Response of Chinese sea buckthorn clonal growth and photosynthetic physiological mechanisms toward a soil moisture gradient
iForest - Biogeosciences and Forestry, Volume 14, Issue 4, Pages 337-343 (2021)
doi: https://doi.org/10.3832/ifor3564-014
Published: Jul 15, 2021 - Copyright © 2021 SISEF
Research Articles
Abstract
Studies have reported on the regulation of clonal growth in Chinese sea buckthorn in response to environmental resource availability, but these studies have been limited to external mechanisms. In this report, we controlled irrigation to generate a soil moisture gradient in order to examine the photosynthetic physiological mechanisms regulating clonal growth in this species. The results indicated that as irrigation intensity increased, the soil water content increased vertically and tissue water content first increased and then decreased. Furthermore, Rubisco activase (RCA) and Mg-chelatase H subunit (CHLH) gene expression levels, photosynthetic capacity (net photosynthetic rate, transpiration rate, chlorophyll content, and stomatal conductance), and clonal growth (ramet growth, clonal proliferation, clonal propagation) all showed a quadratic parabolic change (i.e., first increasing and then decreasing). In addition, gene expression levels and tissue water content, photosynthetic capacity and gene expression levels, and clonal growth and photosynthetic capacity were all significantly positively correlated. When irrigation intensity (soil water content) is exceedingly low or high, the tissue water content is also low, RCA and CHLH gene expression levels are low, photosynthetic capacity is weak, clonal growth ability is inhibited, and clonal growth layout tends toward the “guerrilla type.” This type manifests as fewer and smaller clonal daughter ramets that are sparsely distributed with reduced clonal organ extension ability and branching intensity. When irrigation intensity (soil water content) is moderate, the tissue water content, gene expression levels, and photosynthetic capacity is high, clonal growth ability is completely uninhibited, and the clonal growth layout tends toward the “aggregated type.” This type is associated with numerous large clonal daughter ramets that are densely distributed with high clonal organ extension ability and branching intensity. Therefore, as irrigation intensity continuously changes from inordinately low to moderate to exceedingly high, Chinese sea buckthorn regulates clonal growth by photosynthetic capacity through photosynthetic gene expression. This results in a clonal growth layout continuum of “guerrilla-aggregated-guerrilla” that depends on irrigation intensity.
Keywords
Clonal Growth, Irrigation Intensity, Tissue Water Content, Photosynthetic Genes, RCA and CHLH Gene Expression, Hippophae rhamnoides ssp. sinensis, Mu Us Sandy Land
Introduction
Clonal growth refers to the process of asexual reproduction in plants to produce genetically identical, morphologically and physiologically independent or potentially independent individuals for spatial expansion under natural conditions, such as in stoloniferous or rhizomatous plants ([29], [26]). Currently, research on clonal growth regulation is focused on external mechanisms, such as the response of clonal growth to environmental resource availability or heterogeneity and its ecological adaptation significance ([6], [30], [32], [18], [36]). However, clonal growth regulation in plants is a complex process, as internal changes are closely associated with clonal growth regulation. Following the removal of top growth in the shortleaf pine (Pinus echinata) and lobolly pine (Pinus taeda), 139 genes were differentially expressed in response to sprouting. The functions of these genes included substance metabolism, stress responses, growth and development, signal transduction, and hormonal regulation ([17]).
Individual morphological variation among populations may originate from genetic changes ([24]). It is evident from this that studying the relationship between internal changes and clonal growth might aid in further understanding the internal mechanisms of regulation, particularly the material and energy basis by which photosynthetic intensity determines clonal growth ([31]). At the same time, plant photosynthesis is regulated by genes. An example is ribulose-1.5-bisphosphate carboxylase (EC 4.1.1.39 - Rubisco), which is a key enzyme that participates in the first rate-limiting step of carbon assimilation ([8]). The activity status of Rubisco is regulated and controlled by Rubisco activase (RCA). In other words, Rubisco must undergo activation by the RCA gene to elicit its carboxylase and oxygenase activity, thereby increasing photosynthetic efficiency ([22], [2]). Conversely, chlorophylls are major pigments by which photosynthetic plants absorb and transfer light energy, and their biosynthetic route is completed by magnesium chelatases. Among magnesium chelatases, Mg-chelatase H subunit (CHLH) is a functional gene that regulates chlorophyll synthesis ([20], [23], [25]). Therefore, RCA and CHLH play important roles in photosynthesis. This solicits an assessment of the causal relationship between RCA and CHLH gene expression, photosynthetic physiological characteristics, and clonal growth.
The Chinese sea buckthorn (Hippophae rhamnoides ssp. sinensis) is an important versatile woody species used for afforestation in arid and semiarid regions in Northern China. It is a classic clonal plant that propagates via suckers, exhibiting extremely strong lateral root horizontal extension and branching abilities, and generating large amounts of sucker plants during propagation. This not only provides vegetation cover for areas that are difficult to afforest but also assists in the expansion of the forest edge and renewal of forest gaps, thereby maintaining population stability and clonal persistence ([10], [11], [13], [4], [3]). After the parental plant has died, its clonal daughter ramets can continue to undergo asexual propagation ([10], [29], [26], [3]). Therefore, its clonal attributes have conferred the Chinese sea buckthorn with the potential for forest formation from a single tree and long-living. However, large areas of artificial forests have undergone premature senescence ([16], [7]). The cause of this was determined to be drought stress, which weakens the clonal growth abilities of Chinese sea buckthorn ([14], [5], [38], [37], [1]). However, studies on the relationship between soil moisture and clonal growth have only explained the clonal growth regulatory mechanisms from an ecological perspective ([14], [5], [37], [1]), and the photosynthetic physiological regulatory mechanisms for clonal growth are not well understood. In contrast, research on the photosynthetic physiology of sea buckthorn has focused on the response laws of photosynthetic physiology characteristics to water levels ([12]), and there is a lack of analysis on the causal relationship between photosynthetic physiological characteristics and plant growth. In a preliminary research ([1]), the relationship between clonal growth and irrigation intensity was studied with 2-year-old sea buckthorn, and it was found that the optimal irrigation intensity was close to 6 times the local annual precipitation, which is the highest irrigation intensity employed in that experiment. Therefore, the result could not fully describe the response of clonal growth to the irrigation from deficit to balance to surplus. In our study, the irrigation intensity was adjusted to 3, 6, and 9 times the local annual precipitation, and the experiment was carried out for 3 years. Through regulating soil water content and tissue water content by irrigation, we investigated the response of photosynthetic gene expression, photosynthetic physiological characteristics, and clonal growth ability towards a moisture gradient. The causal relationships between photosynthetic physiology and clonal growth were investigated as well. The aim was to reveal the response laws of Chinese sea buckthorn clonal growth to irrigation intensity and its photosynthetic physiology regulatory mechanisms.
Materials and methods
Study site and plant material
The study site was located at Dingbian County in Shaanxi province (north-central China) at the southern edge of the Mu Us Sandy Land. The geographical coordinates of the site are 107° 15′ ~ 108° 22′ E, 36° 49′ ~ 37° 53′ N. The site has a mid-temperate arid and semiarid continental monsoon climate and experiences droughts and water shortages. The site has four distinct seasons, sufficient light, and frequent sandstorms. The annual mean temperature is 7.9 °C, mean annual precipitation is 316 mm and is mostly concentrated in July to September, the annual evaporation amount is 2490 mm, and the annual mean relative humidity is 53.1%. The geomorphological characteristics of the site include undulating dunes and continuous sand belts, and the area has an altitude of 1303-1418 m a.s.l. The soil consists mainly of aeolian sandy soil and saline-alkaline soil, which are nutrient-deficient and have poor water and nutrient retention abilities. The zonal vegetation is semi-desert grassland which is floristically composed of psammophytes, xerophytes, salt- and alkali-tolerant plants, and mesophytic meadows. The experiment was conducted at the Research and Development Base of Dingbian Forestry Station, and the soil is artificially-leveled aeolian sandy soil.
The Chinese sea buckthorn naturally grows on banks of rivers and lakes and in valleys in the research area, where the soils furnish good water conditions. The identical one-year-old seeded seedlings were used in this experiment. The experiment began in 2011, and after three years of continuous observation, the final survey and measurement were conducted at the end of the experiment in 2013.
Experimental design and setup
A univariate regression design was employed, and different irrigation intensities were applied by adjusting the irrigation period with a fixed flow rate to simulate a natural precipitation gradient. Based on preliminary experimental results ([14], [1]), three irrigation gradients were set up in this study, which were three, six, and nine times the mean annual precipitation of the study site. Flood irrigation from a well was carried out for 280, 560, or 840 sec for each irrigation gradient with a runoff of 6 L sec-1 on the dates of 5th, 10th, 15th, 20th, 25th, and 30th from May to August in 2011-2013, and a non-irrigated group was used as a control. (Tab. 1). A randomized arrangement was used for the field layout, with a plot area of 3 × 10 m. Triplicates were set up, resulting in a total of 12 plots. The ridges between the plots had a width of 0.5 m and a height of 0.3 m. Asphaltic felt and thick plastic films were buried up to a depth of 1 m in the middle of the ridges for separation to prevent water seepage. Thirty seedlings (one-year-old) were planted in every plot, with a distance of 1.0 × 1.5 m between the seedlings. The two sides of every replicate contained guard rows.
Tab. 1 - Irrigation intensity design. (AvIR): average amount of irrigation per plot; (EqPrec): Equivalent precipitation.
| Treatment No. |
Moisture gradient (fold) |
AvIR (kg yr-1) |
EqPrec (mm) |
Irrigation Dates (May-August, 2011-2013) |
|---|---|---|---|---|
| 1 | 0 | - | 300 | Not irrigated |
| 2 | 3 | 40.500 | 900 | 5th, 10th, 15th, 20th, 25th, 30th |
| 3 | 6 | 81.000 | 1800 | 5th, 10th, 15th, 20th, 25th, 30th |
| 4 | 9 | 121.500 | 2700 | 5th, 10th, 15th, 20th, 25th, 30th |
Experiment surveys and measurements
All the measurements were conducted in 2013, the third year of the experiment. Clonal growth parameters including ramet growth ability, clonal proliferation ability, and clonal propagation ability were measured at the end of the growth phase in September ([10], [11], [14], [4], [5], [3], [1]). For ramet (parent and daughter ramet) growth ability, the amount of growth in tree height, base diameter, and crown width were measured for every tree. For clonal proliferation ability, the number of individuals was measured, i.e., the number of individual clonal (sprout) daughter ramets in every experimental plot was tallied. The “tracking and digging” method was used for measuring clonal propagation ability, i.e., starting from a primary lateral root (from the parent plant) of an average standard tree, we followed and excavated the connecting secondary lateral roots (originating from the primary lateral roots) and tertiary lateral roots (originating from the secondary lateral roots) and measured the thickness, length, and quantity of the primary lateral roots and the total number of lateral roots. Soil moisture content and moisture content of leaves, chlorophyll content, and photosynthetic physiological indexes were repeatedly measured for three cycles, i.e., August 16th-19th, 21st-24th, and 26th-29th. The measurements were made from 8:00 to 20:00 every day. Net photosynthetic rate, transpiration rate, stomatal conductance were read at 2-hr intervals and other indicators were measured at 4-hr intervals. The averages of all the data in each cycle were presented as the final measured values. Soil moisture content was measured with an ECH2O EC-5 dielectric water sensor (Decagon Devices inc. Pullman, WA, USA) at fixed positions with depths of 10, 30, and 50 cm. Equal amounts (ca. 3.5 g) of leaves from the top, middle, and bottom of average standard trees were collected and mixed for the tissue water content and chlorophyll content tests. The tissue water content was determined by drying the specimen at 105 °C to constant weight, and then calculated from the formula: moisture content (%) = 100 × (fresh mass - dry mass)/ fresh mass. Chlorophyll content was measured following the method in Li et al. ([14]). The LI-6800® Portable Photosynthesis System (Li-Cor Environmental, Lincoln, NE, USA) was used to measure photosynthetic rate, transpiration rate, and stomatal conductance. The photosynthetic intensity was controlled at 1800 μmol photons m-2 s-1 as this intensity is close to but not greater than the light saturation point for Chinese sea buckthorn. Three fully-expanded leaves with different orientations were selected from average standard plants in every plot.
Twenty-four hours after irrigation, 5 g of young leaves were sampled from the top of average standard plants for the gene expression analyses, then rapidly placed in liquid nitrogen and transported back to the laboratory for storage at -80 °C. In the laboratory, the UNIQ-10 Pillar TRIzol™ total RNA extraction kit (SK1321/SK1322 - Thermo Fisher Scientific Inc., Waltham, MA, USA) was used to extract total RNA. Following that, cDNA synthesis was carried out before real-time PCR was used to measure the relative amounts of the RCA and CHLH genes in the cDNA samples.
Data analysis
All data were analyzed by regression analysis and correlation analysis using the statistical software SPSS® ver. 17.0 (IBM, Armonk, NY, USA).
Results
Responses of soil and tissue water content to irrigation intensity
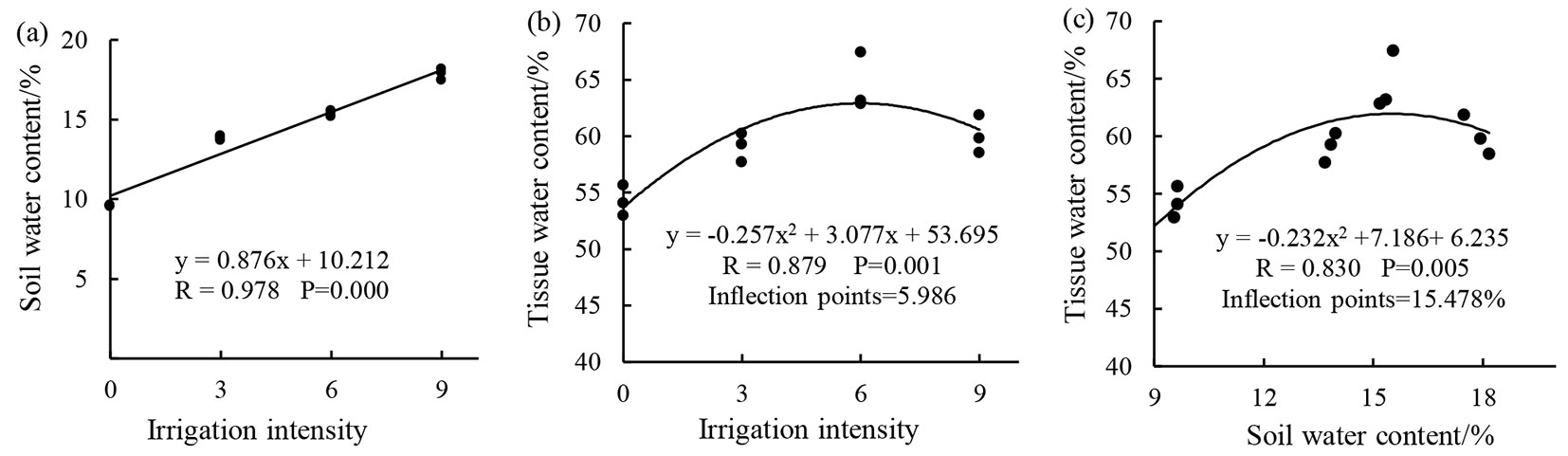
From Fig. 1, it is evident that soil water content (y) and irrigation intensity (x) were highly significantly positively correlated, while tissue water content (y) exhibit a quadratic parabolic change of the downward opening with increasing irrigation intensity and soil water content (x). The inflection point for the regression formula represents the optimal irrigation intensity (or soil water content) when the tissue water content is the highest and values greater or lower than the optimal irrigation intensity (or soil water content) will result in a reduction in tissue water content. This shows that as irrigation intensity increases, the soil water content increases vertically, while tissue water content initially increases before decreasing.
Fig. 1 - Results of the regression analysis of (a) soil water content and (b) tissue water content to irrigation intensity, as well as of (c) tissue water content to soil water content (for more details, see Experiment Surveys and Measurements section).
Responses of photosynthetic gene expression levels to moisture gradient
As reported in Tab. 2, as irrigation intensity (x) increases, RCA and CHLH gene expression levels (y) exhibit a quadratic parabolic change of the downward opening. The inflection point of the formula represents the optimal irrigation intensity when expression levels are the greatest, and values greater or lower than the optimal irrigation intensity will cause gene expression levels to decrease (similar to Fig. 1b). At the same time, the variation trends in gene expression levels with increasing soil water content are consistent with the variation trends when irrigation intensity increases. However, gene expression levels and tissue water content (x) showed a significant positive correlation (similar to Fig. 1a). This indicates that the expression levels of RCA and CHLH increase prior to decreasing as irrigation intensity (or soil water content) and tissue water content increase. Therefore, irrigation intensity (soil water content) has an indirect induction effect on the expression of RCA and CHLH, while tissue water content has a direct induction effect on RCA and CHLH.
Tab. 2 - Results of the regression analysis between gene expression parameters, irrigation intensity, and tissue water content. (R): correlation coefficient; (IP): equation inflection point.
| Predictor (x) | Regression variable (y) | Regression equation | R | p-value | IP |
|---|---|---|---|---|---|
| Irrigation Intensity | RCA | y = -0.162x2 + 1.607x + 0.458 | 0.785 | 0.013 | 4.960 |
| CHLH | y = -0.678x2 + 6.811x + 2.783 | 0.948 | <0.001 | 5.023 | |
| Tissue water content | RCA | y = 0.344x - 17.872 | 0.688 | 0.013 | - |
| CHLH | y = 1.446x - 73.884 | 0.822 | 0.001 | - |
Responses of photosynthetic physiological characteristics to a moisture gradient and photosynthetic gene expression
From Tab. 3, we can see that as irrigation intensity (x) increased, net photosynthetic rate, transpiration rate, chlorophyll content, and stomatal conductance (y) exhibited a quadratic parabolic change of the downward opening. The inflection point of the formula represents the optimal irrigation intensity when these parameters are the highest, and values greater or lower than the optimal irrigation intensity will cause net photosynthetic rate, transpiration rate, chlorophyll content, and stomatal conductance to decrease (similar to Fig. 1b). Additionally, the variation in these indicators with increasing soil water content is consistent with the variation trends when irrigation intensity increases. However, these indicators and tissue water content (x) were highly significantly positively correlated (similar to Fig. 1a). These results show that these indicators increase before decreasing with irrigation intensity (or soil water content) and increase with tissue water content. Therefore, irrigation intensity or soil water content has indirect effects on the changes in photosynthetic physiological characteristics, while tissue water content has a direct effect.
Tab. 3 - Results of the regression analysis between photosynthesis physiological parameters, irrigation intensity, and tissue water content. (R): correlation coefficient; (IP): equation inflection point.
| Predictor (x) | Regression variable (y) | Regression equation | R | p-value | IP |
|---|---|---|---|---|---|
| Irrigation intensity | Net photosynthetic rate | y = -0.306x2 + 3.583x + 16.790 | 0.872 | 0.002 | 5.855 |
| Transpiration rate | y = -0.192x2 + 2.311x + 5.024 | 0.983 | <0.001 | 6.018 | |
| Chlorophyll content | y = -0.012x2 + 0.139x + 1.336 | 0.810 | 0.008 | 5.792 | |
| Stomatal conductance | y = -0.007x2 + 0.076x + 0.356 | 0.813 | 0.008 | 5.429 | |
| Tissue water content | Net photosynthetic rate | y = 1.004x - 36.421 | 0.878 | <0.001 | - |
| Transpiration rate | y = 0.570x - 24.507 | 0.841 | 0.001 | - | |
| Chlorophyll content | y = 0.040x - 0.830 | 0.889 | <0.001 | - | |
| Stomatal conductance rate | y = 0.019x - 0.677 | 0.804 | 0.002 | - |
As indicated in Tab. 4, net photosynthetic rate, transpiration rate, chlorophyll content, and stomatal conductance (y) were significantly positively correlated with the expression levels of the RCA and CHLH genes (similar to Fig. 1a). These results showed that net photosynthetic rate, transpiration rate, chlorophyll content, and stomatal conductance increase with increasing RCA and CHLH gene expression levels. When the aforementioned response laws were combined, it was evident that irrigation intensity determines soil water content, the soil water content in turn determines tissue water content, the tissue water content in turn determines the expression levels of photosynthetic genes, and the expression levels of photosynthetic genes regulate photosynthetic physiological characteristics.
Tab. 4 - Results of the regression analysis between photosynthetic physiology parameters and gene expression parameters. (R): correlation coefficient.
| Predictor (x) | Regression variable (y) | Regression equation | R | p-value |
|---|---|---|---|---|
| RCA | Net photosynthetic rate | y = 1.876x + 18.440 | 0.820 | 0.001 |
| Transpiration rate | y = 0.922x + 7.014 | 0.680 | 0.015 | |
| Chlorophyll content | y = 0.068x + 1.396 | 0.744 | 0.006 | |
| Stomatal conductance | y = 0.035x + 0.387 | 0.730 | 0.007 | |
| CHLH | Net photosynthetic rate | y = 0.563x + 16.462 | 0.867 | <0.001 |
| Transpiration rate | y = 0.319x + 5.532 | 0.828 | 0.001 | |
| Chlorophyll content | y = 0.021x + 1.315 | 0.820 | 0.001 | |
| Stomatal conductance | y = 0.011x + 0.351 | 0.769 | 0.003 |
Response of clonal growth to moisture gradient and photosynthetic characteristics
As irrigation intensity (x) increases, tree height and base diameter; crown width; daughter ramet number; thickness, length, and quantity of primary lateral roots; and total number of lateral roots (y) exhibit a quadratic parabolic relationship (Tab. 5). The inflection point of the formula represents the optimal irrigation intensity when these parameters are the highest, and values greater or lower than the optimal irrigation intensity will cause these indicators to decrease (similar to Fig. 1b). The variation in these indicators with increasing soil water content is consistent with the variation when irrigation intensity increases. However, these indicators (y) and tissue water content (x) demonstrated an extremely significant positive correlation (similar to Fig. 1a). These results indicate that ramet growth, clonal proliferation, and clonal propagation ability increase before decreasing with irrigation intensity (or soil water content) and increase with tissue water content. Therefore, irrigation intensity (or soil water content) has indirect regulatory effects on clonal growth, while tissue water content has direct regulatory effects on clonal growth.
Tab. 5 - Regression relationships between clonal growth parameters, irrigation intensity, and tissue water content. (R): correlation coefficient; (IP): equation inflection point.
| Predictor (x) | Regression variable (y) | Regression equation | R | p-value | IP |
|---|---|---|---|---|---|
| Irrigation intensity | Tree height | y = -0.029x2 + 0.390x + 1.279 | 0.913 | <0.001 | 6.724 |
| Base diameter | y = -0.593x2 + 7.647x + 21.352 | 0.952 | <0.001 | 6.448 | |
| Crown width | y = -0.015x2 + 0.201x + 1.211 | 0.915 | <0.001 | 6.700 | |
| Number of daughter ramets | y = -14.306x2 + 167.317x + 47.450 | 0.952 | <0.001 | 5.847 | |
| Primary lateral root thickness | y = -0.121x2 + 1.799x + 3.065 | 0.929 | <0.001 | 7.434 | |
| Primary lateral root length | y = -1.377x2 + 19.835x + 39.922 | 0.972 | <0.001 | 7.202 | |
| Number of primary lateral roots | y = -0.472x2 + 5.839x + 14.183 | 0.970 | <0.001 | 6.185 | |
| Total number of lateral roots | y = -39.639x2 + 530.628x + 837.883 | 0.922 | <0.001 | 6.693 | |
| Tissue water content | Tree height | y = 0.111x -4.502 | 0.793 | 0.002 | - |
| Base diameter | y = 2.143x - 90.303 | 0.851 | <0.001 | - | |
| Crown width | y = 0.059x - 1.856 | 0.805 | 0.002 | - | |
| Number of daughter ramets | y =42.423x - 2171.986 | 0.869 | <0.001 | - | |
| Primary lateral root thickness | y = 0.621x -29.547 | 0866 | <0.001 | - | |
| Primary lateral root length | y = 6.352x - 291.788 | 0.870 | <0.001 | - | |
| Number of primary lateral roots | y = 1.588x - 68.839 | 0.887 | <0.001 | - | |
| Total number of primary lateral roots | y = 170.729x - 8171.593 | 0.903 | <0.001 | - |
It is evident in Tab. 6 that tree height and base diameter; crown width; number of daughter ramets; thickness, length, and quantity of primary lateral roots; and total number of lateral roots were significantly positively correlated with photosynthetic physiological indicators. These results demonstrated that the growth, clonal proliferation, and clonal propagation abilities of Chinese sea buckthorn increase with increasing net photosynthetic rate, transpiration rate, chlorophyll content, and stomatal conductance.
Tab. 6 - Correlation analysis between clonal growth and photosynthetic physiology parameters. (**): p<0.01; (*): p<0.05.
| Parameter | Net photosynthetic rate (μmolCO2 m-2 s-1) |
Transpiration rate (mmol m-2 s-1) |
Chlorophyll content (mg g-1) |
Stomatal conductance (mol H2O m-2 s-1) |
|---|---|---|---|---|
| Tree height (cm) | 0.809** | 0.887** | 0.705* | 0.635* |
| Base diameter (mm) | 0.864** | 0.922** | 0.763** | 0.728** |
| Crown width (cm) | 0.835** | 0.869** | 0.746** | 0.671* |
| Number of daughter ramets | 0.948** | 0.952** | 0.847** | 0.840** |
| Primary lateral root thickness (mm) | 0.911** | 0.881** | 0.784** | 0.746** |
| Primary lateral root length (cm) | 0.889** | 0.924** | 0.783** | 0.744** |
| Number of primary lateral roots | 0.909** | 0.954** | 0.805** | 0.808** |
| Total number of lateral roots | 0.943** | 0.892** | 0.865** | 0.842** |
Discussion
Responses of soil and tissue water content to irrigation intensity
Soil and plant tissue water content gradients can be affected by regulating irrigation intensity. We observed that soil water content and irrigation intensity were highly significantly positively correlated, while tissue water content showed a quadratic parabolic change of the downward opening with increasing irrigation intensity and soil water content. This was similar to the results obtained by Li ([15]). From the relationship between the three factors, it is evident that as irrigation intensity increases, the soil water content increases vertically and constitutes a passive response process. As irrigation intensity or soil water content increased, tissue water content increased initially before decreasing. This suggests that tissue water content does not only increase when irrigation is increased and an optimal irrigation intensity is present, implying that there are certain internal regulation processes. In addition, irrigation intensity, soil water content, and tissue water content formed a complementary process, i.e., irrigation intensity determines soil water content and soil water content in turn determines tissue water content.
Responses of RCA and CHLH to the moisture gradient
Previous studies have found that gene expression levels in plants are altered by the levels of environmental factors, such as temperature, moisture, and light. In photosynthetic plants, the regulatory mechanisms of RCA towards Rubisco activity are widespread ([22], [2]): Rubisco activity in herbs was found to decrease significantly during long-term drought stress. After three days of watering, the Rubisco activation state recovered to good levels ([35]). Rubisco, RCA activity, and RCA gene expression have been observed to decrease during severe drought stress in many plants ([21], [9]). A study on Arabidopsis thaliana found that CHLH gene expression is regulated by the circadian rhythm, with high expression levels under light conditions and undetectable levels under dark conditions ([19]). From these studies, it is evident that certain external conditions will induce changes in the expression levels of RCA and CHLH. Our study found that as irrigation intensity increases, the expression levels of RCA and CHLH exhibit a quadratic parabolic change, and gene expression levels and tissue water content showed a significant positive correlation. The irrigation intensity when the tissue water content and RCA and CHLH gene expression levels are the highest represents the optimal irrigation intensity. When irrigation intensity is lower than its optimum, tissue water content, and RCA and CHLH gene expression levels increase with increasing irrigation intensity. When the irrigation intensity is higher than its optimum, tissue water content, and RCA and CHLH gene expression levels decrease with increasing irrigation intensity. This variation law conforms to a tolerance law ([28]), i.e., plants have some limit or range of tolerance to any ecological factors. RCA and CHLH gene expression levels increased with increasing tissue water content. The insufficient or excessive water content will lead to reduced tissue water content, while reduced tissue water content inhibits the expression of RCA and CHLH. Appropriate irrigation will cause tissue water content to reach its maximum value. At this point, the tissue water content will promote the expression of RCA and CHLH. From this we can see that irrigation intensity modifies soil water content, the soil water content in turn determines tissue water content, and tissue water content affects the expression levels of RCA and CHLH.
Responses of photosynthetic characteristics to RCA and CHLH
Rubisco is a key enzyme in photosynthetic carbon assimilation, and its activity directly affect the rate of photosynthesis. Therefore, reduced Rubisco activity is often a non-stomatal factor causing reduced photosynthetic rates ([27]), while a stable Rubisco catalytic activation state requires activation by the RCA gene ([22], [2]). Weng et al. ([34]) found that reduced photosynthetic capacity during flag leaf senescence after the heading stage in rice is intimately associated with the decreased expression of Rubisco-activating enzymes (RCA). Their study further showed that initial Rubisco activity was highly significantly positively correlated with stomatal conductance and photosynthetic rate. The activity of the Rubisco activating enzyme (RCA) is significantly positively correlated with transpiration rate and photosynthetic rate ([33]). This study showed that net photosynthetic rate, transpiration rate, chlorophyll content, and stomatal conductance were significantly positively correlated with the expression levels of RCA and CHLH, i.e., net photosynthetic rate, transpiration rate, chlorophyll content, and stomatal conductance increase with increasing RCA and CHLH expression levels.
Photosynthesis is the source of energy for plant growth and all metabolic activities ([31]). Our study found that the growth, clonal proliferation, and clonal propagation abilities of Chinese sea buckthorn increase with increasing net photosynthetic rate, transpiration rate, chlorophyll content, and stomatal conductance. Conversely, Chinese sea buckthorn plants restricts photosynthetic physiology as a means of regulating clonal growth so that clonal populations can adapt to the resource levels. This facilitates the complete utilization of environmental resources by clones and increases population stability ([14], [5], [37], [1]). When irrigation intensity (soil water content) is suitable, clonal growth layout tends toward the “aggregated type”. This not only facilitates environmental resource occupation and utilization by the population but also increases the ability of the population to reject invasive species. When irrigation intensity (soil water content) is inordinately low or high, clonal growth layout tends toward the “guerrilla type.” This allows the clones to search for environmental resources in a wider range and reduce competition between clonal ramets. In addition, this increases the probability of clones placing ramets in a favorable habitat patch. However, the tradeoffs include reduced ramet growth, proliferation ability, and clonal propagation ([14], [5], [36]). Evidently, clonal growth regulation is a manifestation of the ecological adaptation countermeasures of Chinese sea buckthorn populations and is a biological route for the maintenance of clonal persistence and population stability.
As suggested by our results in terms of clonal growth, photosynthetic physiology, and gene expression, both lower and higher irrigation intensities were unfavorable to the plants. The former is obviously attributed to drought. The latter might be ascribed to waterlogging and leaching of soil nutrients. According to our observation, the waterlogging was very short due to the sandy nature of the soil which is poor in water holding capacity.
Conclusions
In summary, irrigation intensity determines soil water content, soil water content in turn determines tissue water content, tissue water content in turn induces changes in gene expression levels, gene expression levels in turn regulate photosynthetic capacity, and photosynthetic capacity in turn regulates clonal growth. This ultimately enables Chinese sea buckthorn to form a clonal growth layout that is adapted to water resource availability. Specifically, when irrigation intensity (soil water content) is low, the tissue water content will be low, RCA and CHLH gene expression levels will be low, photosynthetic capacity will be weak, clonal growth ability will be inhibited, and clonal growth layout will tend toward “guerrilla type”. This type specifically manifests as fewer small daughter ramets that are sparsely distributed with lower clonal organ extension ability and branching intensity. When irrigation intensity (soil water content) is moderate, the tissue water content, gene expression levels, and photosynthetic capacity will be high, clonal growth ability will be uninhibited, and the clonal growth layout will tend toward the “aggregated type”. This type specifically manifests as numerous large daughter ramets that are densely distributed with high clonal organ extension ability and branching intensity. When irrigation intensity (soil water content) is exceedingly high, RCA and CHLH gene expression levels will be low, photosynthetic capacity will be weak, clonal growth ability will be inhibited, and clonal growth layout will once again tend towards the “guerrilla type”. Therefore, as irrigation intensity (soil water content) continuously changes from “overly low to moderate to overly high,” Chinese sea buckthorn restricts photosynthetic physiology as a means of regulating clonal growth in order for populations to form a clonal growth layout that adapts to water levels. This results in a clonal growth layout continuum of the “guerrilla-aggregated-guerrilla” type.
List of abbreviations
The following abbreviations have been used throughout the paper: RCA - Rubisco activase; CHLH - Mg-chelatase H subunit.
Author Contribution
ScB and KhN contributed equally to this paper (co-first authors).
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 31570609/31070551/30371193) and Shaanxi Provincial Key Research and Development Program (Grant No.2017ZDXM-NY-061).
References
Gscholar
Authors’ Info
Authors’ Affiliation
Kaihong Nie
Shengli Ji
Shi Chen
Zengyu Yao 0000-0001-8193-6043
Genqian Li
College of Forestry, Southwest Forestry University, Kunming Yunnan 650224 (China)
Key Laboratory of Forest Resources Conservation and Utilization in the Southwest Mountains of China - Southwest Forestry University, Ministry of Education, Kunming (China)
Wanyuan Forestry Science and Technology Extension Center, Dazhou, Sichuan 635000 (China)
Qianxinan Bouyei and Miao Nationality Autonomous Prefectures Bureau of Forestry, Xingyi, Guizhou 562400 (China)
Corresponding author
Paper Info
Citation
Bai S, Nie K, Ji S, Chen S, Yao Z, Li G, Tang C, Guo F (2021). Response of Chinese sea buckthorn clonal growth and photosynthetic physiological mechanisms toward a soil moisture gradient. iForest 14: 337-343. - doi: 10.3832/ifor3564-014
Academic Editor
Silvano Fares
Paper history
Received: Jun 25, 2020
Accepted: May 19, 2021
First online: Jul 15, 2021
Publication Date: Aug 31, 2021
Publication Time: 1.90 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2021
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 21917
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 19300
Abstract Page Views: 1019
PDF Downloads: 1346
Citation/Reference Downloads: 0
XML Downloads: 252
Web Metrics
Days since publication: 1010
Overall contacts: 21917
Avg. contacts per week: 151.90
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Feb 2023)
(No citations were found up to date. Please come back later)
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Photosynthetic parameters of urban greening trees growing on paved land
vol. 12, pp. 403-410 (online: 13 August 2019)
Research Articles
Arbuscular mycorrhizal colonization in black poplar roots after defoliation by a non-native and a native insect
vol. 9, pp. 868-874 (online: 29 August 2016)
Review Papers
Genomics of the Dutch elm disease pathosystem: are we there yet?
vol. 8, pp. 149-157 (online: 07 August 2014)
Research Articles
Addressing post-transplant summer water stress in Pinus pinea and Quercus ilex seedlings
vol. 8, pp. 348-358 (online: 16 September 2014)
Research Articles
Links between phenology and ecophysiology in a European beech forest
vol. 8, pp. 438-447 (online: 15 December 2014)
Research Articles
A physiological approach for pre-selection of Eucalyptus clones resistant to drought
vol. 13, pp. 16-23 (online: 15 January 2020)
Research Articles
Adaptive response of Pinus monticola driven by positive selection upon resistance gene analogs (RGAs) of the TIR-NBS-LRR subfamily
vol. 10, pp. 237-241 (online: 02 February 2017)
Short Communications
Variation in growth, photosynthesis and water-soluble polysaccharide of Cyclocarya paliurus under different light regimes
vol. 10, pp. 468-474 (online: 04 April 2017)
Research Articles
Effects of abiotic stress on gene transcription in European beech: ozone affects ethylene biosynthesis in saplings of Fagus sylvatica L.
vol. 2, pp. 114-118 (online: 10 June 2009)
Research Articles
Clonal structure and dynamics of peripheral Populus tremula L. populations
vol. 7, pp. 140-149 (online: 13 January 2014)
iForest Database Search
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword