Dispersal and hoarding of sympatric forest seeds by rodents in a temperate forest from northern China
iForest - Biogeosciences and Forestry, Volume 7, Issue 2, Pages 70-74 (2014)
doi: https://doi.org/10.3832/ifor1032-007
Published: Nov 18, 2013 - Copyright © 2014 SISEF
Research Articles
Abstract
Different species of forest trees exhibited great diversity in seed features, and rodents might take different tactics to handle and disperse them. In September 2011, to understand the discriminatory handling by rodents on sympatric seeds, seeds of four plant species, Quercus variabilis, Prunus armeniaca, P. davidiana, and P. persica, were released and tracked in a temperate forest in Yugong area of Jiyuan, Henan, north China. Results showed that: (1) seed removal rates of acorn (Q. variabilis), wild apricot (P. armeniaca) and wild peach (P. davidiana) differed significantly, while almost all (99%) peach seeds (P. persica) remained in situ; (2) acorns (55%) were eaten more than wild apricot (4%) and wild peach (0%), whereas seeds of wild apricot (62%) were scattered-hoarded more than wild peach (13%) and acorns (36%); hull thickness exerted a nonlinear influence on eating and scatter-hoarding; (3) rodents transported wild peach seeds farther (3.81 m ± 2.44 SE) than wild apricot seeds (3.41 m ± 2.05) and acorns (2.49 m ± 2.37); (4) rodents buried multiple wild apricot seeds in some caches, but seeds of wild peach and acorn were stored singly. Results indicated that, for sympatric seeds, rodents would adopt discriminatory processing and storing strategies in eating, burying, dispersal and cache size. Seeds with medium hull thickness were more likely to be dispersed and survived, and consequently have higher probability of future germination and seedling establishment.
Keywords
Seed Traits, Rodent, Discriminatory Dispersal, Cache Size, Dispersal Distance, Seed Fate
Introduction
Interactions between forest seeds and rodents have been widely reported ([38], [2], [24], [3], [4], [10], [28], [48]). Many granivorous rodents are known to store large amounts of plant seeds in the field during seed-rich period ([38], [19], [16], [50], [8], [23]). Rodents’ scattering-hoarding behavior often plays a crucial role on seed dispersal and plant recruitment because scatter-hoarded seeds are buried in microhabitat with temperature and moisture favorable to seed survival and germination ([29], [13], [27], [31], [5], [15], [11], [44]). However, in the field, morphological and physiological differences commonly occur among seeds of sympatric tree species ([41], [51]). Thus, seed-eating rodents usually balance between benefits, e.g. net energy income and nutrients, and costs, e.g. predation risks, during seed scattering-hoarding ([17], [14], [9], [33]). Seed traits can influence animals’ decision concerning seed selection, eating or hoarding ([14], [9], [34]). On one hand, seeds with thinner hulls and lower handling costs are disadvantageous for long-term storage and are more likely to be consumed immediately ([51], [6], [30]). On the other hand, seeds with thick hulls are often lardered or scattered-hoarded because of long-term storage advantage ([36], [37], [20], [51]). Seeds with too thick husks are, however, disadvantageous for long-distance dispersal and feeding by rodents because of lower rewards and high predation risks ([51], [30]).
Sympatric animal and plant species have adaptively co-evolved traits to decrease excessive ecological overlap and avoid intra- and inter-specific competitions ([35], [41]). In the forest, plants disclose seed features to attract possible dispersers but avoiding over-predation at the same time; correspondingly, rodents discriminate seeds depending on their palatability, nutrition and physical characteristics ([38], [25], [30]). For instance, small rodents feed mainly on small-sized seeds, while larger rodents consume seeds of various sizes ([43]).
Studies carried out so far on seed selection and dispersal of sympatric seeds by rodents are limited ([6], [7], [47], [30]) and far from fully depicting the wide variation in the hoarding behavior of rodents in different geographical areas. Hull thickness has been reported to significantly affect seed dispersal ([51]), while other investigations have obtained conflicting result ([47]). To further understand discriminatory hoarding strategies of rodents, seeds from four sympatric forest species differing in seed hull thickness were released and tracked in a temperate forest of China. We expected that rodents were preferably eating on seeds with thinner hull and hoarding medium-thick hull seeds, while seeds characterized by over-thick hull were unlikely to be selected by rodents.
Materials and methods
Study site
The study was conducted in the area of Yugong (750 m a.s.l., 112°16’ E, 35°12’ N) in Jiyuan, Henan province, China. This area is dominated by northern temperate zonal continental monsoon climate. The annual average temperature is 14.3 °C, and average annual precipitation about 600-700 mm. Vegetation can be classified into three types: coniferous forests, broad-leaved forests and shrubs. Our study site fell in a secondary broad-leafed deciduous forest, where the most common tree species included Prunus davidiana, P. armeniaca, Quercus variabilis, P. persica, Populus tomentosa, Robinia pseudoacacia and Platycladus orientalis; while brushwood included mainly Lespedeza bicolor, Cotinus coggygria, Ziziphus jujuba var. spinosa and Rosa xanthine ([53], [21]). The field experiment was carried out in a plot (about 200 x 300 m) where Q. variabilis was the dominant tree species; other species like R. pseudoacacia, P. persica, Vitex negundo var. heterophylla, R. xanthina and C. coggygria were sparsely distributed in the plot. Two parallel transects (separated by at least 25 m) were established, and 5 seed stations (1 x 1 m) were selected along each transect (separated by at least 25 m).
Seed collection and preparation
Ripe seeds of wild apricot (P. armeniaca), wild peach (P. davidiana), peach (P. persica) and Cork oak (Q. variabilis) were collected from different trees during the fruiting season, and kept at field temperature to prevent deterioration and germination.
Healthy seeds of the four species were selected randomly for field tests. All selected seeds were tagged with white plastic tags as described in Zhang & Wang ([52]) and Xiao et al. ([44]). A hole of 0.3 mm in diameter was drilled through the husk far from the embryo of each seed, without damaging the cotyledon and embryo. A plastic tag (2.5 x 3.5 cm, < 0.3 g) was tied through the hole of each seed using a thin 10cm-long steel thread. The plastic tag was consecutively numbered to allow all seeds to be easily relocated and identified.
Seed releasing and tracking
In September 2011, in each seed station twenty seeds per species were released together on the ground surface, for a total of 80 seeds per station. Seeds were checked every five days for two months, and their fates were recorded. Status of the released seeds was defined as: (i) eaten (E) - seeds with kernel eaten at or close to the seed station; (ii) scatter-hoarding (SH) - seeds still intact but buried in soil; (iii) abandoned on the surface (AS) - seeds abandoned on the ground surface after removal; (iv) remained in situ (R) - seeds not removed from the station; and (v) missing (M) - seeds removed but not found ([20], [49]).
Rodent trapping
Main rodent species recorded in the study area are Apodemus peninsulae, A. agrarius, Niviventer confucianus, Sciurotamias davidianus, Cricetulus triton and Eutamias sibiricus ([53], [21]). The potential rodent species and their relative abundance occurring during the experiment were monitored with 80 live traps (30 x 13 x 12 cm) baited with peanut (Arachis hypogaea): 20 traps (separated by at least 5 m) set up in each of four transects (separated by at least 25 m). The traps were examined twice a day (dawn and dusk), and rodent species and gender recorded. Trapped rodents were marked and released in situ. Trapping was conducted for three consecutive days at the end of the experiment to reduce possible interferences with field observations.
Seed traits
Seed weight, kernel weight and husk thickness were measured in 100 healthy seeds per species randomly chosen. Seed and kernel weight was measured by an electronic scale (± 0.01 g), whereas husk thickness was measured with an electronic vernier caliper (± 0.01 mm).
Data analyses
Statistical analyses were carried out by SPSS for Windows (Version 16.0). Kaplan-Meier was used to analyze seed removal curves of different species. General linear model - multivariate test (MANOVA) was used to test possible differences of seed fate among species. One-way ANOVA was used to test differences among different species in dispersal distance and cache size (i.e., number of seeds in one scatter-hoarded cache site - [38]). LSD post-hoc test was used for pairwise comparison of means in MANOVA and ANOVA. The occurrence of possible relations between hull thickness and scatter- hoarding or eating was analyzed by using a nonlinear regression analysis.
Results
Trapped rodents and seed traits
Two species of rodents, A. peninsulae and S. davidianus, were trapped at the study area, with a total trap success rate of 1.3% and 4.2%, respectively.
Seeds of the four tested plant species differed greatly in morphological traits (Tab. 1), in terms of, e.g., seed weights (peach > acorn > wild peach > wild apricot) and hull thickness (acorn < wild apricot < wild peach < peach).
Tab. 1 - Seed characteristics of the four investigated species.
| Species | Seed weight (g) |
Kernel weight (g) |
Thickness of seed hull (mm) |
|---|---|---|---|
| Prunus persica | 3.84 ± 0.11 | 0.37 ± 0.01 | 4.95 ± 0.06 |
| Prunus davidiana | 2.32 ± 0.07 | 0.35 ± 0.01 | 3.72 ± 0.07 |
| Prunus armeniaca | 1.03 ± 0.04 | 0.33 ± 0.01 | 1.48 ± 0.03 |
| Quercus variabilis | 3.11 ± 0.17 | 2.76 ± 0.31 | 0.84 ± 0.04 |
Removal dynamics of tested seeds
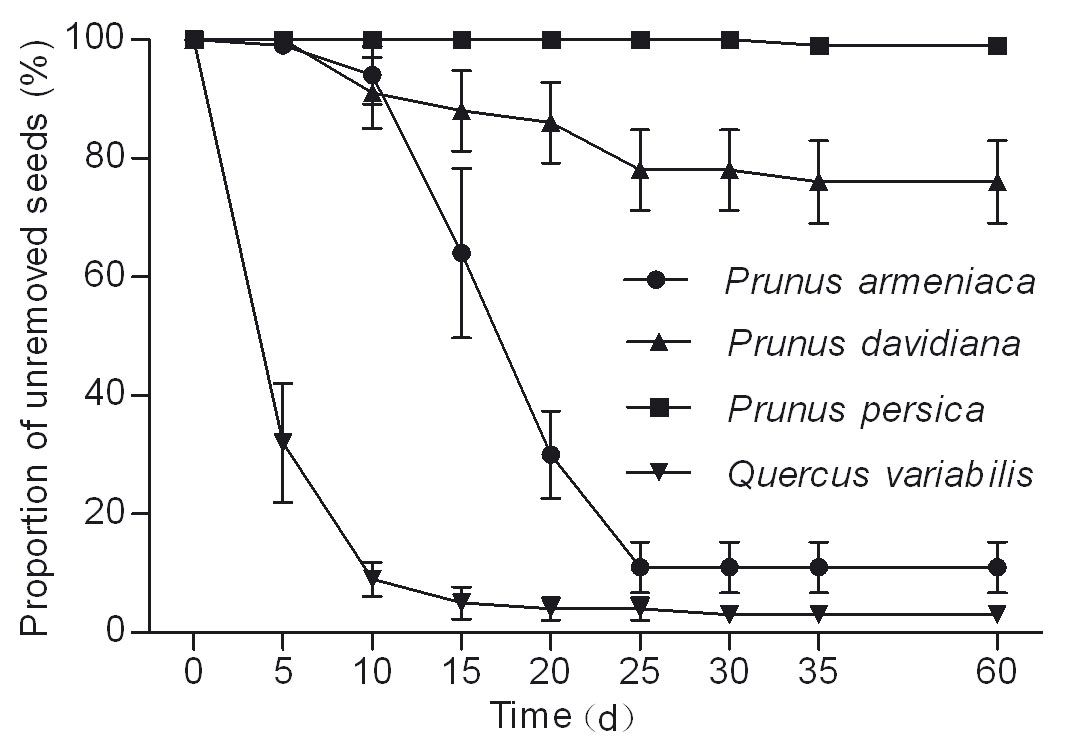
Most of acorns (96%) and wild apricots (89%) were removed within 25 days, while 99% of released peach seeds remained in situ. Removal rates of the released seeds differed significantly among tree species (cork oak, wild apricot and wild peach: χ2 = 107.036, df = 2, P < 0.001 - Fig. 1). The mean survival time of acorns (8.60 ± 0.39 days) was significantly lower than wild apricots (20.90 ± 0.53 days; χ2 = 124.062, df = 1, P < 0.001) and wild peaches (24.50 ± 1.69 days; χ2 = 33.703, df = 1, P < 0.001 - Fig. 1).
Fate of released seeds
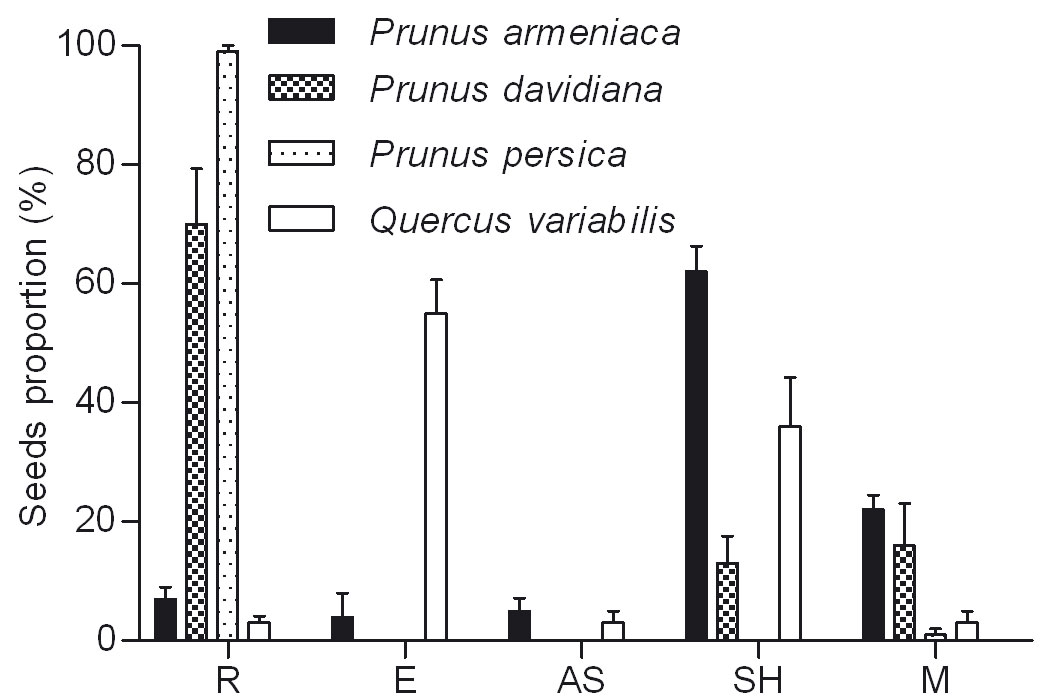
Rodents showed a preference for wild apricot and cork oak higher than wild peach (F = 45.559, df = 2, P < 0.001 - Fig. 2). The proportion of R was significantly higher in wild peach (70%) than in wild apricot (7% - P < 0.001) and cork oak (3% - P < 0.001 - Fig. 2).
Fig. 2 - Fate of released seeds after removal by rodents. (R): remained in situ; (E): eaten; (AS): abandoned on the surface; (SH): scatter-hoarding; (M): Missing.
The proportion of E was significantly different among seed species (cork oak, wild apricot and wild peach - F = 58.165, df = 2, P < 0.001), with cork oak (55 %) higher than wild apricot (4 % - P < 0.001) and wild peach (0 % - P < 0.001 - Fig. 2). Moreover, the proportion of E was strongly correlated (R2 = 0.8265) with hull thickness (y = -40.678 · ln x + 47.069).
Except for peach seeds, many seeds of wild apricot, wild peach and cork oak were in status of SH, with significant differences among tree species (F = 16.541, df = 2, P < 0.001). The proportion of SH cork oak (36 %) and wild apricot (22 %) were much higher than wild peach (13 % - P < 0.001 - Fig. 2). Also in this case the proportion of SH seeds was correlated (R2 = 0.7236) with the hull thickness (y = -9.75 · x2 + 33.05 · x + 18.25).
Variation in cache size among tested seeds
Most scattered cache sites (89.29 %) of wild apricot contained only one seed, whereas 10.71 % contained two or three seeds; cache sites of both wild peach and cork oak had only one seed (Tab. 2). Significant differences were found among the three species for two-seed caches (F = 3.750, df = 2, P < 0.05 - Tab. 2).
Tab. 2 - Scatter-hoarded cache size of seeds from different tree species. (*): significant differences among species (P<0.05).
| Species | Dispersal (m) |
Cache size (%) | |||
|---|---|---|---|---|---|
| 1 seed | 2 seeds | 3 seeds | >3 seeds | ||
| Prunus armeniaca | 3.41 ± 2.05 | 89.29 | 8.93* | 1.78 | 0 |
| Prunus davidiana | 3.81 ± 2.44 | 100 | 0 | 0 | 0 |
| Quercus variabilis | 2.49 ± 2.37* | 100 | 0 | 0 | 0 |
Dispersal distances of tested seeds
The highest dispersal distance of the removed seeds was less than 15 m, although more of 95 % of seeds were dispersed less than 9 m. The mean dispersal distance was 3.41 ± 2.05 m (wild apricot - n = 58), 3.81 ± 2.44 m (wild peach - n = 14) and 2.49 ± 2.37m (cork oak - n = 57) respectively, with significant differences among species (F = 3.365, df = 2, P < 0.05). Especially, the mean dispersal distance of cork oak was remarkably lower than that of the other species (P < 0.05 - Tab. 2).
Discussion
Under natural conditions, different plant seeds usually coexist in given geographical area and provide potential food resources for granivorous animals ([35], [18], [38], [32]). However, sympatric seeds may differ notably in palatability and nutrition value ([38]). To survive and reproduce, seed-eating animals had developed numerous adaptations in treating and consuming various sympatric seeds ([6], [7], [47]).
Discriminatory handling on sympatric seeds
Our results revealed that rodents displayed discriminatory processing strategies in eating and hoarding sympatric forest seeds. Rodents preferred to consume acorns having thinner hulls, while scatter-hoarded wild apricot and wild peach seeds having thicker hulls, and ignored peach seeds with the thickest hulls. The results supported our predictions and indicated that thickness of seed hull produces a nonlinear effect on the scatter-hoarding behavior of rodents. This selectivity in seeds consumption and dispersal may be explained by the trade-off between costs and benefits in handling seeds. Because acorns are vulnerable to microorganism infection and deteriorate easily, they are not suitable for long-term storage ([36], [37], [20], [51]). Their weak hulls are especially convenient for instant consumption by predators. Furthermore, seeds of wild apricot and wild peach are covered with medium-thickness hulls, determining higher consumption costs, as well as longer edibility-guarantee-period ([14], [9], [20], [1]). Almost all peach seeds with very thick hulls were rejected by rodents. This could possibly be attributed to: (1) lower reward and higher predation risk ([18], [51]); (2) the influence of alternative food resources within habitat during study period; or (3) the unsuitable tooth structure of these rodent species to consume such seeds, on which further investigation is needed.
Difference in cache size
In this study, all seeds of cork oak and wild peach were buried singly in each cache site, whereas multiple seeds of wild apricot were found in scatter-hoarded caches. The size of apricot seeds was greatly smaller than that of cork oak and wild peach, so seed size may have accounted for the differentiation in cache size. It is difficult for small rodents to carry many big-sized seeds at one time and the number of seeds in one cache site decreases with increasing seed size ([38], [22], [42]).
Differentiation in hoarding strategy to sympatric species seeds might affect seed fate ([45], [46], [25], [30]). Single-seed caches are favorable for seed germination and seedling establishment compared to multiple-seed and larder-hoarded caches ([11]). Seedlings emerging from clumped seeds often suffered a high mortality rate because of intense competition for limited resources and space ([12]). Also, larger caches were more likely to be found and plundered by conspecific and interspecific foragers ([39]).
Variation in dispersal distance
Dispersal enhances the spreading of plant seeds far away from the mother trees and therefore boost the species colonization ([26], [40]). Some studies demonstrated that larger seeds are transported at a greater distance than smaller ones ([46]); nevertheless other studies showed that seeds with higher predation reward were usually transported and stored at farther distance ([17], [9], [47]). However, we founded that wild peach and wild apricot seeds were moved and hoarded farther than acorns. The reasons might be that seeds of wild peach and wild apricot had moderately thick hulls and were suitable for long-term hoarding compared to acorns. So, dispersal distance may be affected by joint factors such as seed size ([46]), costs and rewards of hoarding ([14], [9], [33], [47]), and the suitability of seeds to storage ([20]).
Conclusions
Rodents exhibited discriminatory selection to sympatric plants when consuming and hoarding their seeds. Consequently, the influence of rodents on seed fate would vary according with seed traits. For instance, hull thickness would produce a non-linear effect on seed dispersal, with species having medium thickness hull being advantaged in seed dispersal and survival. This research might be useful in explaining the co-evolution of plants and animals, and broaden our understanding to the co-existence mechanisms of sympatric forest trees with heavy seeds.
Acknowledgments
We are very grateful to Dr. Terry Boyd-Zhang for revising this manuscript and appreciative to Jiyuan State-owned Yugong Forest Farm for assistance in field work. This study was financially supported by the National Basic Research Program of China (No. 2007CB109106) and the Key Subject Funds of Zoology of Henan Province. Author contributions: Zhang Y and Lu J conceived and designed the experiments; Zhang Y, Wang C and Tian S performed the experiments; Zhang Y analyzed the data; Zhang Y and Lu J wrote the manuscript; Zhang Y originally formulated the idea.
References
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Gscholar
Authors’ Info
Authors’ Affiliation
Chong Wang
Shu-Liao Tian
Ji-Qi Lu
Institute of Biodiversity and Ecology, Zhengzhou University, 450001 Zhengzhou (China)
Zhengzhou Zoo, 450008 Zhengzhou (China)
Corresponding author
Paper Info
Citation
Zhang Y-F, Wang C, Tian S-L, Lu J-Q (2014). Dispersal and hoarding of sympatric forest seeds by rodents in a temperate forest from northern China. iForest 7: 70-74. - doi: 10.3832/ifor1032-007
Academic Editor
Massimo Faccoli
Paper history
Received: May 01, 2013
Accepted: Sep 07, 2013
First online: Nov 18, 2013
Publication Date: Apr 02, 2014
Publication Time: 2.40 months
Copyright Information
© SISEF - The Italian Society of Silviculture and Forest Ecology 2014
Open Access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Web Metrics
Breakdown by View Type
Article Usage
Total Article Views: 54190
(from publication date up to now)
Breakdown by View Type
HTML Page Views: 44665
Abstract Page Views: 3476
PDF Downloads: 4483
Citation/Reference Downloads: 37
XML Downloads: 1529
Web Metrics
Days since publication: 4428
Overall contacts: 54190
Avg. contacts per week: 85.67
Article Citations
Article citations are based on data periodically collected from the Clarivate Web of Science web site
(last update: Mar 2025)
Total number of cites (since 2014): 11
Average cites per year: 0.85
Publication Metrics
by Dimensions ©
Articles citing this article
List of the papers citing this article based on CrossRef Cited-by.
Related Contents
iForest Similar Articles
Research Articles
Seed trait and rodent species determine seed dispersal and predation: evidences from semi-natural enclosures
vol. 8, pp. 207-213 (online: 28 August 2014)
Research Articles
The effect of seed size on seed fate in a subtropical forest, southwest of China
vol. 9, pp. 652-657 (online: 04 April 2016)
Short Communications
Evidence of Alectoris chukar (Aves, Galliformes) as seed dispersal and germinating agent for Pistacia khinjuk in Balochistan, Pakistan
vol. 14, pp. 378-382 (online: 22 August 2021)
Research Articles
Effects of functional traits on the spatial distribution and hyperdominance of tree species in the Cerrado biome
vol. 15, pp. 339-348 (online: 01 September 2022)
Research Articles
Inter- and intra-annual patterns of seed rain in the black spruce stands of Quebec, Canada
vol. 10, pp. 189-195 (online: 13 December 2016)
Research Articles
Seed germination traits of Pinus heldreichii in two Greek populations and implications for conservation
vol. 15, pp. 331-338 (online: 24 August 2022)
Short Communications
Towards an optimal sampling effort for paternity analysis in forest trees: what do the raw numbers tell us?
vol. 5, pp. 18-25 (online: 27 February 2012)
Review Papers
Soil seed banks of pioneer tree species in European temperate forests: a review
vol. 11, pp. 48-57 (online: 25 January 2018)
Research Articles
What if Eurasian jay Garrulus glandarius would larder acorns instead of scatter them?
vol. 11, pp. 685-689 (online: 23 October 2018)
Research Articles
The effectiveness of short-term microwave irradiation on the process of seed extraction from Scots pine cones (Pinus sylvestris L.)
vol. 13, pp. 73-79 (online: 13 February 2020)
iForest Database Search
Search By Author
Search By Keyword
Google Scholar Search
Citing Articles
Search By Author
Search By Keywords
PubMed Search
Search By Author
Search By Keyword