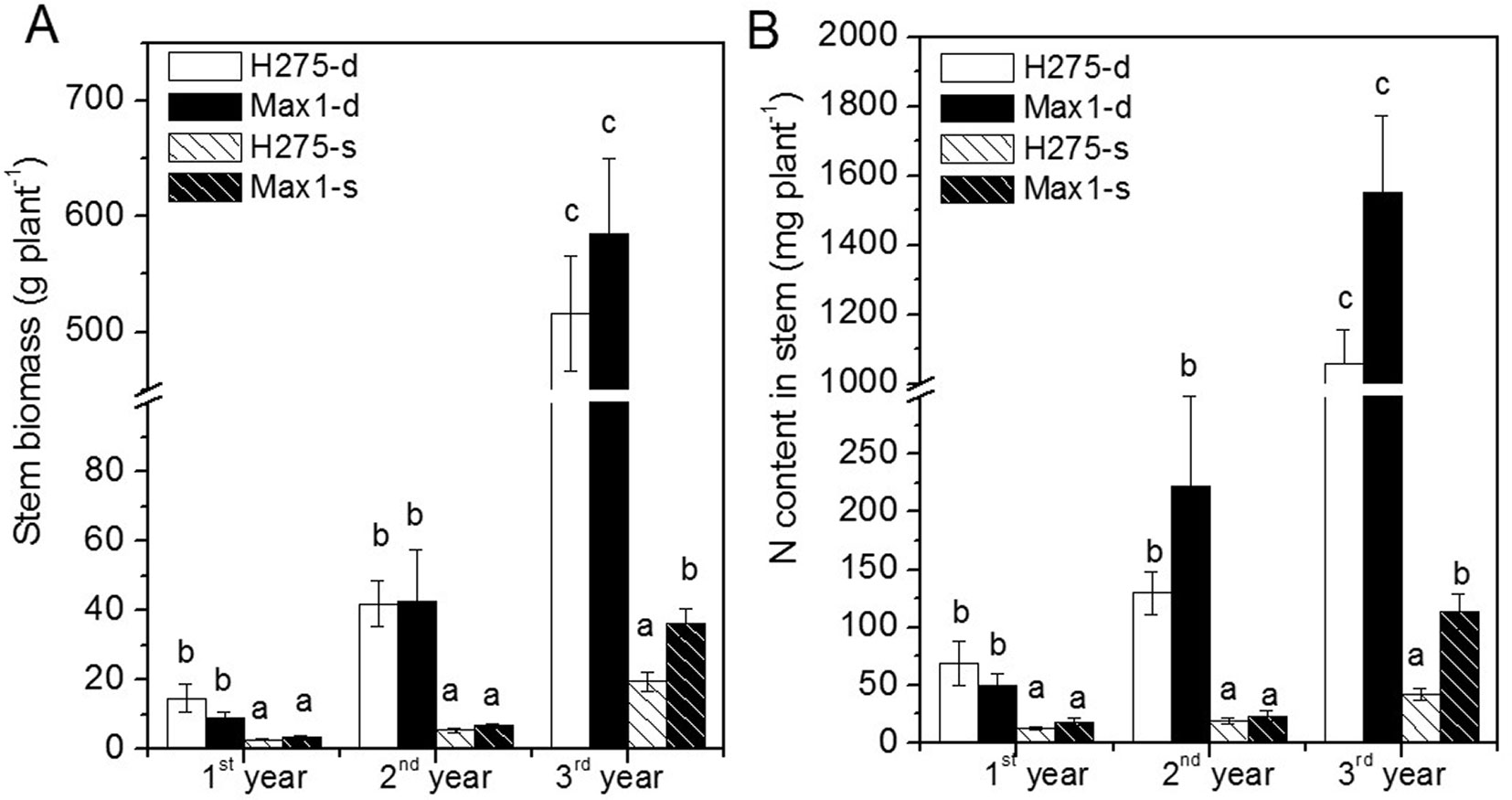
Soil depth was the main factor influencing biomass production of both clones in each year (p < 0.01 - Fig. 4A, Tab. 1). H275 and Max1 produced significant lower stem and total biomass on shallow soil compared with deep soil (Fig. 4A, Tab. 1). Initially, stem and total biomass production of H275 and Max1 were not significantly different on the same soil conditions. In the third year, Max1 produced significantly more stem and total biomass than H275 on shallow soil (Fig. 4A, Tab. 1). H275 and Max1 had significant higher root:shoot ratio on shallow soil compared with deep soil in second year and third year. H275 had lower root:shoot ratio than Max1 on deep soil; root:shoot ratio was significantly different between H275 and Max1 in the second year (Tab. 1).
Fig. 4 - Stem biomass and stem nitrogen content of Max1 and H275 on deep soil (-d) and shallow soil (-s). Data are means ± SE (n=5). Different letters above the bars indicate significant differences between the means after Student’s t-test (p < 0.05).
Tab. 1 - Biomass and shoot:root ratio (g year-1) of Max1 and H275 on deep soil (-d) and shallow soil (-s). (Root:shoot ratio): biomass of roots / biomass of stems and leaves. Data are means ± SE (n=7). Different letters in columns indicate significant differences after Student’s t-test (p < 0.05). P-values of the two-way ANOVA with factor species (Pspecies), soil depth (Psoil) and the interactions of species and soil depth (Pspecies × soil) are given.
| Parameter | Clone / Effect | 1st year | 2nd year | 3rd year |
|---|---|---|---|---|
| Biomass - whole plant | H275-d | 25.58 ± 7.28 b | 66.69 ± 9.83 b | 750.12 ± 68.50 c |
| Max1-d | 14.95 ± 3.33 b | 72.53 ± 26.39 b | 882.53 ± 90.83 c | |
| H275-s | 4.97 ± 0.44 a | 7.64 ± 0.80 a | 36.52 ± 4.80 a | |
| Max1-s | 5.88 ± 1.04 a | 9.08 ± 0.72 a | 73.76 ± 9.36 b | |
| Pspecies | 0.25 | 0.83 | 0.23 | |
| Psoil | < 0.01 | < 0.01 | < 0.01 | |
| Pspecies × soil | 0.17 | 0.90 | 0.49 | |
| Root:shoot ratio | H275-d | 0.06 ± 0.01 a | 0.06 ± 0.01 a | 0.06 ± 0.01 a |
| Max1-d | 0.08 ± 0.01 b | 0.09 ± 0.00 b | 0.07 ± 0.00 ab | |
| H275-s | 0.12 ± 0.02 b | 0.14 ± 0.02 c | 0.07 ± 0.01 bc | |
| Max1-s | 0.08 ± 0.02 ab | 0.16 ± 0.01 c | 0.10 ± 0.01 c | |
| Pspecies | 0.72 | 0.04 | 0.19 | |
| Psoil | 0.21 | < 0.01 | 0.03 | |
| Pspecies × soil | 0.07 | 0.19 | 0.73 |
N concentration, N uptake and N use efficiency
Poplars on deep soil exhibited significantly higher leaf N concentrations than those of on shallow soil (Tab. 2). Leaf and stem N concentrations of Max1 were significantly higher than those of H275 (Tab. 2). There were no significant differences in N concentrations of roots between Max1 and H275 (Tab. 2). N contents of poplar stems were lower on shallow soil compared with those on deep soil (Fig. 4B). There were no significant differences between stem N contents of H275 and Max1 on the same soil conditions in the first two years. In the third year, stem N content of Max1 was higher than that of H275 on shallow soil (Fig. 4B).
Tab. 2 - Nitrogen concentration in stems, leaves and roots of Max1 and H275 on deep soil (-d) and shallow soil (-s). (*): N stem % in the basal stem is shown for the 3rd year. Data (n=5) are means ± SE (g year-1). Different letters in columns indicate significant differences after Student’s t-test (p< 0.05). P-values of two-way ANOVA test with factor species (Pspecies), soil depth (Psoil) and the interactions of species and soil depth (Pspecies × soil) are given.
| Parameter | Clone / Effect | 1st year | 2nd year | 3rd year |
|---|---|---|---|---|
| N leaf % | H275-d | 1.29 ± 0.12 b | 1.07 ± 0.02 a | 1.71 ± 0.07 c |
| Max1-d | 1.45 ± 0.07 bc | 1.35 ± 0.08 b | 1.78 ± 0.06 c | |
| H275-s | 0.96 ± 0.08 a | 1.01 ± 0.04 a | 1.11 ± 0.05 a | |
| Max1-s | 1.17 ± 0.10 a | 1.04 ± 0.02 a | 1.48 ± 0.04 b | |
| Pspecies | 0.06 | 0.01 | < 0.01 | |
| Psoil | < 0.01 | < 0.01 | < 0.01 | |
| Pspecies × soil | 0.82 | 0.04 | 0.11 | |
| N stem %* | H275-d | 0.46 ± 0.02 a | 0.32 ± 0.03 a | 0.21 ± 0.01 a |
| Max1-d | 0.60 ± 0.04 b | 0.54 ± 0.03 b | 0.26 ± 0.01 b | |
| H275-s | 0.48 ± 0.04 a | 0.34 ± 0.01 a | 0.22 ± 0.02 ab | |
| Max1-s | 0.56 ± 0.03 b | 0.34 ± 0.04 a | 0.31 ± 0.02 c | |
| Pspecies | < 0.01 | < 0.01 | < 0.01 | |
| Psoil | 0.74 | 0.02 | < 0.01 | |
| Pspecies × soil | 0.41 | < 0.01 | 0.02 | |
| N root % | H275-d | 0.35 ± 0.01 a | 0.37 ± 0.04 ab | 0.28 ± 0.02 a |
| Max1-d | 0.42 ± 0.02 b | 0.36 ± 0.02 b | 0.24 ± 0.03 a | |
| H275-s | 0.42 ± 0.03 b | 0.29 ± 0.02 a | 0.20 ± 0.04 a | |
| Max1-s | 0.47 ± 0.07 b | 0.35 ± 0.03 ab | 0.25 ± 0.03 a | |
| Pspecies | 0.16 | 0.5 | 0.72 | |
| Psoil | 0.14 | 0.19 | 0.43 | |
| Pspecies × soil | 0.77 | 0.37 | 0.35 |
Poplars on deep soil had significantly higher N uptake rate than those of on shallow soil (Tab. 3). There were no differences between clones for N uptake in the 2nd year, but Max1 had significantly higher N uptake rate than H275 in the 3rd year (Tab. 3).
Tab. 3 - Nitrogen uptake rate (g year-1) of Max1 and H275 on deep soil (-d) and shallow soil (-s). Data are means ± SE (n=5). Different letters in columns indicate significant differences after Student’s t-test (p < 0.05). P-values of the two-way ANOVA carried out with factor species (Pspecies), soil depth (Psoil) and the interactions of species and soil depth (Pspecies × soil) are given.
| Year | H275-d | Max1-d | H275-s | Max1-s | P species | P soil | P species × soil |
|---|---|---|---|---|---|---|---|
| 2nd year | 0.31 ± 0.06 b | 0.49 ± 0.22 b | 0.02 ± 0.00 a | 0.03 ± 0.01 a | 0.42 | < 0.01 | 0.44 |
| 3rd year | 4.34 ± 0.46 c | 5.74 ± 0.67 d | 0.19 ± 0.03 a | 0.56 ± 0.10 b | 0.04 | < 0.01 | 0.22 |
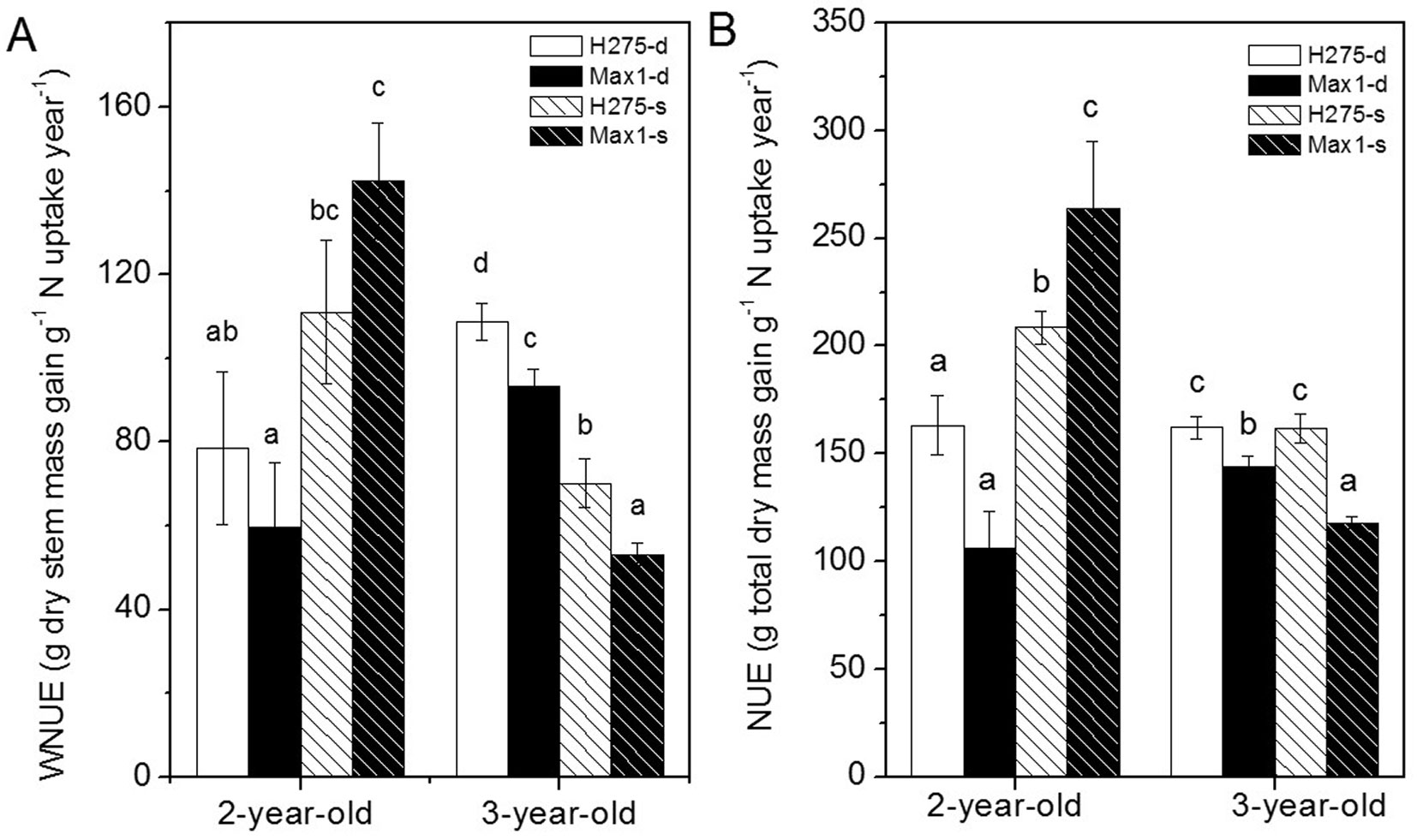
N use efficiency for wood biomass production (WNUE) and for whole plant biomass production (NUE) was lower in H275 and Max1 on the deep soil than in poplars on shallow soil in the 2nd year (Fig. 5A and 5B). However, in the 3rd year, WNUE of both poplar clones on deep soil was higher than on shallow soil (Fig. 5B); NUE was similar for H275 on deep and shallow soil but Max1 had lower NUE on shallow soil than on deep soil (Fig. 5B). No clone differences were found for WNUE and NUE in the second year, but lower nitrogen use efficiency for wood and total biomass production of Max 1 than those of H275 in the third year.
Fig. 5 - Annual nitrogen use efficiency in stem and whole plant of Max1 and H275 on deep soil (-d) and shallow soil (-s). Data (n=5) are means ± SE. Different letters above the bars indicate significant differences after Student’s t-test (p < 0.05).
Discussion
Shallow soil restricts poplar productivity
Root production is an important process driving the acquisition of soil resources and affecting the adaptation of plants to suboptimal soil conditions ([27], [37]). Shallow soils, often occurring in marginal lands, restrict the rooting depth of trees ([9]). In this study, we demonstrated that poplar biomass was remarkably decreased on shallow soil (3- to 6-fold initially and more than 10-fold in the 3rd growth year), indicating that shallow soil drastically hindered both root and shoot formation in poplar plantations. The insufficient moisture storage capacity that usually characterizes shallow soils makes drought stress one of the main threats for poplar plantations ([16]). Indeed, we observed an increase in specific leaf area for poplar clones growing on shallow soil, which may represent an adaptation to drought stress, as thicker leaves may have lower transpiration rates ([24], [15]). Moreover, the root:shoot biomass ratio was higher in plants grown on shallow soils, suggesting that they were affected by water shortage (Tab. 1, Fig. 3). It was notable that in, contrast to the aforementioned relationships, the specific leaf area of poplars on shallow soil was decreased in the 3rd growth year. A possible explanation for this unexpected finding is that trees may regulate water and nutrient balance at the whole-plant level. In the 3rd year, the leaf area of poplars on deep soil was massively increased, while the trees on shallow soil produced only small increments in leaf area compared to the preceding growth phases. Under these conditions, poplars with large total leaf area may also have to cope with water limitations.
In this study, both poplar clones exhibited a decreased survival in the shallow soil area of the plantation. The shallow soil effect was strongest in the third growth year, resulting in the increased mortality of both clones (Fig. 2). The survival of H275 on shallow soil was much lower than that of Max1, especially in the 3rd year after planting (Fig. 2). The difference in stress resistance of hybrid poplars Max1 and H275 may be explained by different origins of their parents, P. maximowiczii,P. trichocarpa and P. nigra. In fact, P. maximowiczii, which is a common parent of both hybrids, is naturally distributed along the Pacific coast of eastern Asia (China, Korea, and Japan - [16]). P. trichocarpa is natively found along rivers and streams of western North America, and may be sensitive to water limitation ([42], [3], [16]). P. nigra is native to Europe and can be found in riparian habitats as well as in arid regions ([43], [19]). P. nigra shows a wide range of adaptive mechanisms in response to drought, which is probably caused by its genomic plasticity ([45]). Poplar hybrids with P. nigra as parent have proven to be more resistant to drought than those having P. trichocarpa as parent ([44]). Accordingly, we found that Max1 (P. nigra × P. maximowiczii) was more tolerant to shallow soil stress than H275, which originated from crossing P. trichocarpa and P. maximowiczii. It is likely that an important factor contributing to the improved performance of Max1 under shallow soil stress was a higher drought adaptability of this clone as compared with H275.
Decreases in wood nitrogen use efficiency as an adaptation to soil constraints
Max1 showed significantly higher N uptake rate than H275 in the 3rd year (Tab. 3). It has been reported that the rate of N uptake of poplars can vary during plant development as well as between poplar species and sites ([28], [36], [30]). N uptake of poplars is adjusted by molecular regulation of nitrate transporters and by N metabolism in response to different environmental conditions ([10], [22], [2]). Furthermore, several studies reported that drought-stress related genes are significantly regulated at the transcription level by N fertilization or starvation ([12], [26]), suggesting that the N metabolism is linked with stress tolerance. This is supported by the finding that poplars overexpressing glutamine synthetase showed higher N uptake rates and higher drought tolerance than wild-type plants ([31]). Therefore, the higher N uptake and N concentrations in leaf and stem tissues of the clone Max1 found in this study (Tab. 2) may also have contributed to its better adaptation to shallow soil compared to H275.
In general, nutrient use efficiency in plants decrease with increasing soil fertility ([46], [5]). Our results of the second year agree with this finding , when a higher WNUE was observed for poplar clones grown on shallow soil, but not with the results of the third year of this study. Indeed, there seemed to be a trade-off between biomass production and environmental stress adaptation for nitrogen utilization in the third year. Clone Max1 decreased its nitrogen usage for wood (WNUE) and whole plant biomass production (NUE) on shallow soil, while H275 decreased only WNUE on shallow soil. These findings indicate that the acquisition of N was relatively higher than biomass production in Max1, which may have contributed to its better performances under shallow soil stress compared with H275.
A trade-off between NUE and water use efficiency (WUE) within species has been demonstrated in many studies ([13], [20], [33], [39], [5]). For example, Broeckx et al. ([5]) showed that a trade-off between intrinsic water use efficiency and photosynthetic N use efficiency existed among six poplar genotypes, but only when soil water availability was restricted. Considering that drought stress was likely an important limiting factor on shallow soil, the variation in WNUE or NUE might be related to differences in water availability among different years. Overall, Max1 exhibited higher growth and lower NUE and WNUE than H275 in the third year, when the mortality of H275 was high. Our results suggest that the high capacity of N acquisition was combined with higher stress tolerance in the clone Max1, thereby this genotype is more suitable for growth on marginal land. However, the total biomass yield of both clones was low at the study site compared with the expected mean yield of 10 tons ha-1 y-1 ([23]). Therefore, genotypes that can produce reasonable yields under the study conditions still have to be identified. Nonetheless, it should be noted that these genotypes have substantial beneficial economic and ecological effects on arable soil, as they also contribute to prevent N leaching ([4]).
Conclusions
In conclusion, we demonstrated that soil depth was an important factor limiting the growth, biomass production and survival of the studied poplar clones. Max1 and H275 showed different adaptation to soil depth constraints, the former showing higher survival and nitrogen concentrations than the latter. Enhanced N uptake may positively affect water usage during dry season. In the context of global change, understanding the mechanism of adaptation to environmental changes is needed to design more efficient and water-saving poplar cropping systems. This study contributes to better understanding the consequences of a changing environment on poplar growth and species adaptation. The comparison between the performance of clones H275 and Max1 suggest that the decrease in nitrogen use efficiency is a necessary trade-off to adapt to a stressful environment.
With regard to plantation management, our results show that not only low productivity but also high mortality may impinge on the yield of poplar plantations on marginal land. We demonstrated that the clone H275 was unsuitable for these conditions. Based on our results, testing a wider range of clones with high drought tolerance and an improved ability to form root biomass for their performance on shallow soils is advised. Biotechnological approaches such as protoplast fusion lines may also be a valid alternative to conventional breeding or genetic engineering for achieving more stress tolerant poplar genotypes ([15]).
Acknowledgements
Author’s contribution: DE carried out the field measurements, sampling and writing the manuscript; AP conceived the study and helped to draft the manuscript; MJ carried out soil depth characterization; CG, JT and SA performed field measurements and sampling.
We are grateful to B. Faust for helping with growth measurements and sample preparation and to G. Langer-Kettner for N analyses. We acknowledge financial support by the BMBF via the program “BEST - Bioenergie-Regionen stärken”. JT acknowledges financial support by a postdoctoral fellowship awarded by the China Scholarship Council (CSC).
References
Gscholar
CrossRef | Gscholar
Gscholar
Gscholar
Gscholar
Online | Gscholar
Authors’ Info
Authors’ Affiliation
Sam Ayegbeni
Jing Tu
Clessio Gomes Da Silva
Andrea Polle
Büsgen-Institut, Abteilung Forstbotanik und Baumphysiologie, Georg-August Universität, Büsgenweg 2, D-37077 Göttingen (Germany)
Büsgen-Institut, Abteilung Ökopedologie der gemäßigten Zonen, Georg-August Universität, Büsgenweg 2, D-37077 Göttingen (Germany)
College of Environmental Science and Engineering, Southwest Forestry University, No. 300 Bailongsi, 650224, Kunming City, Yunnan Province (P. R. China)
Corresponding author
Paper Info
Citation
Euring D, Ayegbeni S, Jansen M, Tu J, Gomes Da Silva C, Polle A (2016). Growth performance and nitrogen use efficiency of two Populus hybrid clones (P. nigra × P. maximowiczii and P. trichocarpa × P. maximowiczii) in relation to soil depth in a young plantation. iForest 9: 847-854. - doi: 10.3832/ifor2016-009
Academic Editor
Gianfranco Minotta
Paper history
Received: Feb 18, 2016
Accepted: Jul 19, 2016
First online: Sep 22, 2016
Publication Date: Dec 14, 2016
Publication Time: 2.17 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2016
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 52304
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 43411
Abstract Page Views: 3368
PDF Downloads: 4212
Citation/Reference Downloads: 54
XML Downloads: 1259
Web Metrics
Days since publication: 3433
Overall contacts: 52304
Avg. contacts per week: 106.65
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2016): 11
Average cites per year: 1.10
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Energy production of poplar clones and their energy use efficiency
vol. 7, pp. 150-155 (online: 23 January 2014)
Research Articles
Soil nutrient status, nutrient return and retranslocation in poplar species and clones in northern Iran
vol. 6, pp. 336-341 (online: 29 August 2013)
Research Articles
Links between phenology and ecophysiology in a European beech forest
vol. 8, pp. 438-447 (online: 15 December 2014)
Research Articles
The effect of clear-cut age on soil organic carbon and nitrogen indices in Scots pine (Pinus sylvestris L.) stands
vol. 18, pp. 146-153 (online: 09 June 2025)
Research Articles
Gas exchange, biomass allocation and water-use efficiency in response to elevated CO2 and drought in andiroba (Carapa surinamensis, Meliaceae)
vol. 12, pp. 61-68 (online: 24 January 2019)
Research Articles
Effective woody biomass estimation in poplar short-rotation coppices - Populus nigra × P. maximowiczii
vol. 16, pp. 202-209 (online: 25 July 2023)
Research Articles
Effects of tree species, stand age and land-use change on soil carbon and nitrogen stock rates in northwestern Turkey
vol. 9, pp. 165-170 (online: 18 June 2015)
Short Communications
Is microbial biomass measurement by the chloroform fumigation extraction method biased by experimental addition of N and P?
vol. 14, pp. 408-412 (online: 04 September 2021)
Research Articles
Climate-wise models of biomass productivity for hybrid poplar clones in Europe
vol. 16, pp. 188-194 (online: 30 June 2023)
Research Articles
Evaluation of fast growing tree water use under different soil moisture regimes using wick lysimeters
vol. 6, pp. 190-200 (online: 08 May 2013)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword