Rapid spread of a fleshy-fruited species in abandoned subalpine meadows - formation of an unusual forest belt in the eastern Carpathians
iForest - Biogeosciences and Forestry, Volume 9, Issue 2, Pages 337-343 (2015)
doi: https://doi.org/10.3832/ifor1470-008
Published: Nov 20, 2015 - Copyright © 2015 SISEF
Research Articles
Abstract
In recent decades, most subalpine hay meadows and pastures have been abandoned, and trees have been recolonizing these sites where forest existed before agricultural activity. This study examined how woody vegetation, dominated by the deciduous fleshy-fruited tree Sorbus aucuparia (rowan), expanded on subalpine meadows in the Western Bieszczady Mountains (eastern Carpathians, Poland) after the cessation of agricultural use. The aims were to determine the abundance of rowan in the woody vegetation, to estimate the rate of rowan expansion in the studied area, and to characterize the variability of rowan stands and growth forms. Rowan dominated the current plant community of abandoned subalpine meadows, though this species is not considered a rapid colonizer of open areas and was not frequent in the uppermost forest belt before the colonization. The whole area was encroached by rowans in a very short period of time 60-70 years ago. Rowan tree density was similar throughout the elevational gradient but the growth form changed, becoming more shrub-like with increasing elevation. Rowan stands will likely be the main element of the subalpine belt in this region in the upcoming decades. At present, no tree species can be considered a rapid successor to rowan in the area.
Keywords
Abandoned Subalpine Meadows, Forest Recolonization, Land Use Change, Subalpine Forest, Succession of Woody Vegetation
Introduction
Recent studies on vegetation of European mountains reported the re-establishment of the forest in subalpine meadows where the upper forest limit was formerly shifted downward by human activity ([26], [4], [21], [16], [48], [6], [1], [45], [9], [50]). It is thought that forest expansion in the subalpine belt is primarily due to land use changes in the last century, mainly the abandonment of hay meadows and pastures. However, the effect of climate change can not be excluded, since favorable climatic conditions may facilitate forest encroachment into subalpine scrubland and grassland ([16], [50]).
Carpathians subalpine forests cover an elevation range from about 1200-1500 to 1250-1650 m a.s.l. ([53], [45]), and are mostly formed of Picea abies (L.) H. Karst., accompanied in some areas by other coniferous species: Larix decidua Mill., Pinus cembra L. and Pinus sylvestris L. The uppermost forest belt may also include deciduous tree species, mostly beech (Fagus sylvatica L.) and less frequently sycamore (Acer pseudoplatanus L.), birches (Betula pendula Roth and B. pubescens Ehrh.), or rowan (Sorbus aucuparia L.). During the expansion of subalpine forest, the most dynamic changes usually occur along the timberlines composed of conifers such as P. abies ([45]), Pinus uncinata Ram. ([8]) or L. decidua ([11]) which are anemochorous species. Over the entire Carpathian range, F. sylvatica forest is less responsive to favorable climatic changes and agriculture cessation than coniferous forests, resulting in a relatively stable timberline ([30], [50]).
Generally, tree species colonizing subalpine non-forest areas are those belonging to the uppermost forest belt ([1], [22], [9]), with several exceptions. Sitko & Troll ([45]) found abandoned meadows above the anthropogenic beech timberline in the Ukrainian Carpathians commonly colonized by wind-dispersed spruce. An unusual situation occurs also in the Western Bieszczady Mountains (eastern Carpathians, Poland), where the cessation of agriculture also led to the recolonization of abandoned meadows by the forest, though its species composition is different as compared with those prevailing in the uppermost forest belt. Indeed, the anthropogenic upper forest limit was previously formed by beech forests with small admixtures of A. pseudoplatanus and S. aucuparia. Such meadows were first colonized by Alnus viridis (Chaix) DC. in LAM. & DC. ([43], [53]). Surprisingly, after about 70 years of secondary succession, rowan was the woody species that expanded most abundantly in the subalpine belt of the Western Bieszczady Mountains ([13], [14]). In particular, rowan is currently the dominant species in some areas of this region, resulting in the formation of a new forest belt above the former upper limit of beech forests (Fig. 1).
Fig. 1 - Vegetation belt dominated by rowan tree stand on the Bukowe Berdo ridge (eastern Carpathians, southern Poland).
In this study, we examined two aspects of rowan stand formation after colonization of abandoned subalpine meadows in the Western Bieszczady Mountains, namely: (i) how quickly rowan has spread over meadow areas; and (ii) what was the structure of stand after approximately 70 years of recolonization. The specific aims of this study were to: (1) determine the abundance of rowan in the woody vegetation that developed in abandoned subalpine meadows; (2) estimate the rate of rowan expansion; and (3) examine the variability of rowan stand and growth form characteristics in relation to habitat conditions.
Methods
Study site
The study was conducted in the subalpine area of the Western Bieszczady Mountains (eastern Carpathians, southern Poland), where the original vegetation was likely made by scattered clumps of S. aucuparia and A. viridis stretching from the natural upper timberline (1150-1260 m a.s.l. - [53]) to the mountain top (up to 1350 m a.s.l.). Starting since 15th century, pastures and hay meadows have been created by clearcut, determining a shift downward of the natural timberline. Such deforestation activity ended in the middle of the 19th century: in the 1940s the agricultural use of subalpine meadows in the Western Bieszczady Mountains was completely abandoned, and woody vegetation began to encroach ([29]). In 1973, the whole area was included into the Bieszczady National Park and became part of the Eastern Carpathian International Biosphere Reserve in 1998.
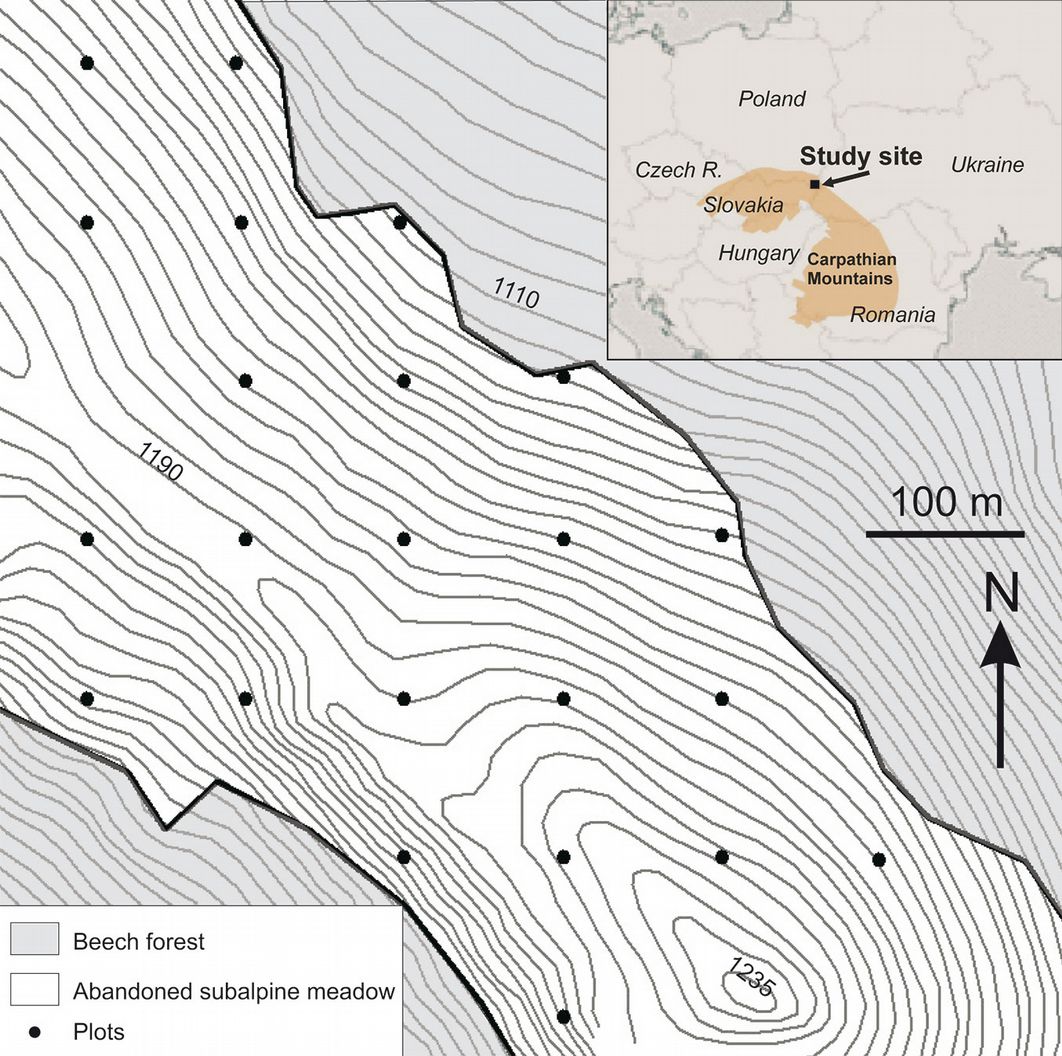
The study area comprised the subalpine belt of the Bukowe Berdo ridge (49° 6′ 0″ N, 22° 43′ 50″ E, 1238 m a.s.l.). According to archival cadastral maps, this area was used as pasture for cattle in 1852 and in later decades. Agriculture in this area was moderately intensive, and further decreased after World War I. Indeed, some scrublands are reported on the Bukowe Berdo ridge on the 1937 map, suggesting that the abandonment of subalpine meadows in this area started earlier than in other parts of the region ([27], [28], [29]).
The study site encompassed approximately a 22-hectare area of a former subalpine meadow, now largely colonized by rowan stands, just above the beech timberline that represented the upper forest limit before the abandonment. The site stretched over the NE and SW slopes of the massif (mean slope: 18.5°; range: 5-35°), from the sheltered vicinity of the beech forest limit to the most elevated places of the mountain ridge (range 1111-1214 m a.s.l.). Soil type varied across the study site, with dystric cambisols at lower elevations and lithosols, regosols, rankers and umbric rankers at higher elevations closer to the ridge ([46]). Only the area where rowan saplings were present was considered in this study.
Study species
S. aucuparia (Rosaceae, Maloideae) is a deciduous monoecious fleshy-fruited tree living up to 100-150 years and reaching 15-20 meters in height ([31], [19]). Seeds are dispersed by animals, mainly birds ([40]). In central European highlands, rowan occurs from foothills up to subalpine scrubland at 2000 m a.s.l., in various forest types and different climatic and edaphic zones ([42], [40]). Rowan is usually a minor component of the plant communities, reaching fairly higher frequencies in species-poor coniferous forests or disturbed stands ([19], [20]), where it may colonize newly-formed gaps ([54], [24]). This process largely relies on the recruitment from seedling and sapling banks established before gap formation ([58], [56]).
Data collection
A regular grid (100 × 100 m) of 23 circular plots (0.01 ha) was established in the study site in 2012 (Fig. 2). Plots were positioned with GPS (accuracy 2-3 m) and characterized in terms of several topographic and habitat parameters. Plot aspect and slope were measured in the field using a compass and a clinometer. Elevation was derived from a digital elevation model (GRID with cell size 5 m). The soil type was determined on the basis of a soil map ([46]). The distance between each plot and the upper limit of the beech forest was measured using an aerial photo.
Within each plot, individuals of all woody species were counted and characterized by their height, the number of shoots (longer than 5 cm) per individual, and the diameter of each stem at breast height (dbh). Youngest seedlings (height <5 cm) were not included in view of their high mortality and problems with their detection (due to low stature). Tree species nomenclature followed Mirek et al. ([34]).
For each plot, the age of the five thickest rowans was measured, under the assumption that the thickest ones were the oldest. Trees were cored with an increment borer (diam. 5 mm) at ca. 30 cm above the ground. The cores were mounted on wood slats, dried and progressively polished with a belt sander (up to grid 600) to make the tree ring sequences clearly visible. The samples were scanned at a resolution of 2400 dpi and annual rings were counted using the WinDendro® software ([41]).
Data handling and analysis
Prior to statistical analysis, the plot aspect was converted from the azimuth values (decimal degrees) into values ranging from -1 to 1 using the formula sin(α-π/2), where α represents the azimuth value given in radians. This transformation produced a continuous variable, whose maximal and minimal values corresponded to opposite facing slopes: N (-1) and S (1). This variable was assumed to represent the gradient of microclimatic conditions in the sampling area. A categorical variable was used for soil type, with value “0” for initial soils such as lithosols, regosols, rankers and umbric rankers, or “1” for more developed soils, i.e., dystric cambisols.
For each plot, tree density and total basal area (per hectare) were calculated for each woody species. For rowan, the density of all shoots (per hectare) was also determined. The relative number of individuals and relative basal area of each woody species in each plot (100 m2) were also calculated. Basal area was calculated using the dbh of all stems taller than 1.3 m. The age of the oldest rowan tree sampled at a given plot was taken as the approximate time of tree establishment. We recorded the maximum height, mean stem diameter, mean number of stems per individual (at ground level) and mean number of twigs per stem (at breast height) of live rowans in each plot.
Non-normally distributed data (continuous variables) were transformed using logarithmic or exponential functions, according to Okland et al. ([38]). To explain the variability of rowan tree stand and growth form characteristics, multiple regressions were performed usin the following predictors : aspect, elevation, slope and soil type. Significant predictors were identified using a forward stepwise selection procedure.
Results
Tree stand characteristics
The studied stand was mainly formed by rowan, which averaged 81.7% (range: 36.4-100%) of all tree individuals per plot. Other tree species were A. pseudoplatanus (10.4%), F. sylvatica (3.5%), A. viridis (3.4%), Abies alba Mill. (0.6%), P. abies (0.2%) and Salix silesiaca Willd. (0.2%). Rowan basal area averaged 18.4 m2 ha-1(range: 3.6-34.3 m2 ha-1), accounting for 90.3% (range: 28.1-100%) of the total stand basal area. The density of rowan trees ranged from 300 to 2700 individuals ha-1 (mean: 1330 ind. ha-1), and the density of rowan shoots ranged from 500 to 27 100 ha-1 (mean: 6990 ha-1). Stand age, as indicated by the age of the oldest rowan tree, ranged from 57 to 75 years (mean: 65 years). Some of the tree stand characteristics were highly correlated (Appendix 1).
In a preliminary analysis, Pearson’s correlation tests were used to explore the strength of associations between variables within the explanatory data set (environmental variables) and response data set (rowan variables - Appendix 1). This analysis showed that plot elevation was strongly correlated with the distance between plots and the edge of the beech forest (r=0.62; p<0.002). The nature of the relationship between these parameters and rowan variables suggested that plot elevation is a causative variable. Therefore, the distance to the edge of the beech forest was selected and excluded from further analysis to reduce multicollinearity in the environmental data.
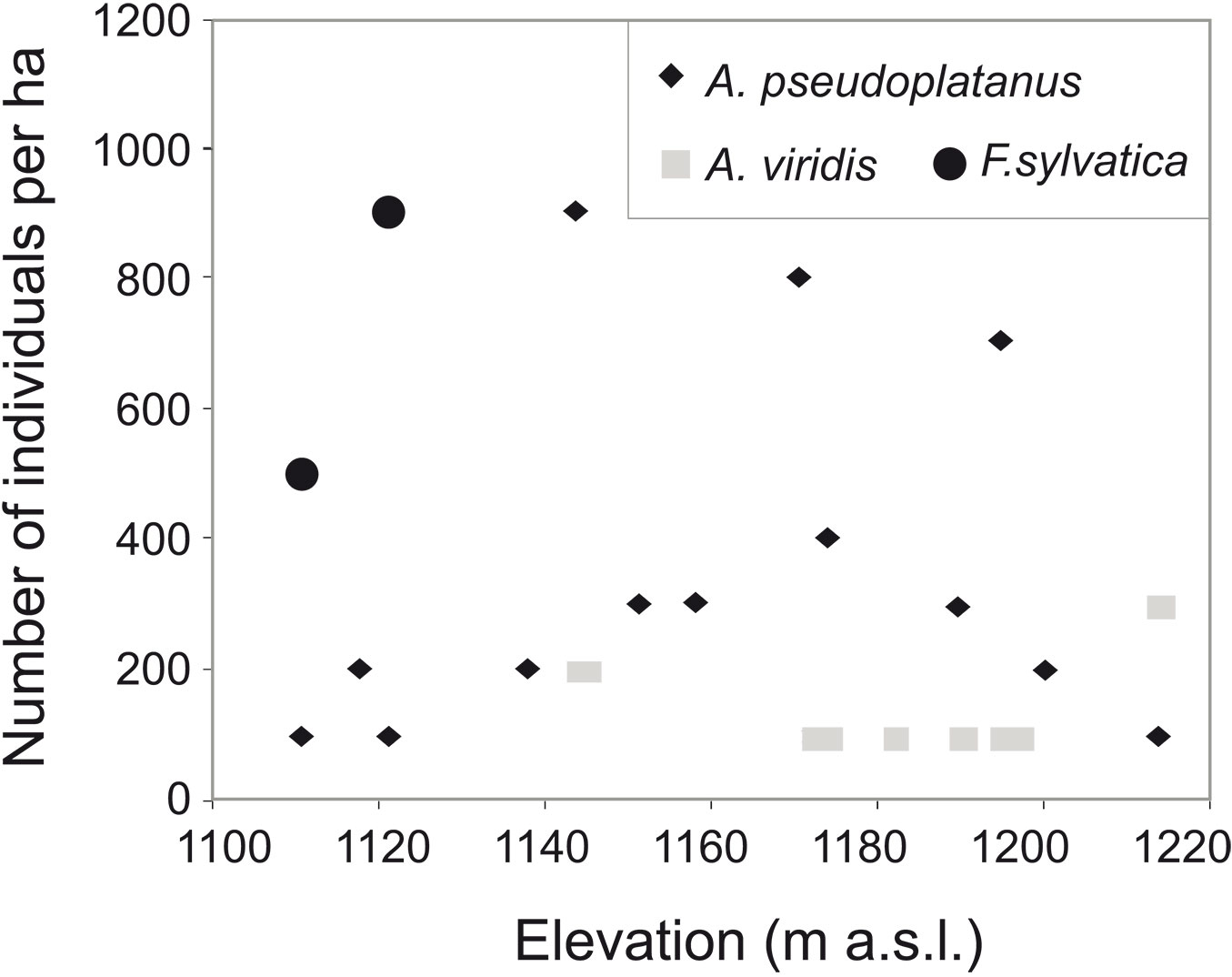
Multiple regressions showed that almost all stand characteristics were independent of the environmental variables (Tab. 1). Only the density of rowan shoots was significantly associated to a topographic gradient, increasing with elevation. Elevation also seemed to affect the distribution of some other species. For example, F. sylvatica was present only at lower elevations close to the upper limit of the beech forest, whereas A. viridis was absent from the lowest plots (Fig. 3). However, the total share of non-rowan species in the total number of tree individuals and the total stand basal area was the same regardless of elevation.
Tab. 1 - Effects of plot parameters on the characteristics of rowan tree stand and growth form illustrated by standardized (Beta) coefficients of multiple regression. (R2adj): adjusted R2; (F): the F ratio; (p): significance level; (-): variable was not included in the model; (*): p <0.05; (**): p <0.01; (***): p <0.001.
| Group | Dependent variable | Regression summary | Beta coefficients | |||||
|---|---|---|---|---|---|---|---|---|
| R 2 adj | F | p | Aspect | Elevation | Slope | Soil | ||
| Tree stand |
Age of oldest rowan | 2.7 | 1.3 | 0.294 | - | - | 0.271 | 0.228 |
| SD of age of oldest rowans | 4.0 | 1.5 | 0.255 | - | 0.213 | -0.252 | - | |
| Density of live rowans (ind. per ha) | 10.2 | 3.5 | 0.075 | - | - | -0.378 | - | |
| Density of rowan shoots (per ha) | 33.9 | 4.8 | 0.012 | - | 0.731** | -0.254 | 0.435 | |
| Percentage of species other than rowan | 7.6 | 1.9 | 0.176 | - | -0.552 | - | -0.338 | |
| Percentage of species other than rowan in total basal area | 14.7 | 2.9 | 0.078 | -0.316 | -0.344 | - | - | |
| Total basal area of rowans (m2 ha-1) | 3.0 | 1.7 | 0.208 | - | - | 0.273 | - | |
| Growth form |
Mean basal area of individuals | 28.9 | 9.9 | 0.005 | - | - | 0.567** | - |
| Maximum height of individuals (m) | 57.1 | 10.8 | <0.001 | - | -0.790** | 0.307 | -0.205 | |
| Mean dbh of individuals (cm) | 53.2 | 9.4 | <0.001 | - | -0.850*** | 0.172 | -0.222 | |
| Mean number shoots per individual | 17.7 | 3.4 | 0.055 | -0.276 | 0.428* | - | - | |
| Mean number of twigs per stem | 34.4 | 12.5 | 0.002 | - | 0.611** | - | - | |
Rowan growth form
The diameter of rowan individuals averaged 7.4 (range: 1.3-16.5 cm) and the maximum height of individuals averaged 9.7 (range: 4.5-17.5 m). The mean basal area of rowan ranged from 36.1 to 422.9 cm2 ha-1 (mean: 171.4 cm2 ha-1). The mean number of shoots per individual averaged 5.5 (range: 1-13.5) and the mean number of twigs per stem averaged 78.8 (range: 4-399). Some of the rowan growth form characteristics were highly correlated (Appendix 1).
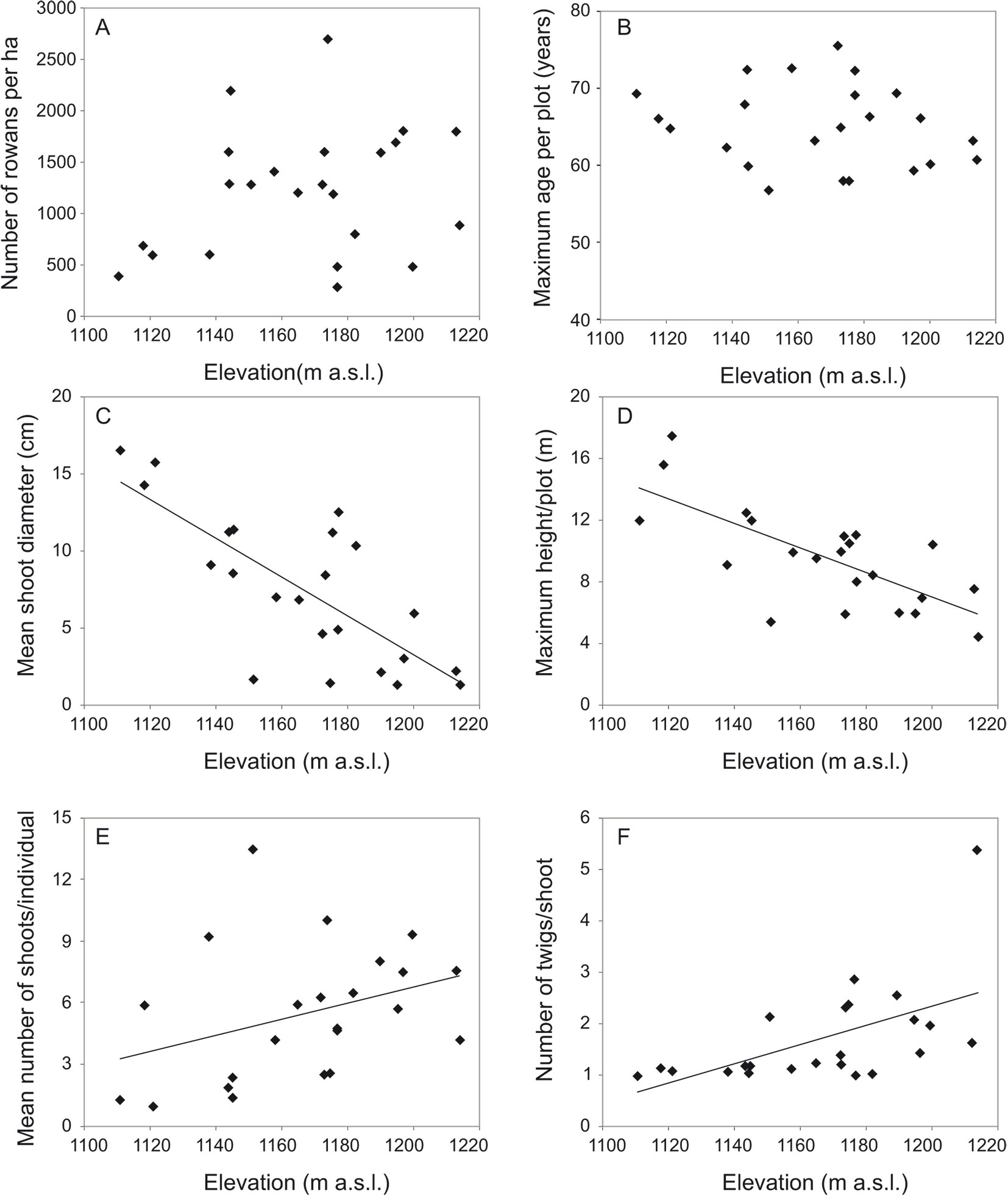
In contrast to tree stand characteristics, rowan growth form clearly varied along environmental gradients. In multiple regression analysis, environmental variables accounted for 17.7 to 57.1% of the variation in growth form characteristics of rowan. Elevation was the most influential factor, that negatively affected mean shoot diameter and the associated maximum height, and positively affected the mean number of twigs per shoot at breast height and mean number of shoots per individual (Tab. 1, Fig. 4). As regards other explanatory variables, slope had a positive effect on mean total basal area, while the effect of aspect and soil type was not significant.
Fig. 4 - Relationship between elevation and: (A) rowan tree density; (B) age of oldest rowans in the plot; (C) mean shoot diameter; (D) maximum height of rowan trees; (E) mean number of stems per rowan tree; and (F) mean number of twigs per stem at breast height.
Discussion
Rowan was the main woody species encroaching on the studied meadows along the whole elevational gradient. Moreover, rowan dominated on both the NE and SW slopes of the massif, from the beech forest limit to the mountain ridge. Similar situations have been reported in other abandoned subalpine meadows on Western Bieszczady Mountains ([51], [12], [14]). This kind of forest community is quite uncommon in the scientific literature. In the subalpine zone of the Massif Central in France, Schaminée et al. ([42]) described scrub communities dominated by Sorbus species on abandoned pastures, with S. aucuparia being the most common species. Borysiak ([5]) found both shrublands and woodlands formed by rowans at the natural upper forest limit on the Babia Góra Massif (western Carpathians, Poland). Kullman ([31]) reported a high frequency of rowan trees in the subalpine zone of the Scandes in Sweden. Subalpine communities with only a small admixture of rowan in the woody species composition were reported more frequently ([35], [47], [57]).
Before their abandonment, subalpine meadows in the study area bordered the forest dominated by F. sylvatica, with small admixtures of A. pseudoplatanus, P. abies, A. alba and S. aucuparia ([43], [53]). Soon after abandonment, scrubland dominated by A. viridis grew above the forest limit, with S. aucuparia, A. pseudoplatanus, S. silesiaca and Rosa pendulina being less frequent ([43], [53]). All these species still occur in the vegetation on the abandoned meadows, though rowan became dominant since 1990 ([51]) and now is largely the most abundant species, in particular in terms of basal area. The role of rowan in the stand increased significantly with elevation, which suggests that this species is more able to colonize subalpine meadows than any other woody species present in the area. This is surprising because rowan was rare in the studied meadows in the 1950s and 1960s, and because its mode of dispersal by animals (mainly birds) does not facilitate the fast colonization of large open areas. Hill et al. ([18]) showed rowan invading abandoned hill pastures at sites located in the close vicinity of bird perches. However, it is possible that birds were not the main seed dispersers in the studied area. Rowan seeds could have been supplied as a result of past pasture management practices and the former presence of livestock. In this case, rowan regeneration may have started in the meadow area long before its abandonment. Ungulates can be effective seed dispersers ([23]), facilitating seedling emergence by trampling and exposing the bare soil, by reducing competition with grasses, and by promoting upslope migration as seed dispersers. Unlike mowing, grazing may facilitate seedling establishment in grassland ([7], [25]). In an experimental study it was found that intense grazing by sheep increased rowan seedling recruitment ([17]). Rowan can survive for decades under suppression and grazing pressure, due to its ability to replace dead stems with new ones ([56]). This ability gives it an advantage over the other woody species in the area, and could have supported the presence of a rowan population in subalpine meadows before abandonment.
In the studied subalpine meadow, rowan expanded at approximately the same time along the entire elevational range, covering areas from the sheltered vicinity of the beech forest limit to exposed fragments on the mountain ridge. The recorded age of the oldest rowans differed only slightly between plots, and their mean age matched the time since the abandonment of meadows. These findings might be explained in two ways. The first hypothesis is that the seeds were dispersed and seedlings were established throughout the whole area at the same time. This assumes that the probability of seed supply and seedling establishment was high and equal throughout the whole area. This seems unlikely, as rowan is considered a slow colonizer of open areas ([58]). Moreover, the dispersal by animals usually results in a very uneven distribution of seeds and seedlings ([44], [10]). The second explanation is that seedling development in the meadow vegetation was inhibited by adverse biotic or abiotic conditions. Most likely the inhibiting factor was pastoralism. Rowan seedlings lasted under grazing pressure in pasture vegetation for a long period of time, and their vertical growth accelerated when such pressure ceased. Under this second scenario the age of individuals should differ, but this difference could not be detected in this study as we took core samples at 30 cm above the ground, i.e., a height threshold not reached by woody plants growing in pastures. In the case of long-term persistence under grazing pressure, the growth increments at ground level would hardly ever show the real age of individuals. As rowan could survive under grazing pressure, it was likely that suppressed individuals accumulated over time ([56]). Previous works have shown that young rowans can markedly increase their growth rate after suppression ceases ([17], [55]). All these features of the regeneration strategy of rowan make the second explanation much more probable. Whatever the case of seedling establishment, rowan tree stands expanded in the studied area almost simultaneously, as evidenced by the narrow range of ages of the measured trees.
In this study, density of rowan trees did not decrease with increasing elevation and distance to the forest limit and decreasing distance to the mountain ridge. This result suggests that environmental conditions are suitable for regeneration and growth along the whole elevational gradient, confirming the broad edaphic and climatic amplitude of rowan. This species is adapted to a short growing season in that it stops shoot growth early, allowing buds to harden before freezing ([2], [40]) and tolerates an extremely low stem water potential corresponding to the minimum values in sclerophyllous plants ([49]). Furthermore, rowan bears mechanical damage very well, forming the wound periderm rapidly ([52]), and it has a high vegetative regeneration capacity ([31]). Indeed, plants are often multi-stemmed and the sprouting mechanism can very effectively extend their lifespan ([56]). All these features facilitated rowan survival in the harsh conditions of the mountain ridge.
The rowans’ growth form changed along with elevation. At higher elevations rowans were shorter, their stems were thinner, and they had more shoots per plant than at lower elevations; in other words, they were more compact and more shrub-like. This trend is well known in mountain woody species ([4], [22]). Short-stature plants are less affected by temperature and snow cover better protects low-growing plants from freezing ([22]). A multi-stemmed form and the ability to sprout are important features enabling the rowan to survive the harsh mountain conditions, especially close to ridges. Vegetative reproduction by stump sprouts gives an advantage under stem breakage by wind, avalanches or heavy snow loads ([3], [22]). Rowan shows high sprouting ability ([31], [56]), and multi-stemmed individuals can survive for decades or longer through shoot turnover.
An interesting question is whether the rowan tree stands will be a long-lasting forest formation in this area, or rowan is rather only a pioneer species which will be displaced by other woody species in the long run. In other Carpathian ranges, rowan trees are usually overtopped and replaced by spruce, and it is reported that young spruces take advantage of improved soil conditions resulting from deposition of rowan litter ([15]). At present, no tree species can be considered a quick successor of rowan in the subalpine area of the Bieszczady mountains. It is most probable that beech stands will expand and replace rowans, though this will take a very long time due to constrains on dispersal of beech nuts. In fact, we recorded this species only in the lowermost plots. However, beech forest is thought to have covered the mountain ridges up to 1250 m a.s.l. until the 15th century ([27], [39]). Another candidate for replacing rowan is sycamore, which was recorded on every second plot with similar frequency along the whole elevational gradient but with much lower abundance. Its seeds are dispersed by wind and can easily reach anywhere in subalpine areas. However, A. pseudoplatanus is less shade tolerant than F. sylvatica ([37]). It has been found that more shade tolerant species can maintain foliage in deeper shade, support larger leaf area indexes and harvest more light ([36]). It might be hypothesized that coexistence would be more probable if light demanding rowan grows together with moderate shade tolerant sycamore than with very shade tolerant beech. Such mixed rowan-sycamore stands are well known in the Polish Carpathians ([33], [32]). Therefore, we hypothesize that rowan or mixed rowan-sycamore stands will be most likely the main element of the subalpine belt in this region in the upcoming decades.
Acknowledgements
We thank the authorities and staff of Bieszczady National Park for their kind cooperation and for granting permission to conduct research in the Park. This study was supported by the National Science Center (grant no. N N305 390739). The statutory funds of the University of Rzeszów and the W. Szafer Institute of Botany of the Polish Academy of Sciences also provided partial funding.
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Online | Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Supplementary Material
Authors’ Info
Authors’ Affiliation
Department of Botany, University of Rzeszów, ul. Zelwerowicza 4, PL-35-601 Rzeszów (Poland)
Pawel Kapusta
Institute of Botany, Polish Academy of Sciences, ul. Lubicz 46, PL-31-512 Kraków (Poland)
Adam Mickiewicz University, Faculty of Biology, Department of Plant Ecology and Environment Protection, ul. Umultowska 89, PL-61-614 Poznan (Poland)
Corresponding author
Paper Info
Citation
Durak T, Zywiec M, Kapusta P, Holeksa J (2015). Rapid spread of a fleshy-fruited species in abandoned subalpine meadows - formation of an unusual forest belt in the eastern Carpathians. iForest 9: 337-343. - doi: 10.3832/ifor1470-008
Academic Editor
Emanuele Lingua
Paper history
Received: Oct 10, 2014
Accepted: Jul 17, 2015
First online: Nov 20, 2015
Publication Date: Apr 26, 2016
Publication Time: 4.20 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2015
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 49341
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 41358
Abstract Page Views: 2772
PDF Downloads: 3889
Citation/Reference Downloads: 24
XML Downloads: 1298
Web Metrics
Days since publication: 3711
Overall contacts: 49341
Avg. contacts per week: 93.07
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
(No citations were found up to date. Please come back later)
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Short Communications
Culturable fungi associated with wood decay of Picea abies in subalpine forest soils: a field-mesocosm case study
vol. 11, pp. 781-785 (online: 28 November 2018)
Research Articles
Former charcoal platforms in Mediterranean forest areas: a hostile microhabitat for the recolonization by woody species
vol. 10, pp. 136-144 (online: 06 October 2016)
Research Articles
Soil stoichiometry modulates effects of shrub encroachment on soil carbon concentration and stock in a subalpine grassland
vol. 13, pp. 65-72 (online: 07 February 2020)
Research Articles
Soil respiration along an altitudinal gradient in a subalpine secondary forest in China
vol. 8, pp. 526-532 (online: 01 December 2014)
Research Articles
The effects of post-pasture woody plant colonization on soil and aboveground litter carbon and nitrogen along a bioclimatic transect
vol. 6, pp. 238-246 (online: 13 June 2013)
Research Articles
Carbon storage and soil property changes following afforestation in mountain ecosystems of the Western Rhodopes, Bulgaria
vol. 9, pp. 626-634 (online: 06 May 2016)
Research Articles
Assessment of land sensitivity to degradation using MEDALUS model - a case study of Grdelica Gorge and Vranjska Valley (southeastern Serbia)
vol. 15, pp. 163-170 (online: 07 May 2022)
Technical Reports
The treatment of land use, land use change and forestry in the post-2012 climate agreement: a perspective from non-Annex I Parties
vol. 3, pp. 56-58 (online: 17 May 2010)
Research Articles
Role of forest cover, land use change and climate change on water resources in Marmara basin of Turkey
vol. 8, pp. 480-486 (online: 31 October 2014)
Review Papers
Soil fungal communities across land use types
vol. 13, pp. 548-558 (online: 23 November 2020)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword