Richness and abundance of granivorous vertebrates determine acorn removal patterns in a human modified oak forest
iForest - Biogeosciences and Forestry, Volume 11, Issue 2, Pages 329-337 (2018)
doi: https://doi.org/10.3832/ifor2216-011
Published: Apr 18, 2018 - Copyright © 2018 SISEF
Research Articles
Abstract
Most forests of the Earth have been affected by human activities and this can alter the plant-animal interactions on which depend the functional integrity of these ecosystems. In this study, we assessed the relationships between acorn removal rates and the richness and abundance of granivorous vertebrates along a forest-edge-clearing gradient. We also evaluated whether removal rates differed among oak species with different acorn size. To this purpose, a field experiment was performed including acorns of five oak species, which were exposed to seed consumers in the three different habitats (forest interior, man-made clearings and the edge between these habitats). The experimental units consisted in five paper trays containing 50 acorns of each oak species located at different distances from the edge towards the forest and the man-made clearing (0, 20 and 50 m). Experimental sites were equipped with phototraps to record the identity of the visiting granivorous vertebrates. Richness and abundance of granivores increased from the edge towards the forest interior, while the converse patterns were observed in the man-made clearing. For most oak species, acorn removal patterns was positively correlated with richness and abundances of granivores, though in all habitats small-sized acorns were removed much faster and in larger proportions than big-sized acorns. Although these results are specific for the study site, they suggest that man-made clearings reduce the richness and abundance of granivores, thus negatively affecting the secondary dispersion of zoochoric tree species towards open habitats. Further, it also seems that large-seeded oak species face greater dispersal limitations than small-seeded oaks, because of the lack of animals able to scatter them from the forest to the clearings.
Keywords
Acorn Size, Forest Gaps, Land Use Change, Man-made Clearing, Species Diversity
Introduction
Large well-preserved forests are currently rare, as most forest ecosystems persist as part of human-modified landscapes ([30]). In the past century, human activities reduced the world’s forests cover by almost 50% and this decline is expected to rise due to the expansion of the agricultural frontier ([11]). These activities, however, strongly interfere with key ecological processes that take place in the forests, including seed dispersal, tree recruitment, seed predation and herbivory, among several other ecosystem functions that depend on local biodiversity ([8]).
In forests dominated by zoochoric trees, the recruitment of these species in gaps generated by natural disturbances (e.g., tree fall) mainly depends on seed consumers that unintentionally disperse plant propagules from the forest interior towards open sites ([38]). Small vertebrates, such as mice and birds, are important seed dispersers in forest ecosystems, but the opening of clearings for agriculture and cattle grazing alters their activity and distribution patterns ([6], [32]). The opening of man-made clearings also affects large and medium sized seed consumers. These animals, rather than disperse seeds, contribute to maintain the flows of matter and energy in forests, but deforestation and habitat fragmentation are dramatically reducing their diversity and abundance in these ecosystems ([34], [5]). Indeed, most granivores usually avoid visiting man-made clearings because these habitats lack of “biological legacies” (e.g., remaining trunks and branches) that provide them refuge from predators ([36], [46], [38], [5]).
Another important feature of human modified forests (hereafter, HMF) are the edge effects, which embrace all processes occurring in the boundaries between forests and man-disturbed sites ([3]). Edge effects can alter the richness and abundance of granivorous vertebrates, which usually decrease from the forest interior towards the edge, while only a few species visit large man-made clearings ([18], [7], [50], [33]). Therefore, most seed consumers can be expected to be concentrated within the forest remnants, while a few of them would visit man-made clearings. Further, several studies have assessed how increasing distance to edges affects seed removal by vertebrates, but their results are not fully consistent. Some authors have reported increased seed removal rates inwards the forest ([42], [2], [32]), while others found higher removal rates at forest edges ([9], [22], [24]) or even in man-made clearings ([10]). These discrepancies have been attributed to the chemical composition of seeds, as highly palatable seeds are quickly removed irrespectively of the habitat they are located, or differences in seed size, as bigger seeds are only removed by large granivores visiting the different habitats in the HMF ([9], [2], [35]). Nevertheless, it remains largely unexplored whether changes in the richness and abundance of granivores are correlated with seed removal patterns along forest-edge-clearing gradients. Indeed, these relationships are likely to vary across different tree species, as they can differ in seed size and seed palatability ([40], [44]). Given the elevated rates of land-use change to which forests are currently subjected, evaluating these relationships is critical to understand how human activities affect the processes linked to seed dispersal in these ecosystems, which in turn could help to design better conservation and management strategies addressed to preserve the functional integrity of HMF.
In this study, we assessed these relationships in a HMF dominated by oaks (Quercus spp., Fagaceae). We focused on this forest type because these tree species are widely distributed across temperate biomes of the Northern Hemisphere and their acorns are consumed by several granivorous vertebrates ([1], [29]). Several authors proposed that the structural integrity and biodiversity of these forests largely depend on the trophic interactions between acorns and their consumers ([49], [20], [43], [23], [47], [33]). Temperate forests of Mexico, in particular, are composed by several oak species that produce acorns largely different in size and chemical composition, while they also harbor an elevated diversity of vertebrates that consume them ([29], [51]). However, these oak forests have been intensely affected by man-made clearings during the past two centuries ([37]) and this may have altered these plant-animal interactions.
We performed a field experiment to answer the following questions: (1) how do acorn removal rates vary across oak species along the forest-edge-clearing gradient? (2) do richness and abundance of granivores differ across habitats of these HMF? and (3) are acorn removal rates related with the richness and abundance of granivores along the forest-edge-clearing gradient? Our working hypothesis states that richness and abundance of granivorous vertebrates should decrease from the interior of forests toward man-made clearings, thus acorn removal rates should also decrease in that direction.
Materials and methods
Study area
This study was conducted in the Wildlife Management Unit “La Laguna” (21° 58′ N, 100° 34′ W, elevation 2100 m a.s.l.), located in westernmost section of Sierra Madre Oriental, State of San Luis Potosí, Mexico. Climate in this region is temperate (mean annual temperature 18 °C) and 90% of rainfalls (mean annual precipitation 500-650 mm) are concentrated between June and November ([13]). Vegetation is composed by continuous oak forests, but local people opened clearings of varying size (5-25 ha) that currently cover 30% of the study area. These clearings are used to develop different activities (e.g., subsistence farming, cattle grazing and mixed management practices) and this has generated an extremely complex HMF.
The study was carried out in a single large clearing (14 ha) sporadically used for cattle grazing. This clearing has sharp edges with forests composed by red and white oaks. Red oaks include Quercus affinis Scheidw. (1837), Quercus castanea Née (1801), Quercus jonesii Trel. (1924), Quercus crassifolia Humb. & Bonpl. (1809), Quercus eduardii Trel. (1924), Quercus mexicana Bonpl. (1809) and Quercus viminea Trel. (1924). White oaks include Quercus laeta Liebm. (1954), Quercus resinosa Liebm. (1954) and Quercus rugosa Née (1801). All these species bloom between April and June and release their fruits between August and November ([25]).
Oak species and acorn collection
Most oak species at the study site have mast-seeding years interposed with several years of low seed production ([51]), thus we focused on those species that had mast acorn production in 2015. This included three red oaks (Q. affinis, Q. castanea and Q. eduardii), and two white oaks (Q. resinosa and Q. rugosa). These oak species strongly differ in acorn sizes and they can be serialized in the following decreasing order: Q. resinosa (3.0-6.0 cm length × 1.5-3.0 cm width), Q. rugosa (2.0-2.5 cm length × 1.5-2.5 cm width), Q. castanea (1.0-2.0 cm length × 1.0-1.5 cm width), Q. affinis (0.5-1.3 cm length × 0.8-1.0 cm width), and Q. eduardii (0.8-1.0 cm length × 0.4-0.6 cm width - [25]).
Between September and October 2015, after the occurrence of primary dispersion, we collected mature acorns beneath the canopy of ten parental trees of each species in the study area. However, because acorns collected in the field are often parasitized by insect or fungi ([14], [33]), we took off the cupules of acorns and placed the nuts in 20-liter containers filled with water. After 2 h in water, sunken acorns were assumed to be viable ([15]) and then used in the experiment, while floating acorns were discarded.
Experimental design and data collection
To assess whether richness and abundance of acorn consumers differed across the forest-edge-clearing gradient, as well as to assess how this influences acorn removal rates, we located four sampling stations at the forest edge, spaced 50 m from each other. From each of these stations, we laid out a 100-m line transect perpendicular to the forest edge, taking care of maintaining equivalent distances in all cardinal directions between these sites and forest edges to avoid interference (Fig. 1a). We established sampling stations along each transect at 0 m, 20 m and 50 m from the edge. Hereafter we will refer to the forest edge as distance 0, while distances inwards the forest and the clearing will be respectively referred as positive (20 m and 50 m) and negative values (-20 m and -50 m).
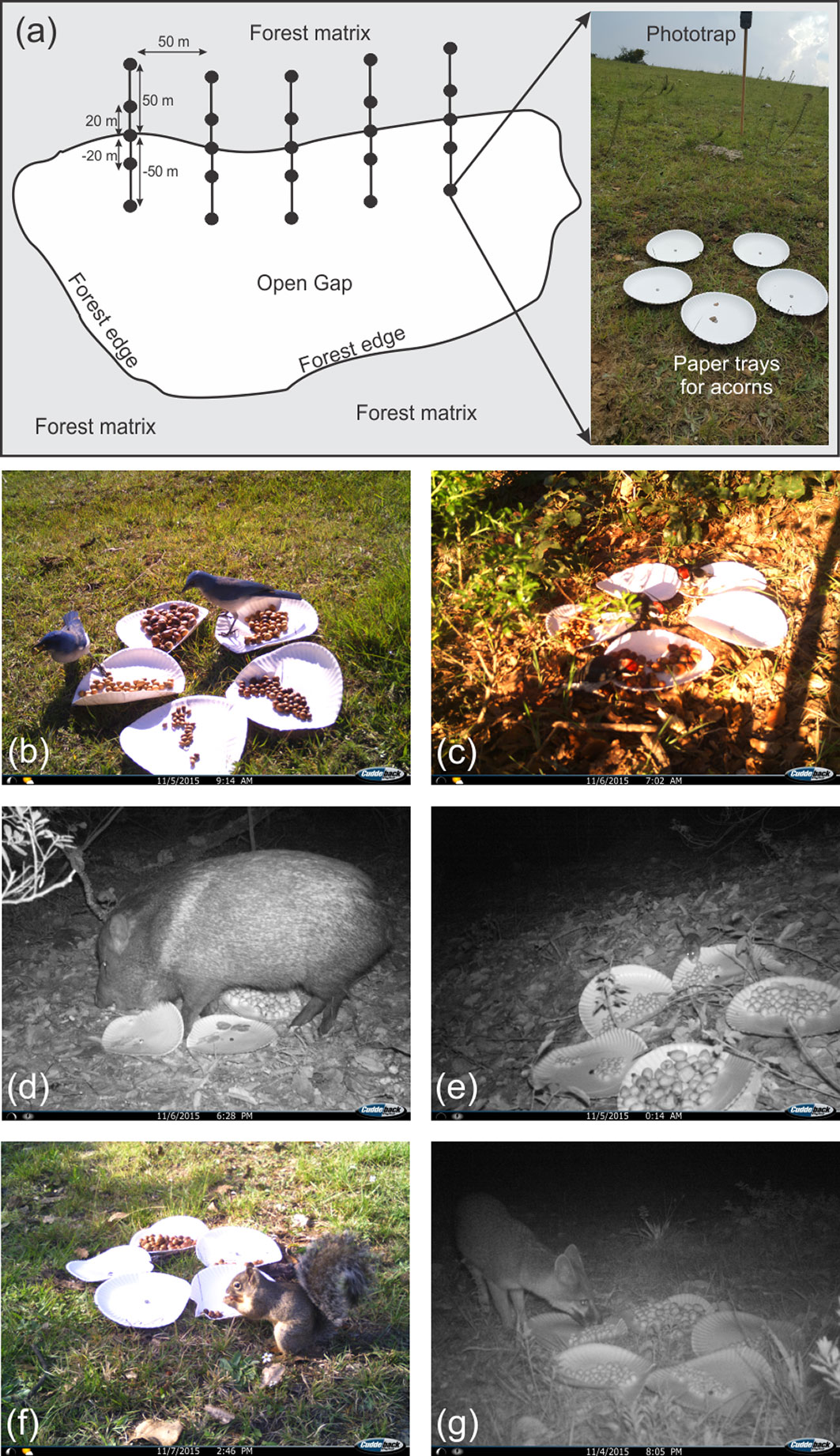
Fig. 1 - Schematic view of the experimental design (a) including two distances inwards the forest (20 m and 50 m), the forest edge (0 m), and two distances towards the open gap (-20 m and -50 m). The figure also shows the paper trays on which acorns were placed at experimental sites and the phototraps used to record the granivores. Consumers captured by phototraps during daytime (color pictures) and night (black-white pictures) were: Aphelocoma ultramarina (b), Melanerpes formicivorus (c), Pecari tajacu (d), Peromyscus sp. (e), Sciurus oculatus (f) Urocyon cinereoargenteus (g).
At each experimental site, we fixed five circular paper trays (20-cm diameter, 1-cm deep) to the soil with 8-cm iron nails (Fig. 1a). Each tray was randomly assigned to contain 50 acorns of an oak species (Q. affinis, Q. castanea, Q. eduardii, Q. rugosa and Q. resinosa). The acorn removal experiment started at 18:00 h on November 3rd 2015 and, to estimate acorn removal rates, we counted the number of remaining acorns in each tray every 12 h (06:00 and 18:00). The experiment was finished on November 7th at 06:00 h (after 84 h), when more than 70% of acorns were removed from trays. To register the identity of animals that visited the trays, we mounted two digital phototraps on each experimental unit (Digital Scouting Camera Model 1231®, Cuddeback, De Pere, WI, USA). These phototraps were fixed 1 m above the soil on wood sticks located 75 cm away from acorn trays. Wood sticks had an inclination of 45° to focus the lens of cameras directly on the trays (Fig. 1a). Cameras were adjusted to the highest resolution (20 megapixels) and movement sensors were adjusted to maximum sensitivity to record both large and small vertebrates.
Statistical analyses
We used failure-time-analyses to compare acorn removal rates among oak species and distances from the forest edge. In these analyses, all acorns had a value of 1 at the beginning of the experiment, while the removal of an acorn from a given tray at a given monitoring time was considered a “failure” and its value was changed to zero. Acorn removal rates were estimated with the Kaplan-Meier’s method ([17]) and the Gehan’s generalized Wilcoxon test was used to compare these values among treatments ([21]). When differences were found, pairwise Gehan’s Wilcoxon tests were used to assess differences between treatments (=sites). These analyses were firstly used to assess overall species and distance-to-forest effects in acorn removal rates. After that, we compared acorn removal rates among oak species at each distance from the forest edge, as well as those of each oak species across the forest-edge-clearing gradient. All the statistical analyses were conducted in R ver. 3.0 ([31]).
Data obtained with phototraps were used to determine the habitat preferences of the different acorn consumers. To this purpose, we pooled the total visitation frequencies of each animal species (i.e., number of times each species was shot by phototraps) across sampling stations located at each distance from the forest edge. Photographs where animals did not consume acorns were discarded from these analyses. These data were used to perform Monte Carlo randomization tests to determine whether visitation frequencies of each animal species at each distance from the forest edge were higher or lower than expected by chance. For this, we generated randomly expected distribution frequencies of each animal species by resampling 1000 times their observed visitation frequencies across all distances from the forest edge and the probability (p-value) for coincidence between observed and expected frequencies was computed ([39]). The null hypothesis is that, if the spatial distribution of species is due to stochastic processes, then they must display random distribution patterns across distances from the forest edge. When p<0.05, the hypothesis is rejected and the spatial distribution of species is considered to be regulated by deterministic processes ([12]). Therefore, a visitation frequency either higher or lower than expected by chance indicates the animal species is positively or negatively associated to that habitat, respectively. These analyses were conducted with the Monte Carlo module of PopTools 3.2 ([16]).
Simple linear regressions were used to determine whether richness and abundance of acorn consumers were related with the distance to the forest edge, as well as to assess whether acorn removal patterns were related with these two variables. Richness of acorn consumers (i.e., number of species recorded with phototraps at each sampling site during the experiment) and the total abundance of granivores at each site (i.e., total number of granivores recorded at each sampling station across the entire experiment), were regressed against the distance to the forest edge. Also, we tested the correlation of richness and abundance of granivores with the total number of removed acorns at each site as well as with the number of removed acorns of each oak species. Finally, to assess whether some granivores had stronger effects than others on acorn removal, we regressed the total number of removed acorns against the individual abundance of each granivore species. No analyses were conducted to assess the effects of each acorn consumer on the different oak species because this would require knowing the acorn preferences of each consumer, but our experimental design did not allow to gather these data. All regression analyses described above were conducted in R 3.0 ([31]).
Results
Acorn removal patterns
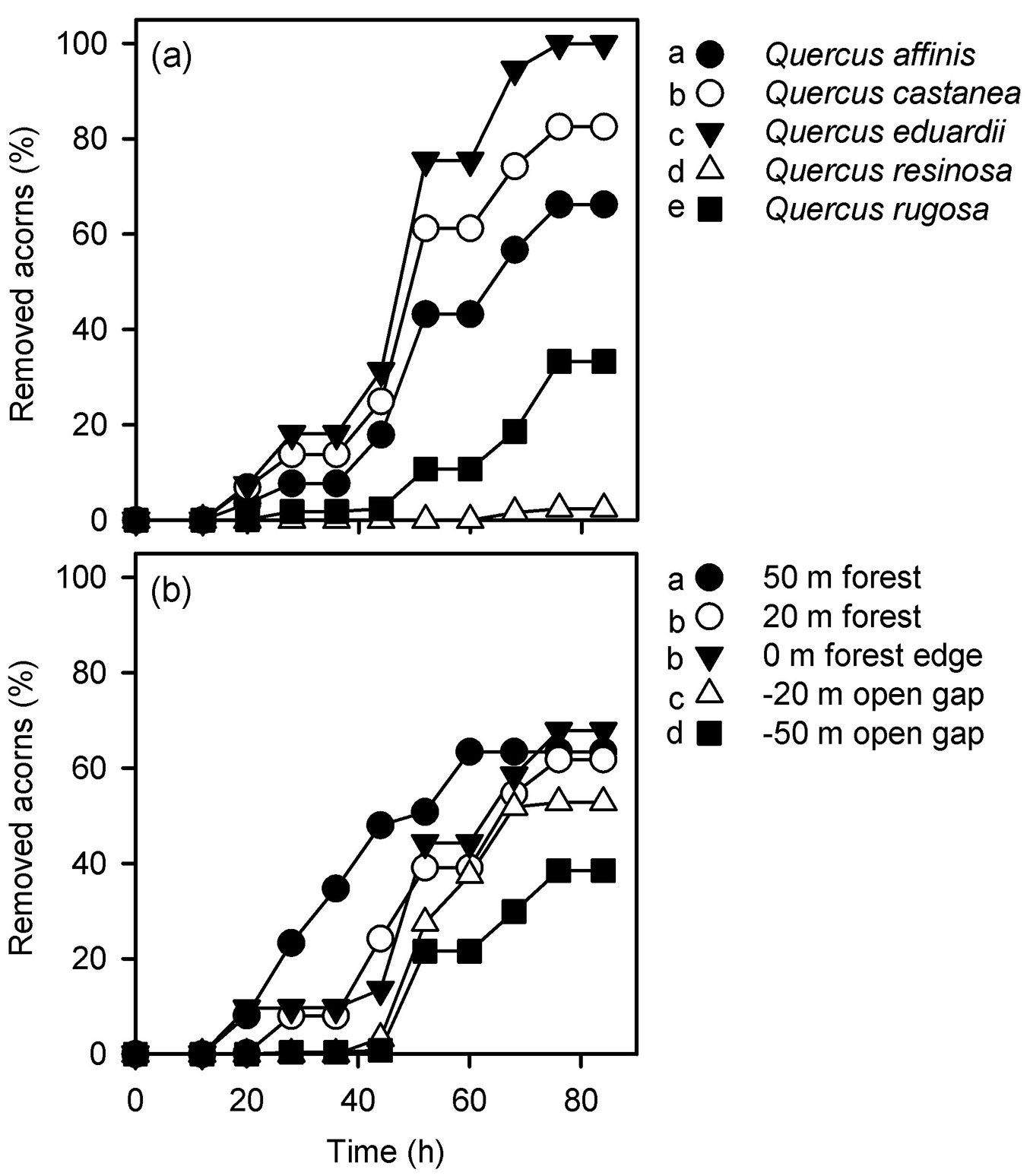
Acorn removal rates estimated irrespectively of the distance to the forest edge significantly differed among oak species (Gehan’s Wilcoxon statistic = 2462.635, df = 4, p < 0.001). Pairwise comparisons indicated that removal rates were always higher for red oaks (Q. affinis, Q. castanea and Q. eduardii) than for white oaks (Q. resinosa and Q. rugosa - Fig. 2a). The highest removal rate was recorded for the oak species with smaller acorns (Q. eduardii), whose acorn trays were completely depleted after 72 h (Fig. 2a). In contrast, the oak species with bigger acorns (Q. resinosa) had the lowest removal rate, with more than 90% of its acorns remaining on the trays by the end of the experiment (Fig. 2a). Removal rates estimated irrespectively of the oak species were also found to differ among distances from the forest edge (Gehan’s Wilcoxon statistic = 364.130, df = 4, p < 0.001). Acorns were removed faster from experimental units located 50 m inwards the forest, while no differences were found between trays located 20 m inwards the forest and those located at the forest edge (Fig. 2b). Acorn removal rates in the clearing were lower than within the forest and the edge, although in this habitat trays located at -50 m had lower removal rates than trays located at -20 m (Fig. 2b).
Fig. 2 - Acorn removal rates estimated for each oak species irrespective of the habitat in which they were located (a) and acorn removal rates estimated at each distance from the forest edge irrespective of the oak species (b). Different letters on the side of symbols indicate significant differences (p<0.05) between mean removal rates after Gehan’s Wilcoxon test.
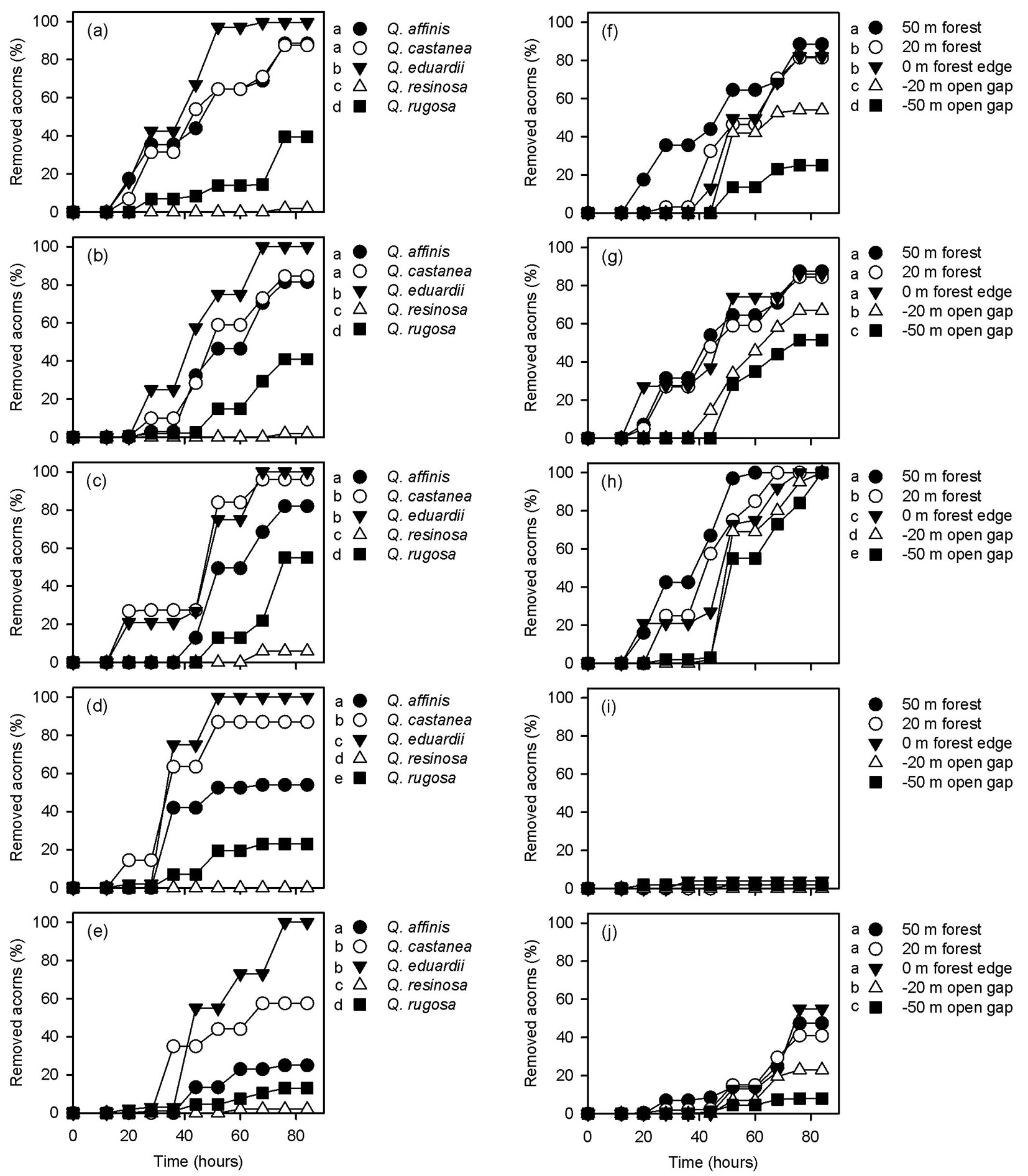
Significant differences were found when acorn removal rates were compared among oak species at each distance from the forest edge (Tab. 1). Within the forests and in the clearing, Q. eduardii had higher removal rates than all the other oak species (Fig. 3). At the forest edge, on the other hand, acorns of Q. eduardii and Q. castanea were more intensely removed than those of the other oak species (Fig. 3). At all distances from the forest edge, white oaks (Q. resinosa and Q. rugosa) had lower acorn removal rates than red oaks (Q. affinis, Q. castanea and Q. eduardii), but removal rates of Q. resinosa were always lower than those of Q. rugosa (Fig. 3). On the other hand, removal rates of all oak species differed among distances from the forest edge, except for Q. resinosa (Tab. 1). As mentioned above, only the acorns of Q. eduardii were fully removed in all experimental sites. However, removal rates of this oak species were higher 50 m inwards the forest and they sequentially decreased towards the clearing (Fig. 3). Removal rates of Q. affinis were also higher 50 m inwards the forest, but trays located 20 m inwards the forests and at the forest edge had higher removal rates than trays located in the clearing (Fig. 3). Acorns of Q. castanea and Q. rugosa had similar removal rates at both distances inwards the forest (20 m and 50 m) and at the forest edge (0 m), and these values were always higher than those estimated at both distances (-20 m and -50 m) inwards the clearing (Fig. 3).
Tab. 1 - Results of failure-time analyses to compare acorn removal rates among oak species at each distance from the forest edge (a), as well as among distances from the forest edge for each oak species (b). Gehan’s Wilcoxon statistic (GWS), the degrees of freedom (df) and the associated p-value of each analysis are reported.
| Comparison | Class | GWS | df | Prob. |
|---|---|---|---|---|
| (a) Oak species at each distance from the forest edge |
50 m (forest) | 577.625 | 4 | < 0.001 |
| 20 m (forest) | 530.907 | 4 | < 0.001 | |
| 0 m (edge) | 595.081 | 4 | < 0.001 | |
| -20 m (clearing) | 559.534 | 4 | < 0.001 | |
| -50 m (clearing) | 513.109 | 4 | < 0.001 | |
| (b) Distances from the forest edge for each species |
Q. affinis | 257.628 | 4 | < 0.001 |
| Q. castanea | 175.13 | 4 | < 0.001 | |
| Q. eduardii | 273.22 | 4 | < 0.001 | |
| Q. resinosa | 5.548 | 4 | 0.235 | |
| Q. rugosa | 530.907 | 4 | < 0.001 |
Fig. 3 - Acorn removal rates of the different oak species at different distances from the forest edge (a: 50 m; b: 20 m; c: 0 m; d: -20 m, e: -50 m) and at different distances from the forest edge for each oak species (f: Q. affinis; g: Q. castanea; h: Q. eduardii; i: Q. resinosa; j: Q. rugosa). In all cases, different letters on the side of symbols indicate significant differences (p<0.05) between mean removal rates after Gehan’s Wilcoxon test.
Distribution patterns of acorn consumers
Six vertebrate species of consumers were recorded in the experimental trays (Fig. 1). Acorn consumers included Mexican jays (Aphelocoma ultramarina), acorn woodpeckers (Melanerpes formicivorus), collared peccaries (Pecari tajacu), Mexican field mice (Peromyscus sp.), Peter’s squirrels (Sciurus oculatus) and gray foxes (Urocyon cinereoargenteus). Jays, woodpeckers and squirrels were only detected during daytime (from 06:00 to 18:00), while peccaries, mice and foxes were only recorded during night (from 18:00 to 06:00). At all distances from the forest edge, the frequency of occurrence for jays and mice was much higher than that recorded for the other acorn consumers (Tab. 2).
Tab. 2 - Tab. 2 - Frequency of occurrence of granivorous species (number of times they were captured with phototraps) visiting the acorn trays located within the forest (20 and 50 m), at the forest edge (0 m) and in the clearing (-50 and -20 m). Symbols on the side of each number indicate the results of Monte Carlo randomization tests. (+): Visitation frequency higher than expected by chance (i.e., positive association between the species and a given distance to forest edge); (-): visitation frequency lower than expected by chance (negative association); (0): visitation frequency consistent with that expected by chance (neutral association pattern).
| Family | Species | Within the forest | Forest edge |
Clearing | ||
|---|---|---|---|---|---|---|
| 50 m | 20 m | 0 m | -20 m | -50 m | ||
| Corvidae | Aphelocoma ultramarina (Bonaparte, 1825) | 347 (+) | 303 (0) | 309 (0) | 157 (-) | 124 (-) |
| Picidae | Melanerpes formicivorus (Swainson, 1827) | 5 (+) | 0 (-) | 0 (-) | 0 (-) | 0 (-) |
| Tayassuidae | Pecari tajacu (Linnaeus, 1758) | 7 (+) | 3 (0) | 0 (-) | 0 (-) | 0 (-) |
| Cricetidae | Peromyscus sp. | 92 (+) | 56 (0) | 60 (0) | 8 (-) | 10 (-) |
| Sciuridae | Sciurus oculatus (Peters, 1863) | 12 (+) | 11 (+) | 9 (0) | 0 (-) | 0 (-) |
| Canidae | Urocyon cinereoargenteus (Schreber, 1775) | 8 (+) | 2 (0) | 3 (0) | 3 (0) | 1 (0) |
Acorn consumers decreased their frequency from the forest interior towards the forest edge, and these values decreased even farther inwards the clearing (Tab. 2). The Monte Carlo randomization tests indicated that the visitation frequency of all these animals was higher than expected by chance in 50 m inwards the forest (Tab. 2). Experimental sites located 20 m inwards the forest were also visited by most acorn consumers, but only S. oculatus was positively associated with this habitat, while M. formicivorus was negatively associated (Tab. 2). No animal species was positively associated with the forest edge, while M. formicivorus and P. tajacu displayed negative associations with this habitat (Tab. 2). Visitation frequencies of most granivores were lower than expected by chance in the experimental sites located at -20 m and -50 m in the clearing (Tab. 2), indicating that most of them avoided this habitat. The only exception was U. cinereoargenteus, which displayed neutral associations at both distances inwards the clearing (Tab. 2).
Relationships between acorn removal and the richness and abundance of consumers
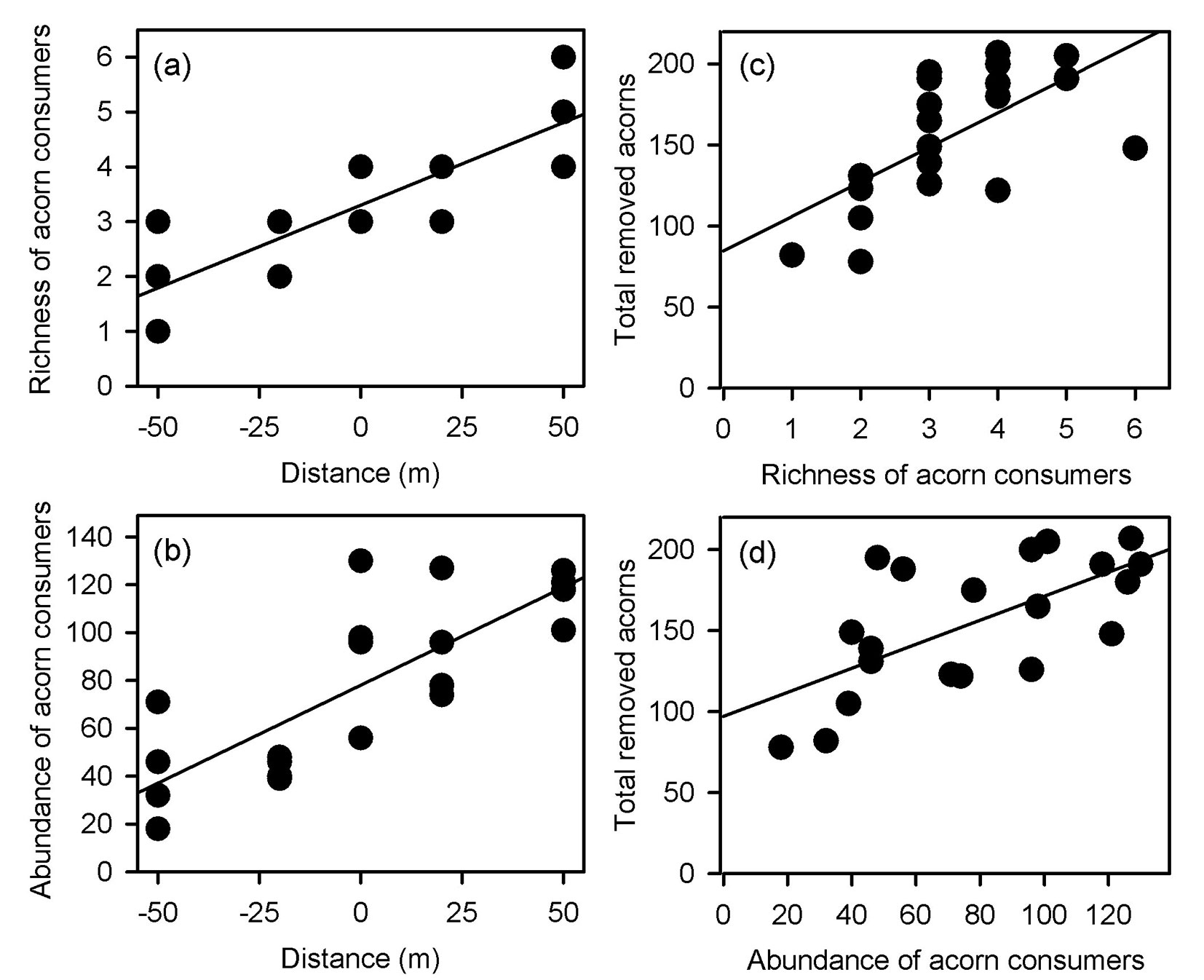
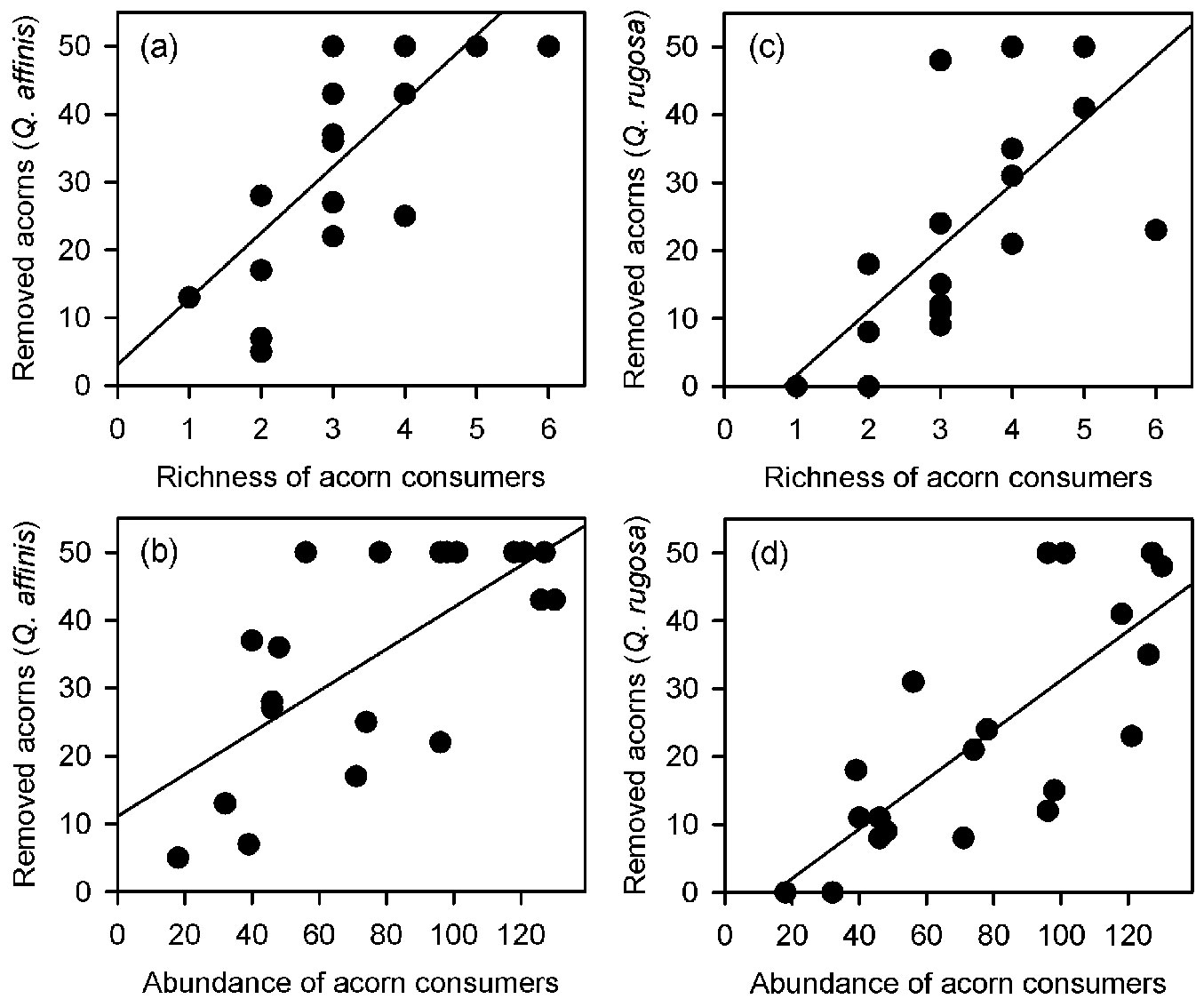
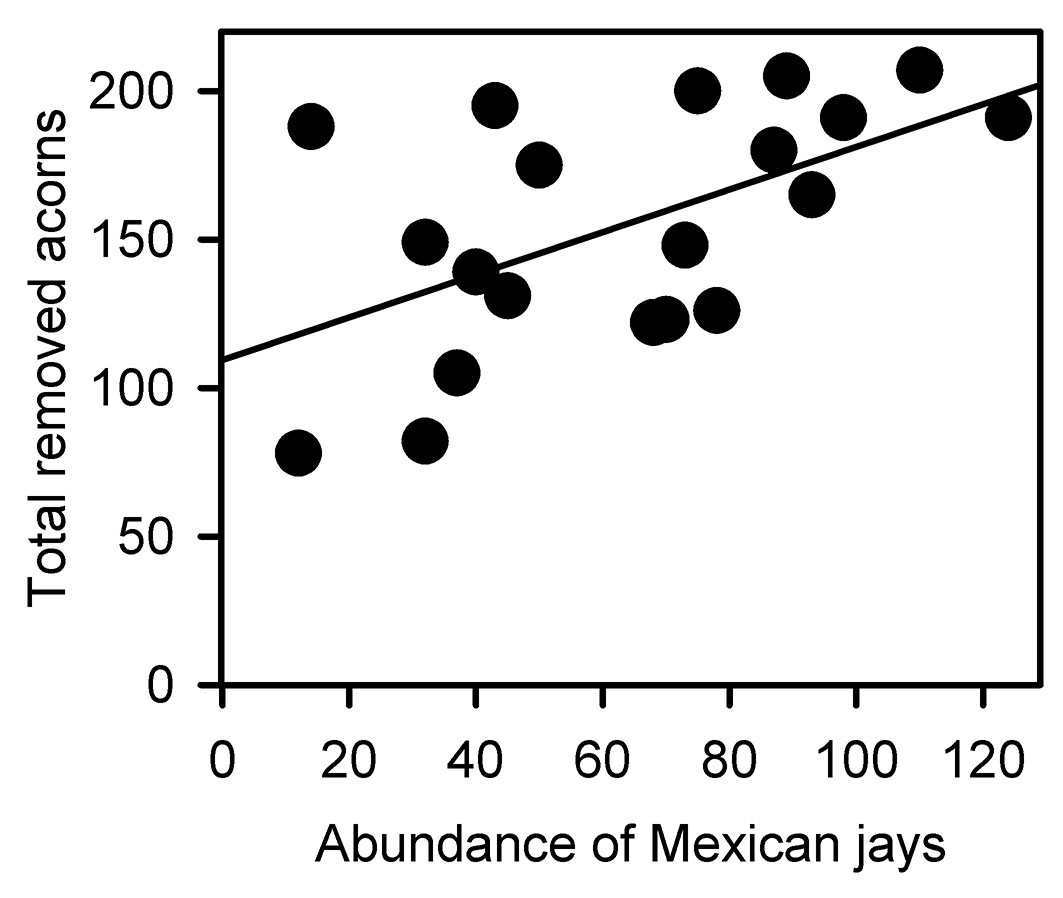
Both richness and total abundance of acorn consumers linearly increased from the maximum distance inwards the clearing towards the forest interior (richness: F[1, 18] = 53.702, p < 0.001, r2 = 0.749 - Fig. 4a; abundance: F[1, 18] = 30.851, p < 0.001, r2 = 0.631 - Fig. 4b). Accordingly, the total number of removed acorns increased with richness and total abundance of granivores (richness: F[1, 18] = 12.563, p = 0.002, r2 = 0.411 - Fig. 4c; abundance: F[1, 18]= 13.771, p = 0.001, r2 = 0.434 - Fig. 4d). However, the regression analyses conducted separately for each oak species did not fully support these overall removal pattern. Positive relationships between the number of removed acorns and richness and abundance of granivores were only found for Q. affinis (richness: F[1, 18] = 22.385, p < 0.001, r2 = 0.554 - Fig. 5a; abundance: F[1, 18] = 16.952, p < 0. 001, r2 = 0.485 - Fig. 5b) and Q. rugosa (richness: F[1, 18] = 14.551, p = 0.001, r2 = 0.447 - Fig. 5c; abundance: F[1, 18] = 16.952, p < 0. 001, r2 = 0.485 - Fig. 5d), while these relationships were not found for Q. castanea (richness: F[1, 18] = 0.243, p = 0.628, r2 = 0.013; abundance: F[1, 18] = 0.414, p = 0.528, r2 = 0.022) and Q. resinosa (richness: F[1, 18] = 1.442, p = 0.245, r2 = 0.074; abundance: F[1, 18] = 0.003, p = 0.961, r2 = 0.001). We did not perform these analyses for Q. eduardii because its acorns were completely removed from all experimental units by the end of the experiment. On the other hand, when the individual effects of granivorous species were analyzed, a weak positive relationship was found between the total number of removed acorns and the abundance of Mexican jays (F[1, 18] = 8.126, p = 0.011, r2 = 0.311 - Fig. 6), but no relationships were found for the other granivores (data not shown).
Fig. 4 - Relationships of richness (a) and total abundance of acorn consumers (b) with distance from the forest edge, and relationship between the total number of removed acorns and the richness (c) and abundance of granivores (d).
Fig. 5 - Relationships of the number of removed acorns of Quercus affinis (a, b) and Quercus rugosa (c, d) with the richness (a, c) and total abundance (b, d) of granivores.
Fig. 6 - Relationship between the total number of removed acorns and the abundance of Mexican jays (Aphelocoma ultramarina). F[1, 18] = 8.126, p = 0.011, R2 = 0.311.
Discussion
Our results suggest that granivorous vertebrates alter their activity patterns along the forest-edge-clearing gradient, which in turn causes changes in their richness and abundance across these habitats. The six species of acorn consumers recorded in this study exhibited a positive association with the forest habitat, while most of them were negatively associated with the man-made clearing. These results agree with the widely accepted hypothesis that impacts of human activities on forest landscapes reduces the diversity of wild animals ([18], [7], [50]). Consistently with these distribution patterns of granivores, acorn removal rates gradually decreased from the forest interior towards the forest edge and, for most oak species, they abruptly decreased in the clearing. Previous studies have reported that seed removal rates in HMF decrease from the interior of forests towards human-impacted areas ([42], [48], [26], [24], [2]), but richness and abundance of seed consumers have been barely considered to explain seed removal patterns. In this study, acorn removal rates correlated with these two variables (richness and abundance of granivores) and their values decreased from the maximum distance inwards the forest (50 m) towards the maximum distance in the man-made clearing (-50 m). To the best of our knowledge, this is the first study reporting the relationship between the reduction of seed removal chance in a man-made clearing and the decrease in richness and abundance of seed consumers.
Our results also indicate that different oak species have differential consumption patterns. Oak species with bigger acorns (Q. resinosa) showed extremely low removal rates (less than 10%), even within the forest, which is the habitat that most granivores seems to prefer. This is somewhat counterintuitive because large animals which manipulate and consume these acorns (e.g., collared peccaries and grey foxes) were positively associated with the forest. Therefore, it could be hypothesized that the acorns of this oak species are not palatable for the fauna that currently inhabit this HMF. Alternatively, Q. resinosa may require highly specialized granivores that are no longer present in the study area. This may be the case of the wild turkey (Meleagris gallopavo), which is an important acorn consumer in most oak forests of North America ([45]), but this bird species was extinguished in most temperate forest of Mexico because of intense poaching ([27], [4]). Such scarce consumption of Q. resinosa acorns deserves more attention in future studies and their chemical composition should be determined to evaluate whether they contain unpalatable compounds for wild animals.
Acorn removal rates of all other oak species (Q. affinis, Q. castanea, Q. eduardii and Q. rugosa) decreased from the forest towards the man-made clearing, though removal patterns not always concurred with changes in richness and abundance of granivores. Removal rates of Q. affinis and Q. rugosa consistently decreased with the reduction in richness and abundance of granivores along the forest-edge-clearing gradient. This agrees with the general suggestion that human-induced disturbances can disrupt the ecological processes that take place in natural ecosystems ([41]). These results suggest that the opening of man-made clearings can change the behaviour of granivores, as most these animals seemed to avoid the clearing even when food resources were available. Thus, the decreasing removal rates of Q. affinis and Q. rugosa from the forest to the clearing may be linked with a simplification of the community of consumers towards the clearing, thus reducing the probability that different animal species could perform the same ecological role. On the other hand, although acorn removal rates of Q. castanea decreased from the forest towards the clearing, they were neither related with changes in richness nor abundance of granivores. As compared with Q. affinis and Q. rugosa, this is likely due to a higher palatability of Q. castanea acorns for most granivores, as reflected by the higher removal rate of its acorns observed in most habitats.
The exception to these removal patterns was Q. eduardii, whose acorns were completely removed irrespectively of the habitat where they were placed. These elevated acorn removal rates, as compared with those of the other oak species, may be attributed to differential preferences of their consumers. Acorns of Q. eduardii are much smaller than those of the other oaks species included in this study, and this facilitates their manipulation by the small-bodied consumers, such as jays and mice. The marked preference of these granivores for this oak species could also be linked with an elevated palatability of these acorns ([40]).
Despite the specific removal patterns recorded for each oak, it is important to highlight that total acorn removal was positively related with the abundance of Mexican jays across the forest-edge-clearing gradient, and this was the only granivorous species that displayed such a relationship. This indicate that Mexican jays are important consumers (and scatterers) of acorns across all habitats of the forest-edge-clearing gradient. Nevertheless, it can be concluded that the elevated acorn removal within the forest is the result of a large number of interactions between these fruits and their consumers.
Conclusions
This study indicates that richness and abundance of granivorous vertebrates decrease along the forest-edge-clearing gradient of our study site. The total number of removed acorns (i.e., irrespective of the oak species) decreased with the decrease in richness and abundance of seed consumers. This relationship provide insights about how the opening of man-induced clearings can alter fundamental ecosystem processes in oak forests. However, it is worth to note that some of the oak species studied departed from this general pattern. Differences among oak species may be due to differential abilities of granivores to manipulate acorns of different size, which in turn may have important consequences on forest regeneration. Large granivores, such as foxes and peccaries, are not likely to contribute to forest regeneration as they directly consume the acorns ([20]). Conversely, small vertebrates, such as mice and squirrels, usually store large amounts of acorns in soil catches ([47], [19]), thus favouring seed dispersal and oak forest regeneration. Indeed, because most granivores avoid visiting forest clearings to reduce their predation risk, some scatter-hoarding rodents locate their acorn catches in these habitats to prevent pilferage of their food reserves ([28]). As several acorn catches are latter forgotten, this promotes acorn germination in safe sites and allows forest regeneration in clearings ([47], [19]). Moreover, our results indicate that Mexican jays can also act as important acorn dispersers in HMF, as these birds may lose the acorns while flying ([43]). Our findings highlight the importance of animal-mediated secondary dispersion in the regeneration of oak forests, and the negative effect of large man-made clearings on the community of consumers, as most potential acorn dispersers seem to avoid these habitats.
Acknowledgments
We are grateful with the owners of the Wildlife Management Unit “La Laguna”, Ana Mayra de la Garza and José Luis Carrera, for the logistic support provided during this study. We thank JP Rodas-Ortiz for his support during the collection of the acorns. We also thank the valuable comments of the three anonymous reviewers that contributed to improve the earlier versions of the manuscript. This study was supported by project SEP-CONACYT CB-2013/221623 to EIB.
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
David Douterlungne
CONACYT-IPICYT, División de Ciencias Ambientales, Instituto Potosino de Investigación Científica y Tecnológica, Camino a la Presa San José 2055, Lomas 4ª Sección, C.P. 78216, San Luis Potosí, SLP (México)
Joel Flores
IPICYT-División de Ciencias Ambientales, Instituto Potosino de Investigación Científica y Tecnológica, Camino a la Presa San José 2055, Lomas 4ª Sección, C.P. 78216, San Luis Potosí, SLP (México)
Corresponding author
Paper Info
Citation
Barragán F, Badano EI, Douterlungne D, Flores J (2018). Richness and abundance of granivorous vertebrates determine acorn removal patterns in a human modified oak forest. iForest 11: 329-337. - doi: 10.3832/ifor2216-011
Academic Editor
Gianfranco Minotta
Paper history
Received: Sep 05, 2016
Accepted: Jan 21, 2018
First online: Apr 18, 2018
Publication Date: Apr 30, 2018
Publication Time: 2.90 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2018
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 36988
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 32312
Abstract Page Views: 1880
PDF Downloads: 2068
Citation/Reference Downloads: 17
XML Downloads: 711
Web Metrics
Days since publication: 2199
Overall contacts: 36988
Avg. contacts per week: 117.74
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Feb 2023)
Total number of cites (since 2018): 1
Average cites per year: 0.17
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Regeneration of Abies pinsapo within gaps created by Heterobasidion annosum-induced tree mortality in southern Spain
vol. 7, pp. 209-215 (online: 27 February 2014)
Research Articles
Effects of gap size and within-gap position on seedlings establishment in silver fir stands
vol. 1, pp. 55-59 (online: 28 February 2008)
Review Papers
Soil fungal communities across land use types
vol. 13, pp. 548-558 (online: 23 November 2020)
Research Articles
Effect of size and surrounding forest vegetation on chemical properties of soil in forest gaps
vol. 8, pp. 67-72 (online: 04 June 2014)
Technical Reports
Effects of different mechanical treatments on Quercus variabilis, Q. wutaishanica and Q. robur acorn germination
vol. 8, pp. 728-734 (online: 05 May 2015)
Technical Reports
The treatment of land use, land use change and forestry in the post-2012 climate agreement: a perspective from non-Annex I Parties
vol. 3, pp. 56-58 (online: 17 May 2010)
Research Articles
Role of forest cover, land use change and climate change on water resources in Marmara basin of Turkey
vol. 8, pp. 480-486 (online: 31 October 2014)
Research Articles
Influences of forest gaps on soil physico-chemical and biological properties in an oriental beech (Fagus orientalis L.) stand of Hyrcanian forest, north of Iran
vol. 13, pp. 124-129 (online: 07 April 2020)
Research Articles
Reforestation and land use change in a drainage basin of southern Italy
vol. 6, pp. 175-182 (online: 08 May 2013)
Research Articles
Land use inventory as framework for environmental accounting: an application in Italy
vol. 5, pp. 204-209 (online: 12 August 2012)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword